Research Article Open Access
Biobutanol Production from Lignocellulosic Biomass: Prospective and Challenges
Guangli Cao1,3*, Yachun Sheng1, Liang Zhang1, Jinzhu Song1, Hua Cong1 and Junzheng Zhang2
1School of Life Science and Technology, Harbin Institute of Technology, Harbin 150001, China
2School of Chemistry, Harbin Institute of Technology, Harbin 150090, China
3State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China
- *Corresponding Author:
- Guangli Cao
School of Life Science and Technology
Harbin Institute of Technology
Harbin 150001, China
Tel: 8645186402652
E-mail: caogl@hit.edu.cn
Received date: June 27, 2016; Accepted date: July 29, 2016; Published date: July 30, 2016
Citation: Cao G, Sheng Y, Zhang L, Song J, Cong H, et al. (2016) Biobutanol Production from Lignocellulosic Biomass: Prospective and Challenges. J Bioremed Biodeg 7: 363. doi:10.4172/2155-6199.1000363
Copyright: © 2016 Cao G, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Biobutanol production from lignocellulosic biomass is considered promising and economically feasible. This paper provides an updated review on development of lignocellulose-based biobutanol production with a focus on the understanding of the structure of the feedstock, pretreatment technologies, and fermentation processes. To enhance butanol production from lignocellulosic materials, strategies in terms of inhibitors detoxification, strains improvement and process integration and optimization are also addressed. Besides, the reviewer attempts to shed light on the challenges and perspectives for the bioconversion of lignocellulosic biomass-to-butanol.
Keywords
Butanol; Lignocellulose; Pretreatment; Fermentation; Bioconversion
Introduction
Considering the fast depletion of fossil fuel-reserves and environmental impact caused by the over-consumption of fossilbased energy, there is an urgent need to develop renewable and environmental friendly alternatives to substitute fossil fuels. Butanol is an idea alternative due to its significant properties such as high energy content, low volatility and hydroscopicity [1]. In particular, it can be used directly or blended with gasoline without the need to change any current vehicles and supplied with existing gasoline pipes [2]. Of the butanol production ways, biological butanol (biobutanol) production by microbial fermentation has been widely concerned [3,4]. Nevertheless, the major obstacle to the commercialization of such a sustainable pathway is the high production cost [4,5]. Currently, biobutanol is mainly produced from conventional sugar-and starchbased feedstocks such as sugarcane, molasses and corn [6]. Taking into account the global population and increased demand for food supply, this feedstock is not sustainable and cost-effective. Therefore, the development of alternative feedstocks that are widely available at low cost and abundant supply is the key issue.
Lignocellulosic biomass, such as agricultural and forest residues, is by far the most abundant and sustainable renewable natural resource on the earth. Particularly, as one of the biggest agricultural countries in the world, China has abundant lignocellulosic biomass resources. It’s reported that nearly 0.73 billion tons of agricultural residues are produced per year, the value is equivalent to 12,000 trillion kJ of energy. Moreover, there is approximately 37 million m3 of forest wastes, which contained 580 trillion kJ of energy [7-9]. Unfortunately, up to now, most of the biomass residuals are burned directly. This not only has low energy efficiency, but also causes serious environmental pollution such as respiratory aerosols, SO2 and CO. Therefore, the conversion of lignocellulosic biomass to high quality bioenergy by advanced processes rather than combustion is a promising way to substitute fossil-based fuels and decrease greenhouse gas emissions [10,11]. However, there are challenges in producing butanol from lignocellulosic biomass due to their recalcitrance to degradation [12] as well as their unique chemical compositions. It has been acknowledged that the use of lignocellulosic biomass involves pretreatment and hydrolysis of raw material followed by fermentation of sugars to butanol. The process can be made costeffective by choosing an efficient pretreatment, hydrolysis method, and fermentation strategy. Although several studies have demonstrated the potential bioconvertion of lignocellulosic biomass to butanol after pretreatment, there is still lack of systematic study on bioconvertion lignocellulosic biomass to butanol to the authors’ best knowledge.
In this paper, an up-to-date review on the bioconversion of lignocellulosic biomass to butanol in terms of the characteristics of the feedstock, pretreatment technologies, fermentation processes and strategies for enhancing butanol production. Remaining challenges and perspectives for the conversion of lignocellulosic biomass to butanol are also presented.
Structure of Lignocellulosic Biomass
Lignocellulosic biomass is a complex substrate. Understanding of its characteristics, particularly its chemical compositions, is a prerequisite for developing efficient pretreatment technologies to deconstruct its rigid structure and conditioning subsequent enzymatic hydrolysis of cellulose to liberate sugars, as well as designing microorganisms to convert sugars into butanol with high yields.
Lignocellulosic material is mainly composed of cellulose, hemicellulose and lignin, which are associated with each other [13]. However, the composition of these constitutes varies widely with plant species, growth of stage and area, time of harvest and other conditions, e.g., softwoods have higher content of cellulose, whereas grass and leaves have more hemicellulose (Table 1).
| Lignocellulosic material | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Hardwood stems | 40-55 | 24-40 | 18-25 |
| Softwood stems | 45-50 | 25-35 | 25-35 |
| Nut shells | 25-30 | 25-30 | 30-40 |
| Corn cobs | 45 | 35 | 15 |
| Paper | 85-99 | 0 | 0-15 |
| Wheat straw | 30 | 50 | 15 |
| Rice straw | 32.1 | 24 | 18 |
| Sorted refuse | 60 | 20 | 20 |
| Leaves | 15-20 | 80-85 | 0 |
| Cotton seeds hairs | 80-95 | May-20 | 0 |
| Newspaper | 40-55 | 25-40 | 18-30 |
| Waste paper from | 60-70 | Oct-20 | 05-Oct |
| chemical pulps | |||
| Fresh bagasse | 33.4 | 30 | 18.9 |
| Swine waste | 6 | 28 | NA |
| Solid cattle manure | 1.6-4.7 | 1.4-3.3 | 2.7-5.7 |
| Switch grass | 45 | 31.4 | 12 |
NA, data not available; aAdapted from Ref. [14]
Table 1: Chemical compositions of various lignocellulosic biomassa.
Cellulose
Cellulose is the most abundant and interesting polysaccharide in biomass [15]. Its molecule is composed of D-glucose subunits that are linked by β-1,4-glycosidic bonds with cellubiose as the repeating unit. Fermentable D-glucose can be produced from cellulose by breaking the β-1, 4-glycosidic linkages.
The cellulose in biomass consists of two forms: one is organized structure called crystalline cellulose and the other is not well-organized structure called amorphous cellulose [16]. Crystalline structure comprises the major proportion of cellulose, whereas small amount of unorganized structure forms amorphous cellulose. Compared to crystalline cellulose, amorphous cellulose is more susceptible to enzyme. Appropriate measures are therefore needed to change crystalline structure cellulose in biomass into amorphous polymorphs that can be hydrolyzed more efficiently by cellulases.Hemicellulose
Different from cellulose, hemicellulose is a more complex carbohydrate structure that consists of different polymers including pentoses (such as xylose and arabinose), hexoses (such as glucose and galactose), and uronic acids (such as 4-omethylglucuronic and D-galactouronic acids) [17]. In biomass, hemicelluloses are embedded and interact with cellulose and serve as a connection between the lignin and the cellulose fibers which make the whole cellulose–hemicellulose–lignin network more strength and toughness. In contrast to the resistance of crystalline cellulose, the polymers present in hemicellulose are more sensitive to thermalchemically pretreatment and easy to be hydrolyzed to monomer sugars even though they cocrystallize with cellulose chains [18].
Lignin
Of the three components, lignin which presents in the cellular wall is the most recalcitrant to degradation due to its highly organized structure. It is an amorphous heteropolymer consisting of three major monolignols (p-coumaryl, coniferyl and sinapyl alcohols) that are linked together by alkyl-aryl, alkyl-alkyl, and aryl-aryl ether bonds. Lignin is not a sugar-based polymer and cannot be employed as substrate for biobutanol production. However, it exhibits a significant influence on the economic and efficient performance of the bioconversion processes, since most undesirable inhibitors of microbial growth and fermentation released during the pretreatment from this compound [19].
Pretreatment of Lignocellulosic Materials
It is acknowledged that the presence of lignin and hemicellulose makes the cellulose more difficult to cellulases attack. For improving the conversion of lignocellulosic biomass to biobutanol, pretreatment is required to alter the structure and chemical composition of the biomass, so that the hydrolysis efficiency of the carbohydrate to fermentable sugars can be enhanced with high yield and rate [20,21]. In the past decades, various pretreatments methods, roughly divided into four categories: physical pretreatment, chemical pretreatment, solvent fractionation and biological decomposition, have been developed to improve hydrolysis rates [22]. As an ideal pretreatment process, it should maximize sugars yield from pretreated biomass, and in the meantime minimize energy consumption and environmental impact. However, none of them can meet all these criteria. For example, current widely used chemical pretreatment methods like acid/alkaline hydrolysis and ozonalysis which require excessive use of chemicals such as concentrated acids [22,23], alkaline and oxidizing agents that may interfere in the subsequent hydrolysis [24]. Moreover, these chemicals are toxic, corrosive and hazardous, which make the pretreatment more costly due to the need of anti-corrosion equipment. Similarly, physical-chemical methods like steam explosion, ammonia fiber expansion (AFEX), and wet air oxidation which are operated at high temperature and pressure, making the process energy-intensive and economic unfeasible [25]. In addition, operations at such high temperature and pressure lead to the formation of toxic compounds such as furfural and 5-hydroxymethylfurfural (HMF) derived from sugar degradation, acetate from hemicellulose, and a number of soluble aromatic compounds (acids, aldehydes, and alcohols) from lignin, which are known to be inhibitory to enzymatic hydrolysis and fermentation [26,27]. Thus the major challenge for pretreatment is to avoid the degradation or loss of carbohydrate, and minimize the cost of treatment so as to make the process economically feasible.
Table 2 lists the advantages and disadvantages of various pretreatments [28]. Of all pretreatments, a combination of different pretreatments by taking advantage of their specific superiorities might be favorable since the limitations of one pretreatment maybe overcome by the other pretreatment used in the combination. For instance, sulfurcatalyzed steam explosion in which an external acid was added to aid solubilize hemicellulose, this can help lower the optimal pretreatment temperature and pressure and give a better enzymatic hydrolysis ratio [29,30]. In another combination of biological pretreatment using white-rot fungi to treat rice straw followed with AFEX pretreatment gave significantly higher glucan and xylan conversions and less-severe AFEX conditions than with AFEX only [31]. In addition, compared with physical and chemical pretreatments in which excess chemicals and water and intensive energy consumption are needed, biological pretreatment by employing microorganisms to degrade lignocellulosic biomass at mild condition with the advantages of high specificity and low energy requirement maybe another cost-effective strategy [32].
| Pretreatment process | Advantages | Limitations and disadvantages |
|---|---|---|
| Mechanical comminution | Reduces cellulose crystallinity | Power consumption usually higher than inherent biomass energy |
| Steam explosion | Causes hemicellulose degradation and lignin transformation; cost-effective | Destruction of a portion of the xylan fraction; incomplete disruption of the lignin-carbohydrate matrix; generation of compounds inhibitory to microorganisms |
| AFEX | Increases accessible surface area, removes lignin and hemicellulose to an extent; does not produce inhibitors for downstream processes | Not efficient for biomass with high lignin content |
| CO2 explosion | Increases accessible surface area; cost-effective; does not cause formation of inhibitory compounds | Does not modify lignin or hemicelluloses |
| Ozonolysis | Reduces lignin content; does not produce toxic residues | Large amount of ozone required; expensive |
| Acid hydrolysis | Hydrolyzes hemicellulose to xylose and other sugars; alters lignin structure | High cost; equipment corrosion; formation of toxic substances |
| Alkaline hydrolysis | Removes hemicelluloses and lignin; increases accessible surface area | Long residence times required; irrecoverable salts formed and incorporated into biomass |
| Organosolv | Hydrolyzes lignin and hemicelluloses | Solvents need to be drained from the reactor, evaporated, condensed, and recycled; high cost |
| Biological | Degrades lignin and hemicelluloses; low | Rate of hydrolysis is very low |
| energy requirements |
Table 2: Comparative evaluation of different pretreatment strategies.
As mentioned above, lignocellulosic biomass is a complex feedstock whose physicochemical properties and chemical compositions vary with different biomass. It should be noted that it is not always possible to apply the conditions of pretreatment from one type of material to another. In other words, one pretreatment method is efficient for a particular type of material might not work for another material [33]. It therefore can be concluded that the choice of appropriate pretreatment technology and its optimization for a particular biomass depends on the physicochemical properties and chemical compositions of biomass as well as which components of the biomass need to be altered. Additionally, although a variety of studies on optimization of pretreatment at bench-scale have been carried, not much research on optimization of pretreatment at scale-up has been done. Since the results obtained at bench-scale may not feasibly meet the situation at pilot-scale. Thus, it is suggested that much effort should be paid on selection and optimization of pretreatment strategy at scale up.
Fermentation Processes
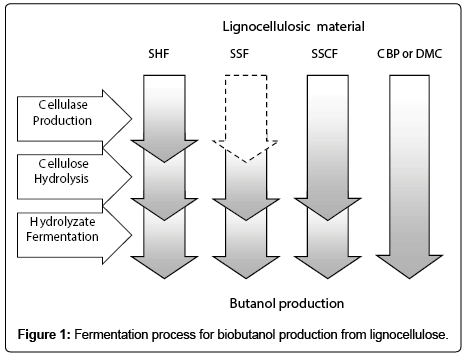
Following pretreatment, enzymatic hydrolysis is needed to convert cellulose into glucose, which can be subsequently used for butanol fermentation [34]. Based on the production of cellulase and the process of cellulose hydrolysis and butanol fermentation, the conversion of lignocellulosic biomass for butanol production can be classified into four major processes: (1) separate hydrolysis and fermentation (SHF), (2) simultaneous saccharification and fermentation (SSF), (3) simultaneous saccharification and co-fermentation (SSCF), and (4) consolidated bioprocessing (CBP) as illustrated schematically in Figure 1.
Separate hydrolysis and fermentation
For the separate hydrolysis and fermentation (SHF) process, cellulose is hydrolyzed to glucose by cellulases under optimum conditions, particularly around 50°C, and then resulting glucose is transferred to fermentation reactor and fermented by butanolproducing microorganisms at temperatures around 35°C [35]. As summarized in Table 3, the process of SHF is currently the most frequently applied in butanol production from lignocellulosic biomass. For example, Cheng et al. evaluated the butanol production from rice straw and sugarcane bagasse by a mixture culture using SHF [10]. Wang et al. discussed the feasibility of using crude enzyme to hydrolyze wheat straw for butanol fermentation in SHF processes [5]. In SHF process, cellulose hydrolysis and fermentation can be carried out at their different optimum conditions. However, the accumulation of glucose during enzymatic hydrolysis may inhibit cellulases activity in the hydrolysis process. Moreover, two separated enzymatic hydrolysis and fermentation processes inevitably increase the cost of equipment and the risk of microbial contamination which makes the process uneconomically feasible.
| Microoganism | Fermentation process | Substrate | Butanol concentration (g/L) | Butanol yield (g/g substrate) | Butanol productivity (g/L·h) | References |
|---|---|---|---|---|---|---|
| Acclaimed mixed bacterial microflora | SHF | Rice straw | 2.92 g/L, | - | 0.042 | [32] |
| Sugarcane bagasse | 2.29 g/L | - | 0.059 | |||
| C. thermocellum+C. saccharoperbutylacetonicum N1-4 | CBP | Crystalline cellulose | 7.9 | 0.198 | 0.037 | [33] |
| C. beijerinckii BA101 | SHF | Corn fiber | 6.4 | 0.138 | 0.073 | [34] |
| C. beijerinckii NCIMB8052 | SHF | Corncob | 5.6 | 0.13 | 0.057 | [35] |
| C. saccharoperbutylacetonicum N1-4 | SHF | Sago starch | 10.4 | 0.29 | 0.072 | [36] |
| C.acetobutylicum MTCC 481 | SHF | Rice straw | 2.1 | 1.04 | 0.017 | [37] |
| C. acetobutylicum ATCC824 | SSF | Seepweed | 3.5 | 0.101 | 0.101 | [38] |
| C. beijerinckii P260 | SSF | Wheat straw | 7.4 | 0.113 | 0.164 | [39] |
| SHF | 8.09 | 0.193 | 0.085 | |||
| C. acetobutylicum ATCC824 | SHF | Wheat straw | 7.05 | 0.155 | 0.141 | [5] |
| SSF | 5.05 | 0.127 | 0.08 |
SHF: Separate hydrolysis and fermentation; SSF: Simultaneous saccharification and fermentation; CBP: Consolidated bioprocessing
Table 3: Biobutanol production from cellulosic substrates.
Simultaneous saccharification and fermentation
Different from SHF, simultaneous saccharification and fermentation (SSF) is the process that cellulose hydrolysis and fermentation takes place in one step. When a similar process is applied in butanol production from lignocellulosic biomass taking into account the unique properties of the hydrolysate that includes both C5 and C6 sugars, the term simultaneous saccharification and co-fermentation (SSCF) is used [36].
Compared with SHF, SSF process has the advantages of lower equipment cost and sugar inhibition. However, the temperatures for enzymatic hydrolysis and butanol fermentation are significantly different, because the optimum temperature for cellulose hydrolysis by cellulases normally occurs at 50°C, while the optimum temperature for butanol-producing strain is around 35°C. This makes the simultaneous optimization of the two unit operations impossible [37]. Generally, the SSF process for butanol production is operated at lower temperatures to accommodate microbial growth and butanol fermentation, usually at 35-37°C. Consequently, the efficiency of the enzymatic hydrolysis is inevitably compromised, and a much longer time is needed to complete the hydrolysis.
Consolidated bioprocessing
Although the processes of SHF and SSF are currently the most widely employed strategies to produce butanol from lignocellulosic biomass, one of the major barriers for the SHF and SSF is the exogenous cellulase addition which increases the cost of butanol production due to the high cost of the cellulase as well as the high enzyme dosage required by the processes [38]. Thus, development of cost-effective and efficient saccharification and fermentation technologies is the key for successful cellulosic butanol production.
An alternative process that aims to eliminate this critical costincreasing is the consolidated bioprocessing (CBP) also called direct microbial conversion, in which cellulase production, cellulose hydrolysis and fermentation are completed in one step [39,40]. This integrated process has been proposed as the most economically attractive configuration for low cost hydrolysis and fermentation of cellulosic biomass. It is estimated that CBP has a potential for cost reduction in excess of 50% compared to the processes of SSF and SHF due to the elimination of the operating and capital costs correlated with the additional steps of cellulase production and enzymatic hydrolysis [40,41]. However, there is no perfect CBP microorganism that can degrade lignocellulosic biomass efficiently and at the same time produce butanol at desired yield. Co-cultures have been widely studied to address the limitations in substrate utilization by individual strains for the production of various bioproducts. For example, it was reported that the co-culture of Bacillus sp. SGP1 and C. tyrobutyricum ATCC 25755T to produce butyric acid from sucrose [42]. Geng et al. discussed the effect of key factors on hydrogen production from cellulose in a coculture of cellulolytic bacterium Clostridium thermocellum DSM 1237 and a non-cellulolytic hydrogen-producing bacterium Clostridium thermopalmarium DSM 5974 [43]. Apparently, co-culture of different microorganisms by taking advantage of their specific metabolic capacities provides a promising method to improve the substrate conversion and the product yield. Recently, Dwidar et al. evaluated the efficiency of butanol production from crystalline cellulose via CBP by cocultured cellulolytic Clostridium. thermocellum JN4 and the butanolproducing strain Clostridium. saccharoperbutylacetonicum N1-4. After 9d fermentation, butanol yield as high as 7.9 g/L was obtained on 4% Avicel cellulose [32]. To the best of authors’ knowledge, this is the first report to produce butanol from cellulosic materials via CBP, indicating that much effort should be paid on butanol production through CBP strategy. Overall, development of CBP microorganisms is the core of the CBP process.
Strategies for Increasing Butanol Production from Lignocellulose
Inhibitors detoxification
Besides monomeric sugars are produced during the pretreatment and hydrolysis steps, some undesirable degradation products, including aliphatic acids (acetic, formic and levulinic acids), furan derivatives (furfural, 5-hydroxymethyl-furfural (5-HMF)), and phenolic compounds (such as syringaldehyde, ferulic and pcoumaric acids) [44,45], are also generated. These inhibitors have been identified to exert a significant influence on the effectiveness of biomass conversion [46,47], removal of these inhibitory compounds before fermentation is therefore pivotal for successful biofuel production. Traditional methods for the removal of inhibitors mostly employ the processes of adsorption, extraction, precipitation and their combinations e.g., acuum evaporation and overliming with calcium hydroxide [48,49]. Biologic abatement (or bio-abatement) can be an alternative method to reduce inhibitory compounds from biomass hydrolysates [50-52]. Bioabatement, in which inhibitors are removed by microbial metabolism, has the advantage of treating liquid-solid mixtures without the need of chemical inputs and with no generation of chemical wastes. Cao et al. utilized a fungus, Coniochaeta ligniaria NRRL30616, to treat dilute acid and liquid hot water-pretreated corn stover liquors and found most acetate and more than 50% of HMF, furfural, and phenolic compounds were successfully removed [50].
Although the detoxication for the removal of inhibitors from lignocellulosic hydrolysates has been proven to improve their fermentability, only part of inhibitors were removed in most cases. So, the selection of appropriate detoxification method depends on the type of hydrolysate to be treated with respect to the efficiency of the detoxification on that kind of hydrolysate and the fermentation microorganisms employed in the butanol production with respect to which inhibitors need to be removed.
Strain improvement
It is acknowledged that one of the biggest challenges for industrialization of butanol fermentation is low butanol yield due to the formation of byproducts acetone and ethanol and low butanol tolerance capability of the microorganism. To overcome this drawback, developing robust strains with improved butanol yield and tolerance is the prerequisite for butanol fermentation. Aiming at this, a lot of works have been made on modifying strains through mutagenesis, evolutionary engineering and metabolic engineering strategies e.g., a mutant strain C. beijerinckii BA101 treated N-methyl-N-nitro-Nnitrosoguanidine could produce 19 g/L butanol, which was 10 g/L higher than by the parent strain C. beijerinckii NCIMB 8052 [53], a another hyper-butanol producing strain C. acetobutylicum JB200, acquired from C. acetobutylicum ATCC 55025 by long term adaptation with intermittently butanol challenges, could tolerate and produce butanol as high as about 20 g/L [54], and a recombinant strain disrupted of acetoacetate decarboxylase gene adc significantly increased the butanol ratio from 70% to 80% [55]. In addition, it should be noted that the hydrolysates of lignocellulosic biomass contain pentose sugars such as xylose and arabinose and hexose sugars of glucose, mannose and galactose. Unfortunately, current butanol-producing microorganisms either C. acetobutylicum or C. beijerinckii cannot ferment the pentose sugars into butanol efficiently. If only hexose sugars from lignocellulosic biomass are fermented with pentose sugars left behind, feedstock consumption for biobutanol production will be significantly high. Therefore engineering pentose-utilizing microorganisms with butanol pathways or butanol producers with pentose-metabolizing pathways is preferred.
Despite the progress made at molecular and physio-ecological levels, a superior butanol producing phenotypes with wide substrates spectrum especially have a capability to utilize pentose and cellulose at high rate still remains to be expected.
Process integration and optimization
Like ethanol production from lignocellulose-based feedstock, butanol production from this material involves various technologies such as pretreatment, saccharification and fermentation. In order to achieve an economical butanol production system, process integration aiming at optimizing these units on the system level is of great important. Considerable successes on the development of ethanol system in the aspects of pretreatment, enzymatic hydrolysis, fermentation, and ethanol recovery have been achieved in the past few years [56]. These successful cases may be good references for the butanol production from lignocellulosic material. In addition, many novel ideas, such as biorefinery and the concept of oriented conversion of classified composition which have been proposed and investigated in ethanol production may also provide practical and promising strategies for developing a feasible butanol production technology from lignocellulosic biomass [12,56,57]. Whatever, to achieve the commercialization of cellulosic butanol, further decrease in the cost of individual process and efficient combination of these processes will result in competitive biobutanol production from biomass.
Conclusions
Bioconversion of lignocellulose-to-butanol provides a sustainable and economical way to produce butanol from raw material via biotechnology. While, a deep understanding of fundamentals of various pretreatment processes and development of more efficient and economical fermentation processes needs relentless effort. Moreover, the development of cost-effective detoxification, more efficient microbial strains and process integration and optimization for reducing energy consumption and increasing butanol yields from raw materials would decrease the cost of butanol production and make it more economically competitive.
Acknowledgements
This project was supported by National Science and Technology Ministry (No. 2014BAJ21B02), Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (No. LBH-Q15054), and State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (No. 2014TS07).
References
- Dürre P (2007) Biobutanol: an attractive biofuel. Biotechnol J 2: 1525-1534.
- Pfromm PH, Amanor-Boadu V, Nelson R, Vadlani P, Madl R (2010) Bio-butanol vs. bio-ethanol: a technical and economic assessment for corn and switchgrass fermented by yeast or Clostridium acetobutylicum. Biomass Bioenerg 34: 515-524
- Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, et al. (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101: 209-228.
- Ni Y, Sun Z (2009) Recent progress on industrial fermentative production of acetone–butanol–ethanol by Clostridium acetobutylicum in China. Appl Microbiol Biotechnol 83: 415-423.
- Wang ZY, Cao GL, Jiang C, Song JZ, Zheng J, et al. (2013) Butanol Production from Wheat Straw by Combining Crude Enzymatic Hydrolysis and Anaerobic Fermentation Using Clostridium acetobutylicum ATCC824. Energy Fuels 27: 5900-5906.
- Ni Y, Wang Y, Sun ZH (2012) Butanol Production from Cane Molasses by Clostridium saccharobutylicum DSM 13864: Batch and Semicontinuous Fermentation. App Biochem Biotechnol 166: 1896-1907.
- Ren NQ, Wang AJ, Cao GL, Xu JF, Gao LF (2009) Bioconversion of Lignocellulosic Biomass to Hydrogen: Potential and Challenges. Biotechnol Adv 27: 1051-1060.
- Fan YT, Zhang GS, Guo XY, Xing Y, Fan MH (2006) Biohydrogen-production from beer lees biomass by cow dung compost. Biomass Bioenergy 30: 493-496.
- Zhang ML, Fan YT, Xing Y, Pan CM, Zhang GS, et al. (2007) Enhanced biohydrogen production from cornstalkwastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy 31: 250-254.
- Cheng CL, Che PY, Chen BY, Lee WJ, Lin CY, et al. (2012) Biobutanol production from agricultural waste by an acclimated mixed bacterial microflora. Appl Energy 100: 3-9.
- Kumar M, Gayen K (2012) Developments in biobutanol production: New insights. App Energy 88: 1999-2012.
- García V, Päkkilä J, Ojamo H, Muurinen E, Keiski RL (2011) Challenges in biobutanol production: How to improve the efficiency? Renewable and Sustainable Energy Reviews 15: 964-980.
- Himmel ME, Ding SY, Johnson DK (2007) Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 315: 804-807.
- Howard RL, Abotsi E, Jansen van Rensburg EL, Howard (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. African Journal of Biotechnology 2: 602-619.
- Klemm D, Heublein B, Fink HP (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int 44: 3358-3393.
- Laureano-Perez L, Teymouri F, Alizadeh H, Dale BE (2005) Understanding factors that limit enzymatic hydrolysis of biomass. Appl Biochem Biotechnol 124: 1081-1099.
- Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61: 263-289.
- Gray MC, Converse AO, Wyman CE (2003) Sugar monomer and oligomer solubility. Data and predictions for application to biomass hydrolysis. Applied Biochemistry and Biotechnology, Humana Press, New York, USA, pp: 179-193.
- Grabber JH (2005) How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Sci 45: 820-831.
- Zhao L, Cao GL, Yao J, Ren HY, Dong D, et al. (2012) Fungal pretreatment of cornstalk with Phanerochaete chrysosporium for enhancing enzymatic saccharification and hydrogen production. Bioresour Technol 114: 365-369.
- Mosier N, Wyman C, Dale B, Elander R, Lee YY, et al. (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96: 673-686.
- Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100: 10-18
- Volynets B, DahmanY (2011) Assessment of pretreatments and enzymatic hydrolysis of wheat straw as a sugar source for bioprocess industry. Int J Energy Environ 2: 427-446.
- Banerjee G, Car S, Scott-Craig J, Hodge D, Walton J (2011) Alkaline peroxide pretreatment of corn stover: effects of biomass, peroxide, and enzyme loading and composition on yields of yield of glucose and xylose. Biotechnol Biofuels 4: 16.
- Pandey MRA (2014) Lignocellulosic biobutanol production: Gridlocks and potential remedies. Renewable and Sustainable Energy Reviews 37: 21-35.
- Palmqvist E, Hahn-Hägerdal B, Galbe M, Zacchi G (1996) The effect of water-soluble inhibitors from steam-pretreated willow on enzymatic hydrolysis and ethanol fermentation. Enzyme Microb Technol 19: 470-476.
- Ximenes E, Kim Y, Mosier N, Dien B, Ladisch M (2010) Inhibition of cellulases by phenols. Enzyme Microb Technol 46: 170-176.
- Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48: 3713-3729.
- Brownell HH, Yu EKC, Saddler JN (1986) Steam-explosion pretreatment of wood: effect of chip size, acid, moisture content and pressure drop. Biotechnol Bioeng 28: 792-801.
- Gregg D, Saddler JN (1996) A techno-economic assessment of the pretreatment and fractionation steps of a biomass-to-ethanol process. Applied Biochemistry and Biotechnology, Humana press, New York, USA, pp: 711-727.
- Yu J, Ji BZ, He J, Liu Z, Yu Z (2009) Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour Technol 100: 903-908.
- Nakayama S, Kiyoshi K, Kadokura T, Nakazato A (2011) Butanol production from crystalline cellulose by cocultured Clostridium thermocellum and Clostridium saccharoperbutylacetonicum N1-4. Appl Environ Microb 77: 6470-6475
- Qureshi N, Ezeji TC, Ebener J, Dien BS, Cotta MA, et al. (2008) Butanol production by Clostridiumbeijerinckii Part I: Use of acid and enzyme hydrolyzed corn fiber. Bioresour Technol 99: 5915-5922.
- Zhang WL, Liu ZY, Liu Z, Li FL (2012) Butanol production from corncob residue using Clostridiumbeijerinckii NCIMB 8052. Lett Appl Microbiol 55: 240-246.
- Hipolito CN, Crabbe E, Badillo CM, Zarrabal OC, Morales Mora MA, et al (2007) Bioconversion of industrial wastewater from palm oil processing to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). J Clean Prod 16: 632-638.
- Ranjan A, Moholkar VS (2013) Comparative study of various pretreatment techniques for rice straw saccharification for the production of alcoholic biofuels. Fuel 112: 567-571.
- Zhao SH, Ma TS, Zhang HB (2011) Butanol production from halophyte seepweed Suaeda salsa by simultaneous saccharification and fermentation. Asian J Chem 23: 5285-528.
- Qureshi N, Saha BC, Hector RE, Hughes SR, Cotta MA (2008) Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part I - Batch fermentation. Biomass Bioenerg 32: 168-175.
- Lynd LR, Van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotech 16: 577-583.
- Talluri S, Raj SM, Christopher LP (2013) Consolidated bioprocessing of untreated switchgrass to hydrogen by the extreme thermophile Caldicellulosiruptor saccharolyticus DSM 8903. Bioresour Technol 139: 272-279.
- Cao GL, Zhao L, Wang AJ, Wang ZY, Ren NQ (2014) Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnol Biofuels 7: 82
- Dwidar M, Kim S, Jeon BS, Um Y, Mitchell RJ, et al. (2013) Co-culturing a novel Bacillus strain with Clostridium tyrobutyricum ATCC 25755 to produce butyric acid from sucrose. Biotechnol Biofuels 6: 35.
- Geng A, He Y, Qian C, Yan X, Zhou Z (2010) Effect of key factors on hydrogen production from cellulose in a co-culture of Clostridium thermocellum and Clostridium thermopalmarium. Bioresource Technol 101: 4029-4033.
- Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxiï¬cation. Bioresour Technol 74: 17-24.
- Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour Technol 74: 25-33.
- Ximenes E, Kim Y, Mosier N, Dien B, Ladisch M (2011) Deactivation of cellulases by phenols. Enzyme Microb Technol 48: 54-60.
- Ximenes E, Kim Y, Mosier N, Dien B, Ladisch M (2010) Inhibition of cellulases by phenols. Enzyme Microb Technol 46: 170-176.
- Larsson S, Reimann A, Nilvebrant NO, Jönsson LJ (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Applied Biochemistry and Biotechnology, Humana Press, New York, USA, pp: 77-79, 91-103.
- Mussatto SI, Roberto IC (2004) Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93: 1-10.
- Cao GL, Ximenes E, Nichols N, Zhang LY, Ladisch M (2013) Biological abatement of cellulase inhibitors. Bioresour Technol 146: 604-610.
- Nichols NN, Dien BS, Cotta MA (2010) Fermentation of bioenergy crops into ethanol using biological abatement for removal of inhibitors. Bioresour Technol 101: 7545-7550.
- Nichols NN, Dien BS, Guisado GM, López MJ (2005) Bioabatement to remove inhibitors from biomass-derived sugar hydrolysates. Appl Biochem Biotechnol 121: 379-390.
- Qureshi N, Blaschek HP (2000) Butanol production using Clostridium beijerinckii BA101 hyperbutanol producing mutant strain and recovery by pervaporation. Applied Biochemistry and Biotechnology, Humana press, New York, USA, pp: 84-86, 225-235.
- Xue C, Zhao JB, Lu CC, Yang ST, Bai FW, et al. (2012) High-titer n-butanol production by Clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol Bioeng 109: 2746-2756.
- Jiang Y, Xu C, Dong F, Yang Y, Jiang W, et al. (2009) Disruption of the acetoacetate decarboxylase gene insolvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11: 284-291.
- Zhao XQ, Zi LH, Bai FW, Lin HL, Hao XM, et al. (2012) Bioethanol from Lignocellulosic Biomass. Adv Biochem Engin/Biotechnol 128: 25-51.
- Demirbas A (2009) Biorefineries: current activities and future developments. Energy Convers Manag 50: 2782-801.
--
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 17947
- [From(publication date):
July-2016 - Jul 14, 2025] - Breakdown by view type
- HTML page views : 16290
- PDF downloads : 1657