Biochemistry and Physiology of Mitochondrial Ion Channels Involved in Cardioprotection
Received: 06-Jun-2022 / Manuscript No. bcp-22-67426 / Editor assigned: 09-Jul-2022 / PreQC No. bcp-22-67426 / Reviewed: 20-Jun-2022 / QC No. bcp-22-67426 / Revised: 25-Jun-2022 / Manuscript No. bcp-22-67426 / Published Date: 30-Jun-2022 DOI: 10.4172/2168-9652.1000383
Abstract
Over the past decades there has been considerable progress in understanding the multifunctional roles of mitochondrial ion channels in metabolism, energy transduction, ion transport, signaling, and cell death. Recent data have suggested that some of these channels function under physiological condition, and others may be activated in response to pathological insults and play a key role in cytoprotection. This review outlines our current understanding of the molecular identity and pathophysiological roles of the mitochondrial ion channels in the heart with particular emphasis on cardioprotection against ischemia/reperfusion injury, and future research on mitochondrial ion channels.
Keywords
Mitochondrial ion channel; ATP-sensitive potassium channel; Calcium-activated potassium channel; Permeability transition pore; Calcium uniporter; Cardioprotection
Introduction
Mitochondria play an important role in energy metabolism within the cell. In addition, the organelles are also involved in regulation of cell death and survival, a crucial determinant of apoptosis or necrosis. However, the physiological roles of mitochondrial ion channels on inner membrane are largely unknown. For example, K+-selective and/or anion-selective pores are believed to operate to regulate mitochondrial volume [1], but it is difficult to achieve in vivo confirmation of this function . Mitochondrial swelling and shrinkage have been proposed to modulate the rate of substrate oxidation under normoxic conditions and could be an important factor in determining the extent of ischemia/reperfusion injury . At a minimum, energy dissipation due to channel opening will increase the flux through the electron-transport chain, and if this effect is uncompensated by increased substrate production, then net oxidation of the matrix, as well as a change in reactive oxygen species (ROS) production, will occur. Thus one potential physiological role of mitochondrial ion channels may be redox regulation, which is an important intracellular signaling mechanism influencing transcription, translation, phosphorylation cascade, and cell death [2].
In the cardiovascular system mitochondria are target organelles of ischemic insults as well as effectors for cardioprotection. As pointed out by Jennings and Ganote 30 years ago, the heart is strictly aerobic and consequently vulnerable to a decrease in oxygen supply. Therefore, myocardial ischemia causes immediate and deep mitochondrial derangements. These include cessation of ATP synthesis, inhibition of respiration, and depolarization of mitochondrial membrane (ΔΨm). This is accompanied by cellular changes, especially an increase in Ca2+ and phosphate during ischemia, and large increases in ROS originating from the respiratory chain during reperfusion . Also from the therapeutic perspective, with reference to ischemia/reperfusion injury in the heart and brain, it is important to note that mitochondrial ion channels especially play a key role. An increase in mitochondrial K+ flux, for example, in hearts treated with K+ channel opener compounds activating mitochondrial ATP-sensitive K+ (mitoKATP) or Ca2+-activated K+ channels (mitoKCa), has been found to significantly decrease infarct size and improve functional recovery after ischemia/ reperfusion. On the other hand, blocking mitochondrial ion channels such as the mitochondrial permeability transition pore (mPTP) can prevent the loss of mitochondrial function that leads to necrotic or apoptotic cell death. Since cardioprotection involves the activation of mitoKATP or mitoKCa channels and a decrease in mPTP opening, it is reasonable to hypothesize that these two phenomena are part of the same signaling pathway. Indeed, this connection has been demonstrated in a previous report [3].
In this review we describe our current understanding of the molecular identity and pharmacological properties of important four mitochondrial ion channels conferring cardioprotection (see the property of these ion channels (Figure 1).
Figure 1: Mitochondrial Cx43 in cardiomyocytes. In cardiomyocytes, the accumulation of Cx43 in mitochondria occurs primarily in the inner membrane of subsarcolemmal mitochondria, where the close proximity to the plasma membrane and internalized annular gap junctions likely favour the transfer of Cx43 to the mitochondria.
The mitochondrial Ca2+ uniporter
Mitochondrial Ca2+ uptake has been recognized for more than 50 years, yet the protein mediating this essential transport process has still not been clarified. However, the mitochondrial Ca2+ uniporter has perhaps the most well-understood transporter in physiology. The Ca2+ uniporter is a relatively fast mechanism and a channel, which transports Ca2+, Sr2+, Mn2+, Ba2+, but not Mg2+ with different selectivity and very low affinity. Because of the low number of uniporters found per mg protein (around 0.001 nmol/mg protein reported and the large value of the estimated Vmax [ ≈1200 nmol Ca2+/(mg min)] the turnover of Ca2+ per site can be calculated to be about 2 × 104 Ca2+/(site s), in the range of a fast gated pore. Studies performed on isolated mitochondria allowed the identification of some regulatory molecules acting on Ca2+ uniporter, in particular the most effective inhibitor is hexavalent cation ruthenium red (RuR). This RuRsensitive Ca2+ uptake pathway is the primary route for Ca2+ entry into the mitochondrial matrix, and there is strong evidence that an increase in matrix Ca2+ is important for stimulating oxidative phosphorylation at several sites, including the Ca2+-sensitive dehydrogenases of the Krebs cycle and one or more sites in the electron-transport chain [4].
This uniporter is modulated by aliphatic polyamines, such as spermine and aminoglycosides, and by the adenine nucleotides, in the order of effectiveness ATP > ADP > AMP (whereas the nucleoside adenosine is ineffective) as well as several plant-derived flavonoids . Recently, an inwardly rectifying, highly Ca2+ selective, low conductance ion channel that is compatible with mitochondrial Ca2+ uniporter was recorded in a patch-clamp study of intact heart mitoplasts . However, the molecular structure of this channel has not yet been determined despite a number of attempts to identify Ca2+ uniporter protein over years. Trenker et al. suggested that the uncoupling proteins UCP2 and UCP3 were promising candidates for enabling Ca2+ uptake in mitochondria since isolated liver mitochondria from UCP2 knockout mice showed no RuR-sensitive Ca2+ uniporter activity. On the opposite, in new data submitted by Brookes et al. there was no effect of UCP2 or UCP3 knockout on either Ca2+ uptake or membrane potential depolarization in mitochondria from liver, skeletal muscle, heart, or kidney [5]. As described below, the inhibition of Ca2+ influx to matrix through mitochondrial Ca2+ uniporter by mitochondrial K+ cannel opening results in decrease of mitochondrial Ca2+ overload. Mitochondrial Ca2+ loading is associated with cell injury although the relative contributions of cytoplasmic vs. mitochondrial Ca2+ overload are often difficult to assess individually due to their interdependence, and there is not always a clear correlation between mitochondrial Ca2+ and survival.
Mitochondrial K+ channel and cardioprotection
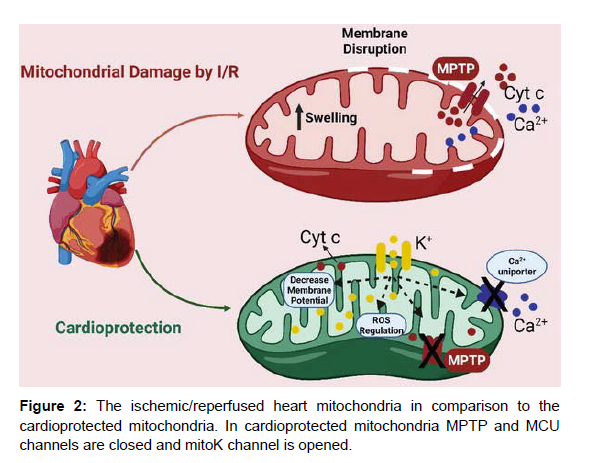
Some 25 years ago Lamping et al. showed that pharmacological agents capable of opening K+ channels have cardioprotective effect against ischemia/reperfusion injury . Earlier studies naturally supposed that the target of these compounds was the sarcolemmal ATP-sensitive K+ (sarcKATP) channel. However, a new door was opened in 1991, with the discovery of mitoKATP channels in the liver mitochondrial inner membrane [6]. This finding was bolstered by evidence that a variety of K+ channel openers and inhibitors influenced mitochondrial function , resulting in the establishment of a link between the mitoKATP channel and cardioprotection against ischemia/reperfusion injury in intact hearts and isolated myocytes . Therefore, the focus has shifted recently to the mitochondria as the primary target of these agents (Figure 2).
Mechanism of cardioprotection by mitochondrial K+ channel
One of the major physiological features of mitochondria is the generation of a large transmembrane potential across the mitochondrial inner membrane. This is a direct consequence of the biochemical reactions that constitute the respiratory chain. Thus, substrates supplied to mitochondria such as pyruvate, products of β-oxidation of fatty acids, and some amino acids enter the TCA cycle and maintain the reduced state of the NADH/NAD+ and FADH2/FAD couples. These substances supply electrons to the respiratory chain, which eventually are transferred to oxygen [7]. The process also transfers protons across the mitochondrial inner membrane, generating a proton gradient – a proton motive force that is largely expressed as ΔΨm usually estimated as 180 mV negative to the cytosol. The Ca2+ uptake into mitochondria is driven primarily by this large negative electrical potential of the matrix . Therefore, partial depolarization of ΔΨm through opening of mitochondrial K+ channels reduces the driving force for Ca2+ influx through the Ca2+ uniporter, which results in the prevention of mitochondrial Ca2+ overload during ischemia. Ishida et al. have reported that in rat cardiomyocytes the selective mitoKATP channel opener diazoxide depolarizes the mitochondrial membrane and attenuates the mitochondrial Ca2+ overload experimentally evoked by ouabain. Ouabain, a Na+/K+-ATPase inhibitor, impairs Na+ extrusion and consequently prevents Ca2+ extrusion via Na+/Ca2+ exchange. Elevation in cytosolic Ca2+ concentration eventually results in the accumulation of mitochondrial Ca2+. Furthermore, we have recently demonstrated that K+ influx via mitochondrial K+ channels accelerates electron transfer by the respiratory chain and leads to net oxidation of mitochondria if uncompensated by electron donors, and attenuates mitochondrial Ca2+ overload with accompanying depolarization of the mitochondrial membrane .
Mitochondrial ATP-sensitive K+ channel
In 1991, Inoue. reported the identification of an ATP-sensitive K+ channel in the mitochondria. They patch clamped mitoplasts (mitochondria stripped of their outer membranes) from rat liver and identified K+ selective channels, sensitive to inhibition by ATP, 4-aminopyridine and glibenclamide. The conductance of these channels was lower than that of ssrcKATP channels (about 10 pS in 100 mM matrix K+ and 33 mM cytosolic K+). Subsequent studies by several groups have demonstrated that mitoKATP may be the key player in cardioprotection.
Garlid’s group has used two different approaches to study the channel. In their first approach, a protein fraction was purified on DEAE-cellulose columns, and an ATP-binding affinity column. The fraction was reported to contain two major proteins of 55 and 63 kDa in size; the larger protein was reported to bind to fluorescently labeled glibenclamide. The protein fraction was then incorporated into liposomes containing a K+-sensitive fluorescent marker. The emission intensity of this marker increases when K+ binds to it. Thus, the rate of fluorescence increase can be used to quantify K+ transport into the proteoliposomes . In their second approach, they assessed steady state matrix volume by measuring light scattering at 520 nm in the intact isolated mitochondria . The opening of mitoKATP channel and influx of K+ result in an increase in the osmotic pressure and water movement into the mitochondrial matrix, which would lead to an increase in the mitochondrial matrix volume . Thus, changes in the matrix volume can be used as an indirect measure of the mitoKATP channel activity.
Our study group has taken a different approach to assay the he autofluorescence of mitochondrial flavoproteins and NADH, which increases with partial uncoupling generated by mitoKATP channel activation.The advantage of this technique is that it can be used in intact cells. However, there are also several limitations with this technique. Since the mitochondrial redox potential is a balance between NADH production and oxidation, it does not represent the overall rate of oxidative phosphorylation of the cell.
O’Rourke et al. have indicated that pharmacological drugs such as diazoxide open mitoKATP channel, and 5-hydroxydecanoate has been used as a blocker of mitoKATP channel . MitoKATP channel openers such as diazoxide, nicorandil, minoxidil and so on have been shown to be cardioprotective in all species examined. The ability of these agents to open mitoKATP channels in their therapeutic dose range was described in 1996. Diazoxide was 1000 times more potent in opening of mitoKATP channel than in opening of sarcKATP channel, making diazoxide a valuable tool to determine whether cardioprotection was mediated by the activation of sarcKATP or mitoKATP channels [8].
The molecular structure of sarcKATP channel has been clarified by cloning of the inwardly rectifying K+ channel subfamily Kir6.0 (Kir6.1, 48-kDa and Kir6.2, 44-kDa) and the receptors for sulfonylureas (SUR1, 177-kDa, SUR2A, 174-kDa, and SUR2B, 174-kDa). From a reconstitution study, this channel on cardiomyocytes has been suggested to comprise SUR2A and Kir6.2. Our functional study using Kir6.2-deficient mice has provided direct evidence that Kir6.2 forms the pore region of cardiac sarcKATP channels. On the other hand, molecular identification of mitoKATP channel has not been achieved yet. Although the molecular structure of mitoKATP channel remains unclear, experiments using dominant negative gene transfer have indicated that neither Kir6.1 nor Kir6.2 is a functionally important part of the mitoKATP channel in intact cardiomyocytes . In addition, the mitoKATP channel function, evaluated by flavoprotein oxidation, was preserved in cardiomyocytes of either Kir6.1-deficient or Kir6.2- deficient mice However, K+ influx activity has been observed when purified mitochondrial proteins in the molecular weight range of 50– 60 kDa have been reconstituted into proteoliposomes , and a 54-kDa protein was tentatively identified as a component of mitoKATP channel . The existence of SUR proteins in cardiac mitochondria is still controversial, whilst a protein of about 28 kDa was labeled by [125I]- glibencamide. Recently, some study has reported that mitoKATP channel is potentially composed of four mitochondrial proteins (mitochondrial ATP-binding cassette protein 1, phosphate carrier, adenine nucleotide translocator, and ATP synthase) and succinate dehydrogenase, and multiprotein complex
Funding
Self-funded.
Acknowledgement
None
Conflict of Interest
None declared.
References
- Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, et al. (2020) Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun 56-58.
- Etienne MS, Hall A (2002) Rho GTPases in cell biology. Nature 629-635.
- Dai L, Zhao T, Bisteau X, Sun W, Prabhu N, Lim YT, Sobota RM, Kaldis P, Nordlund P, et al. (2018) Modulation of Protein-Interaction States through the Cell Cycle . Cell 1481-1494.
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, et al. (2020) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Sci 1078-1081.\
- O'Malley MA, Soyer OS (2012) The roles of integration in molecular systems biology. Stud Hist Philos Biol Biomed Sci 58-68.
- Darden L, Kundu K, Pal LR, Moult J (2018) Harnessing formal concepts of biological mechanism to analyze human disease. PLoS Comput Biol 106-540.
- Moczek AP, Sears KE, Stollewerk A, Wittkopp PJ, Diggle P, et al.(2019)The significanceand scope of evolutionary developmental biology: a vision for the 21st century. Evol Dev 198-219.
- Kampis G, Gulyás L (2008) Full body: the importance of the phenotype in evolution. Artif Life 375-386.
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at,Google Scholar, Cross Ref
Citation: Takoshi H (2022) Biochemistry and Physiology of Mitochondrial Ion Channels Involved in Cardioprotection. Biochem Physiol 11: 383. DOI: 10.4172/2168-9652.1000383
Copyright: © 2022 Takoshi H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2243
- [From(publication date): 0-2022 - Sep 23, 2025]
- Breakdown by view type
- HTML page views: 1752
- PDF downloads: 491