Research Article Open Access
Bioactivity Guided Fractionation of the Aqueous Extract of the Indian Banyan and Evaluation of Immunomodulatory Activity - Validating the Traditional Use
| Tabassum Khan1*, Pratima Tatke2 and Satish Gabhe3 | |
| 1SVKM’s Dr. Bhanuben Nanavati College of Pharmacy, Mumbai, India | |
| 2C.U.Shah College of Pharmacy, SNDT Women’s University, Mumbai, India | |
| 3Bharti Vidyapeeth, Pune University, Pune, India | |
| Corresponding Author : | Tabassum Khan Assistant Professor, SVKM’s Dr. Bhanuben Nanavati College of Pharmacy Gate No-1, Mithibai College Campus, First Floor V.M.Road, Vile Parle (W), Mumbai- 400 056, India Tel: 91-22-26134557, 91-22-26134558 Fax: 91-22-26132905 E-mail: tabassum.khan@bncp.ac.in |
| Received May 02, 2014; Accepted June 15, 2014; Published June 17, 2014 | |
| Citation: Tabassum K, Pratima T, Satish G (2014) Bioactivity Guided Fractionation of the Aqueous Extract of the Indian Banyan and Evaluation of Immunomodulatory Activity - Validating the Traditional Use. J Homeop Ayurv Med 3:158. doi: 10.4172/2167-1206.1000158 | |
| Copyright: © 2014 Tabassum K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
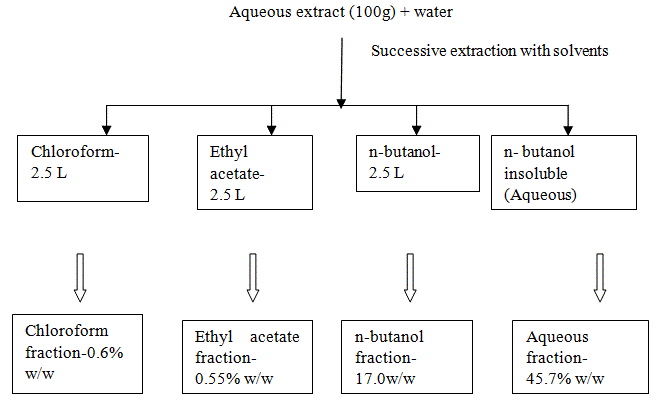
The Indian Banyan is a large tree, bearing many aerial roots. An aqueous decoction of the fresh aerial roots of this tree has been used by traditional ayurvedic medical practitioners to boost the immune system in various diseases. In AIDS, death occurs eventually due to the failing immune system hence new therapies directed towards augmenting the functional capacity of the immune system are the need of the hour. Studies carried out in our laboratory have indicated the immunostimulant activity of the methanol and aqueous extracts of the aerial roots. The aqueous extract was found to enhance the cell mediated and antibody mediated immune responses in rats. The aqueous extract was subjected to fractionation using solvents of varying polarity. This process generated four fractions namely chloroform fraction, ethyl acetate fraction, n-butanol fraction and the aqueous fraction. These fractions were screened for immunomodulatory activity using the in vitro lymphocyte proliferation assay in an effort to identify the most bioactive fraction. The results of this assay indicated that the aqueous fraction exhibited maximum immunostimulatory activity (80% stimulation of lymphocytes at 1 μg/ml) while the other three fractions did not exhibit stimulation of lymphocytes at the concentrations tested (0.001- 10 μg/ml). Hence the aqueous fraction was taken up for evaluation of in vivo immunomodulatory activity. The aqueous fraction was evaluated at doses ranging from 25 mg/kg to 200 mg/kg body weight in rats using the hypersensitivity reaction assay and the hemagglutination reaction assay. The maximum response was observed at 50 mg/kg. The aqueous fraction of the aqueous extract exhibited significant stimulation of cell mediated and antibody mediated immunity and could prove beneficial in diseases involving suppression of the immune system like AIDS.
Ayurvedic concept of Rasayanas and the modern concept of new class of plant derived drugs, the “adaptogens”, appear to induce a state of nonspecific increase in resistance of an organism to diverse aversive assaults that threaten the internal homeostasis [5]. Thus leads derived from traditional medicines through scientific studies appear to be influential for the discovery of new immunomodulatory compounds and novel mechanisms of action [6].
Natural product derived immunomodulators can be classified as low and high molecular weight compounds. A more specific classification reveals that active compounds belong to the following classes: carbohydrates, terpenes, steroids, phenolics, coumarins, aminoacids, peptides/proteins, glycoproteins, alkaloids and other nitrogen containing organic compounds [7].
Ficus benghalensis was selected based on ethnomedical use. A decoction of aerial roots of Ficus benghalensis has been used by Ayurvedic practitioners in the rural areas (Dhule region) of Maharashtra to boost the immune system in a variety of diseases that are accompanied by impairment of the immune system. However no phytochemical and pharmacological investigations of aerial roots have been reported so far. Our initial studies indicated a significant immunomodulatory activity of the methanol and aqueous extracts of the aerial roots of Ficus benghalensis [8,9]. This paper reports the bioactivity-guided fractionation of the active aqueous extract and the subsequent evaluation of its fractions for immunomodulatory activity using in vitro and in vivo studies.
Peripheral blood lymphocyte (PBL) was obtained from the buffy coat residues by the Ficoll Hypaque method. Under sterile conditions, 50 μl of PBL suspension (5×106 cells/ml), 50 μl sample dilutions and 50 μl of Phytohemagglutinin (PHA) (5 μg/ml) were mixed in sterile 96 well flat-bottomed microtitre plates. A solution of Ashwagandha capsules in DMSO was used as a positive control (0.25 mg/ml) in this assay. The plates were incubated at 37oC in a 5% CO2 incubator for 48–72 hours. After incubation cell growth was quantitated using MTT. For this 25 μl of MTT (1 mg/ml) was added to each well after which the plates were incubated at 37oC for 4 hours. Next 50 μl of acidified propanol (0.04 M hydrochloric acid in isopropanol) was added and the contents of each well mixed thoroughly. Plates were read on an automated ELISA reader at 540 nm. Controls consisted of PBL with PHA (100% activity), PBL with RPMI-1640 medium (0% activity) and sample with PHA (background). The four fractions were screened at concentrations ranging from 0.001- 10 μg/ml.
Test wells
PBMC (50 μl) + PHA (50 μl) + sample (50 μl)
Background wells
PHA (50 l) + sample (50 μl)
Control wells
Activity (100%): PBMC (50 μl) + PHA (50 μl)
Activity (0%): PBMC (50 μl) + RPMI-1640 medium (50 μl)
The absorbance values of test fractions and control were recorded and percentage
proliferation of lymphocytes was calculated using the formula given below.
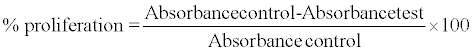
In vitro cytotoxicity assay: The chloroform fraction was found to be cytotoxic at 10 μg/ml while the other three fractions did not exhibit cytotoxicity at 10 μg/ml.
In vivo hypersensitivity reaction assay: The aqueous fraction was found to be the most bioactive fraction in the in vitro lymphocyte proliferation assay, hence was taken up for in vivo bioassays in rats. The aqueous fraction was evaluated at doses of 25, 50, 100 and 200 mg/kg. It produced a dose related increase in the early and delayed hypersensitivity reaction thereby indicating a significant stimulation of cellular immune responses at doses ranging from 25-200 mg/kg. The maximum response was observed at 50 mg/kg (Table 2). This indicates that phenolic compounds, flavonoids, triterpenoids, saponins, carbohydrates present in the aqueous fraction could be responsible for the immunostimulant activity.
Hemagglutination reaction assay: The aqueous fraction was evaluated for its effect on humoral immunity at doses ranging between 25-200 mg/kg body weight. The aqueous fraction produced a dose related increase in the antibody titer in rats. The aqueous fraction exhibited a maximum response at a dose of 50 mg/kg (Table 3).
In this assay, the aqueous fraction exhibited a better immunostimulatoryresponse than the reference standard. This augmentation of the humoral response to sheep red blood cells as evidenced by the increase in the hemagglutination antibody titers in rats indicated the enhanced responsiveness of B-lymphocyte subset, involved in antibody synthesis.
There are no previous reports on the bioactivity guided fractionation of the aqueous extract of the aerial roots of Ficus benghalensis. The activation and proliferation of T lymphocytes and cytokine production post stimulation with antigens play important roles against bacterial and viral infection. Immune stimulation is important in many disease conditions where there is a suppression of normal immune responses viz. AIDS. In AIDS, the HIV infects the T-helper cells that are the central controllers of immune responses to antigens. When these cells become infected with HIV, many other cell types fail to function normally and this results in a severe depression of the immune system. The body becomes highly susceptible to infections with opportunistic pathogens eventually leading to death. Hence immune restoration becomes imperative in the overall management of AIDS because death eventually is a result of failed immune system rather than the virus itself. Thus improving immune health is an important part of a long-term strategy for managing AIDS. The bioactive aqueous fraction of Ficus benghalensis has a strong potential to be explored further as an immune based therapy along with anti-HIV drugs in the overall management of AIDS. The present study establishes the in vitro and in vivo cellular and humoral immunomodulatory activity of the aqueous fraction of the aqueous extract of the aerial roots of Ficus benghalensis.
References
- Cordell GA, Angerhofer CK, Pezzuto JM (1994) Recent studies on cytotoxic, anti-HIV and antimalarial agents from plants. Pure Appl Chem 66: 2283-2286.
- Wagner H (1990) Search for plant derived products with immunostimulatory activity (recent advances). Pure Appl Chem 62: 1217-1222.
- Anjali G, Chitalwar G (1997) Immunomodulatory properties of stem extracts of Tinosporia cardiofolia: Cell targets and active principles in Immunomodulation. Upadhyay, S.N Narosa Publishing House, New Delhi, India.
- Bhattacharya SK, Bhattacharya A, Chakrabarti A (2000) Adaptogenic activity of Siotone, a polyherbal formulation of Ayurvedic rasayanas. Indian J Exp Biol 38: 119-128.
- Acharya Sharma PV (1996) Dravyaguna Vigyan. (17th edn), Chaukhamba Bharati academy, Sanskrit Sansthan Varanasi. 2:20-39.
- Brekhman II, Dardymov IV (1969) New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol 9: 419-430.
- Labadie RP (1993) Immunomodulatory compounds in bioactive natural products. CRC Press INC:279-286.
- Gabhe SY, Tatke PA, Khan TA (2006) Evaluation of the immunomodulatory activity of the methanol extract of Ficus benghalensis roots in rats. Ind J Pharmacol 38: 271-275.
- Khan T, Tatke P, Gabhe SY (2008) Immunological studies on the aerial roots of the Indian banyan. Indian J Pharm Sci 70: 287-291.
- Ndip RN, Ajonglefac AN, Mbullah SN, Tanih NF, Akoachere J-F TK, et al. (2008) In vitro anti-Helicobacter pylori activity of Lycopodium cernuum(Linn)Pic Serm. Afr J Biotech 7: 3989-3994.
- Benencia F, Courrèges MC, Coulombié FC (2000) In vivo and in vitro immunomodulatory activities of Trichilia glabra aqueous leaf extracts. J Ethnopharmacol 69: 199-205.
- Davis L, Kuttan G (2000) Immunomodulatory activity of Withania somnifera. J Ethnopharmacol 71: 193-200.
- Doherty NS (1981) Selective effects of immunosuppressive agents against the delayed hypersensitivity response and humoral response to sheep red blood cells in mice. Agents Actions 11: 237-242.
- Nelson DS, Mildenhall P (1967) Studies on cytophilic antibodies. 1. The production by mice of macrophage cytophilic antibodies to sheep erythrocytes: relationship to the production of other antibodies and the development of delayed-type hypersensitivity. Aust J Exp Biol Med Sci 45: 113-130.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 14146
- [From(publication date):
June-2014 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 9514
- PDF downloads : 4632
