Research Article Open Access
Bioactive-Functionalized Interpenetrating Network Hydrogel (BIOF-IPN): A Novel Biomaterial Transforming the Mechanism of Bio-Repair, Bio-Adhesion and Therapeutic Capability – An In Vitro Study
Tamara Perchyonok V1*, Vanessa Reher2, Sias Grobler3, Annette Oliver3 and Shengmiao Zhang4
1Research and Development Department, VTPCHEM PTY LTD., Southport 4215, Australia
2School of Dentistry and Oral Health, Gold Coast campus, Griffith University, QLD 4222, Australia
3Oral and Dental Research Institute, Faculty of Dentistry, University of the Western Cape, Private Bag X1, Tygerberg 7505, Cape Town, South Africa
4School of Material Science and Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
- Corresponding Author:
- Tamara Perchyonok V
Research and Development Department
VTPCHEM PTY LTD., Southport 4215, Australia
Tel: +61-414-596-304
Email: tamaraperchyonok@gmail.com
Received Date: December 16, 2014; Accepted Date: January 24, 2015; Published Date: January 27, 2015
Citation: Tamara Perchyonok V, Vanessa Reher, Sias Grobler, Annette Oliver, Shengmiao Zhang (2015) Bioactive-Functionalized Interpenetrating Network Hydrogel (BIOF-IPN): A Novel Biomaterial Transforming the Mechanism of Bio-Repair, Bio-Adhesion and Therapeutic Capability – An In Vitro Study. J Interdiscipl Med Dent Sci 3:166. doi: 10.4172/2376-032X.1000166
Copyright: © 2015 Perchyonok VT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
The purpose of this investigation was to evaluate and report the in vitro performance of a few selected Bioactive- Functionalized Interpenetrating Network Hydrogels (BIOF-IPN); a novel biomaterial with a build-in mechanism of biorepair capacity, bio-adhesion and therapeutic capability. The study also evaluates the cytotoxicity of the newly developed material, and its ability to be used in the dental field. In this study, four bioactive-functionalized interpenetrating network hydrogels (BIOF-IPN) were prepared by dispersion of different therapeutic agents. The dentin bond strength is tested, the bio-adhesivity, modulus of elasticity and cytotoxicity of the materials were investigated, and the performance of the therapeutic-agents release profile, evaluated. The gels used in this study demonstrated excellent capacity in leading to high internal surface areas with low diffusional resistance. An increase in bond strength of the dentin treated with the BIOF-IPNs compared to the bond strength of the conventionally bonded teeth was indicated, and a significant increase in the modulus of elasticity observed. BIOF-INPs showed high adhesive force promotes binding to the negative surface of skin or dentin structure. It was found that chitosan alone increased the cell survival rate remarkably (113%) and its presence with naproxen and ibuprofen increased the cell survival rate [naproxen (93%), chitosan/naproxen (96.6%), ibuprofen (76.6%), and chitosan/ibuprofen (89.1%). The use of BIOF-IPNs has increased the time of the release of therapeutic agents, and protected the active ingredient from any type of free radical damage produced in and around the active site. Present results demonstrate the capability of the BIOF-IPNs to play an important role in the defense mechanism, and in the functional multidimensional restorative repair materials. The findings suggest that the new material would definitely find applications in functional dental composites and regenerative dentistry.
Keywords
Interpenetrating network hydrogel; Chitosan; β-cyclodextrin; Drug release; Antioxidants; Reactive oxygen species (ROS); Cytotoxicity
Introduction
The field of biomaterials has recently been focused on the design of “intelligent materials” with a purposely build in functionality to address specifics shortcomings of the conventional clinical protocols at the molecular level. In designing such novel biomaterials, researchers have sought not merely to create bio-inert materials, but rather materials that can respond to the cellular environment around them, to improve and promote device integration, tissue regeneration, changes in pH or cell-associated enzymatic activity, in materials with build-in bio-active capability.
Despite the immense advances achieved in adhesive dentistry over the years, research still demonstrates that resin-dentin bond performs poorly when compared with the resin enamel bond [1]. It can be attributed to the degradation of either the hydrophilic resin components following water sorption, as well the deterioration of collagen fibrils within the bonded interface by dentin-bond enzymes [2,3]. Furthermore, the biocompatibility of some dentin bonding agents is questionable, and its cytotoxicity and adverse biological effects influence the biological behavior of the resin restorations [4]. Over the years the 3T3 mouse fibroblast cell-line has extensively been used as a standard cell line to test the cytotoxic effect of various dental materials and constituents thereof [4]. It is believed that the cytotoxicity towards this cell-line gives one a good indication to what would happen towards human pulp fibroblast cells. Recently in the dental field, several studies have been developed regarding the use of IPNs components to overcome the common failures in bio-adhesion, and to improve drug release and bio-repair [5] in target-designed biomaterials.
The purpose of this investigation is to evaluate the performance of a novel biomaterial, the Bioactive-Functionalized Interpenetrating Network Hydrogel (BIOF-IPNs), and its ability to be used in dentistry. The study also evaluates the BIOF-IPN build in bio-repair, bio-activity and bio-adhesion capability, its cytotoxicity, and possible therapeutic uses.
Methods
Preparation of Bioactive-Functionalized Interpenetrating Network Hydrogel (BIOF-IPNs)
BIOF-IPN was prepared by dispersion of therapeutic agent such as naproxen, ibuprofen or aspirin powder 0.4 gm in glycerol (5% w/w) using a mortar and a pestle using protocol previously discussed in detail in previous studies [6] and summarized in Appendix 1. The chitosan: Vit C (5: 1 mixture) is commercially available composition and is used as received.
Dentin bond strength testing
The shear bond strength tests for dentin bonding of the BIOF-IPNs were evaluated by using previously reported and published protocol [6] and experimental details are summarized in Appendix 1.
An Instron Universal Testing Machine at a crosshead speed of 0.5 mm/minute was used to test the de-bonding strength. All data tests were analyzed using the non-parametric ANOVA test.
Bio-adhesive investigation
Bioadhesion studies were done using Chatillon apparatus for force measurement using previously reported protocol that has been published in detail [6]. This method determines the maximum force and work needed to separate two surfaces in intimate contact [6]. The strength was recorded as a function of the displacement, which allowed to determine the maximal detachment force, Fmax, and the work of adhesion, W, which was calculated from the area under the strength-displacement curve.
Modulus of elasticity (MOE)
Modulus of elasticity was measured using 3-point bend test (50 g load cell, crosspeed 0.5 ml/min) following the methodology previously reported [6] and detailed protocol described in Appendix 1. Load/displacement curves were converted to stress-strain curves and the apparent MOE was calculated at 3% strain. Displacement (D) during compression was displayed in millimeters and calculated at a maximum strain of 3% using the formula D = eL2/ 6T, where e, strain; L, support span length and T, the thickness of the specimen. The MOE of the specimens was expressed in MP (megapascals) and calculated using the following formula E = PL3/4DbT, where P, the maximum load; L, the support span length; D, the displacement; b, width of the specimen, and T, the thickness of the specimen.
Cytotoxicity testing: cell maintenance and culture
Cell maintenance and culture: A Balb/c 3T3 mouse fibroblast cell line (The National Repository for Biological Materials, Sandringam) was maintained and cultured in standard conditions (37°C under 5% carbon dioxide and 95% humidity) in Dulbecco’s Modified Eagles Medium (DMEM). The medium was supplemented with 10% fetal bovine serum and penicillin (10.000 U/ml) streptomycin (10.000 μg/ml) mix (Biochrom Ltd), changed every second day and cells sub-cultured using routine trypsin/EDTA procedures. The pH of this growth medium was adjusted when necessary to 8.0 [7-10]. To test the cytotoxicity towards 3T3 cells, the cells were first grown to near confluency [7-10] and the detailed experimental protocol is described in Appendix 1.
The Kruskal-Wallis Multiple-Comparison and Bonferroni test was used to test for significant differences amongst the 7 mentioned compounds (significant level p<0.05).
Therapeutic agents release profile
The in vitro release of the therapeutic agents was evaluated using previously reported protocol and it has been published in detail [6] and the detailed experimental protocol is described in Appendix 1.
Results
The characterization of BIOF-IPNs (Gel 1-4)
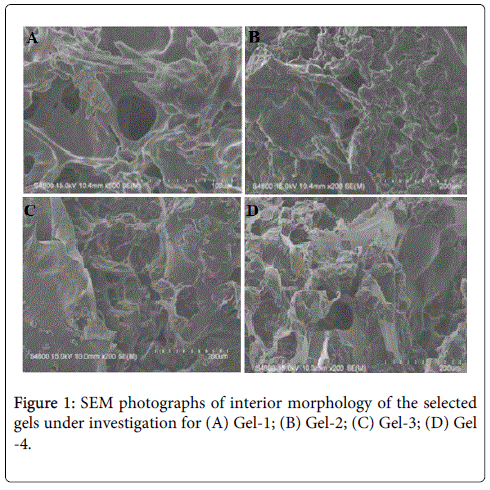
The SEM images were obtained to characterize the microstructure of the freeze-dried newly prepared gels, and are presented in Figures 1A-1D. It could be seen that the gels displayed a homogeneously pore structure. It was thought that the micro-porous structure of the gels could lead to high internal surface areas with low diffusional resistance in the gels.
Dentin shear bond strength
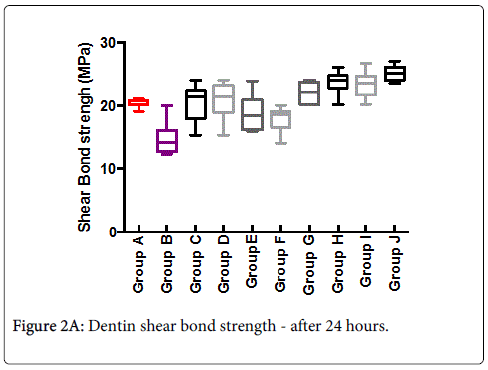
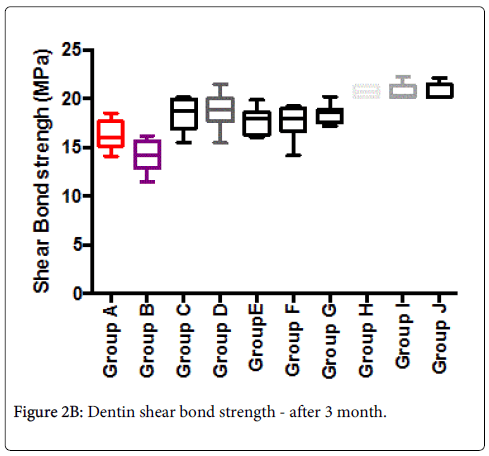
Mean shear-bond strength values and difference between the groups are summarized in Figure 2A for bonding to dentin after 24 hours. In general, there was an increase in bond strength of the dentin treated with the BIOF-IPNs compared to the bond strength of the conventionally bonded teeth. An increase in the shear bond strength was also previously reported for groups of hydrogen peroxide exposed samples [6], suggesting that additional benefits associated with chitosan: antioxidant systems are in need of further investigations [9-11]. In general after 3 months the bond strength (Figure 2B) was somewhat lower than at the beginning. The non-parametric ANOVA test was used to test for significant differences amongst the 8 mentioned groups and the corresponding control groups A and B (significant level p<0.05 in both cases).
Bio-adhesion
Higher adhesiveness of the gels is desired to maintain an intimate contact with skin or tooth structure and results are summarized in Table 3. BIOF-INPs showed the highest adhesive force, and this result can be expected because of the well-known intrinsic bio-adhesive properties of chitosan [12].
| Gel Formulation | Chitosan: Vit C 5:1(w/w%) | A (w/w%) | β-CD (w/w) | I | N | pH | |
|---|---|---|---|---|---|---|---|
| Gel-1 | Chitosan: Vit C (5:1) | 5 | 0 | 5 | 0 | 0 | 5.84 |
| Gel-2 | Chitosan: Vit C: A1 | 5 | 1 | 5 | 0 | 0 | 5.92 |
| Gel-3 | Chitosan: Vit C: N1 | 5 | 0 | 5 | 0 | 1 | 6.05 |
| Gel-4 | Chitosan: Vit C: I1 | 5 | 0 | 5 | 1 | 0 | 6.16 |
Table 1: Gel formulation prepared in the study. Where Vit C is vitamin C additive, A is aspirin additive, N is the naproxen, I-ibuprofen, β -CD is β - cyclodextrin. Hydrogels containing β- CD: chitosan: Vitamin C (5:1) (5 %) are synthesized and characterized).
| Group A | 37% of phosphoric acid +primer+ bonding agent immediately (negative control) |
| Group B | Self-etching + primer + bonding agent immediately (positive control) |
| Group C | Gel1 + bonding agent immediately |
| Group D | Gel2 + bonding agent immediately |
| Group E | Gel3 + bonding agent immediately |
| Group F | Gel4 + bonding agent immediately |
| Group G | Gel1 + Self-etching primer + bonding agent immediately |
| Group H | Gel2 + Self-etching primer + bonding agent immediately |
| Group I | Gel3 + Self-etching primer + bonding agent immediately |
| Group J | Gel4 + Self-etching primer + bonding agent immediately |
Table 2: Groups tested (8 teeth per group).
| BIOF-IPN | Adhesive Force (N) ± SD (Dentin) | Work of Adhesion (Ncm) ± SD (Dentin) |
|---|---|---|
| Gel-1 | 1.71 ± 0.35 | 5.92 ± 0.34 |
| Gel-2 | 1.17 ± 0.44 | 2.49 ± 0.42 |
| Gel-3 | 1.12 ± 0.60 | 2.94 ± 0.29 |
| Gel-4 | 1.12 ± 0.35 | 2.12 ± 0.34 |
Table 3: Bio-adhesion to dentin. The presented values are an average (n= 5).
The adequate water absorption capacity together with the cationic nature, which promotes binding to the negative surface of skin or dentin structure, could also explain these results.
Modulus of elasticity
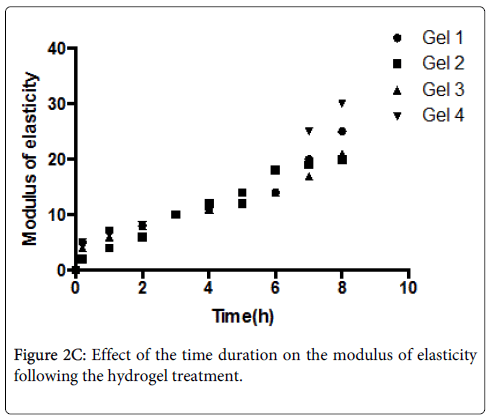
The mean and standard deviations of MOE of de-mineralized dentine treated with flavonoids at different time periods are shown in Figure 2C. The results of two-way ANOVA showed that both factors, “hydrogel treatment’’ and “treatment duration”, had a significant effect on the MOE of demineralised dentine (p < 0.001).
Interaction of the two factors was also significant (p < 0.001). The MOE of BIOF-IPNs treated dentine increased with time. A rapid significant increase in MOE was observed after 10 min treatment with Gels 1-4.
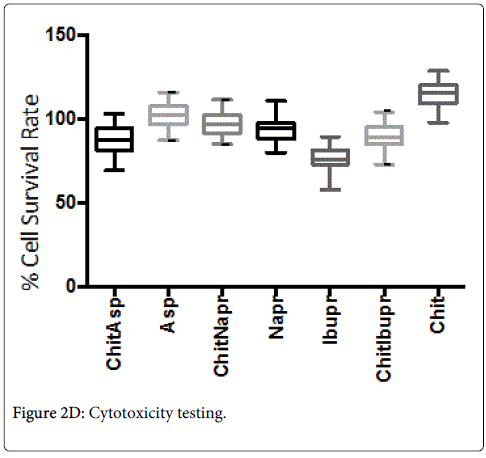
Cytotoxicity
The median cell survival rates were found to be: chitosan (113%), aspirin (102.1%), chitosan/aspirin (88.1%), ibuprofen (76.6%), chitosan/ibuprofen (89.1%), naproxen (93%), and chitosan/naproxen (96.6%).
Chitosan alone was found to have a significantly better (p < 0.05) survival rate than all the other hydrogels and therapeutic agents mentioned above (Figure 2D).
The Box & Whisker plot of the % survival rate of mouse 3T3 fibroblast cells when exposed to corresponding hydrogels and controls. The maximum and minimum values were given. The intermediary box represents the position of 50% of the values and the line within the box shows the median values. The median cell survival rates were found to be: chitosan (113%), aspirin (102.1%), chitosan/aspirin (88.1%), ibuprofen (76.6%), chitosan/ibuprofen (89.1%), naproxen (93%), chitosan/naproxen (96.6%). Chitosan alone was found to have a significantly better (p < 0.05) survival rate than all the other hydrogels and therapeutic agents mentioned above.
In vitro release of therapeutic agents
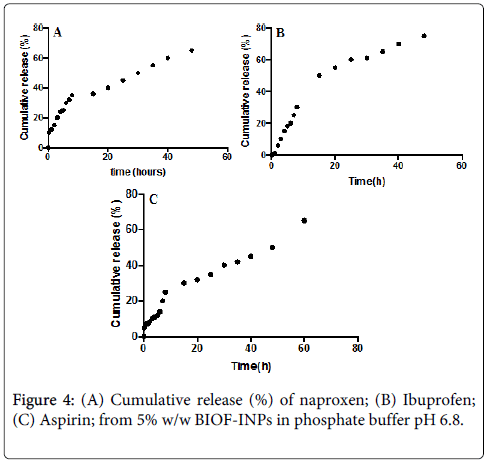
The in vitro release of therapeutic agents from Bioactive-functionalized interpenetrating network hydrogel (BIOF-IPNs) was carried out using USP dissolution apparatus type I. As the regression analysis of the obtained results for two kinetic models including zero order and Higushi’s model showed that Higushi’s model gave the highest value of r2 with significant difference (p < 0.05) [13]. Higushi’s model, where the cumulative amount of the released drug per unit area is proportional to the square root of time, is the more suitable model to describe the release kinetics from the gel preparations examined in the present study. The release of therapeutic agents from the BIOF-IPNs was studied as demonstrated in Figures 3A-3C.
Discussion
Dentin shear bond strength
The results of this study suggest that optimum bond strengthening of dentin can be achieved throughout the immediate or conventional adhesive treatment with BIOF-IPNs. Initial results have proven that this significant increase in bond strength and the durability of resin-dentin bond lasts for a prolonged time (up to 3 months). It is well documented that the hydrostatic pulpal pressure, the dentinal fluid flow, and the increased dentinal wetness in vital dentin can affect the intimate interaction of certain enamel and dentin adhesives with dentinal tissue. Therefore, the newly developed BIOF-IPNs systems might be able to address the shortfalls in the current perspectives in improving bond durability.
Although the correlation between the force and work of adhesion was noticeable for all samples, the chitosan: β-cyclodextrin complex has shown the highest adhesive force. This performance can be expected because of the well-known intrinsic bio-adhesive properties of this material. Also ionic vs covalent bonding of the chitosan: therapeutic agent complex may depend on the pH of the environment as the -COOH groups in naproxen, ibuprofen and/or aspirin ionize at alkaline pH and form covalent "amide" linkage at low pH. The adequate water absorption capacity, together with its cationic nature, which promotes binding to the negative surface of skin or dentin structure, can also explain these results. Hydration of the polymer causes mobilization of the polymer chains and hence influences polymeric adhesion [14]. Appropriate swelling is important to guarantee bio-adhesion; however, over-hydration can form slippery non-adhesive hydrogels [15].
Chitosan and β-cyclodextrin are potent antioxidants with multiple free hydroxyl groups [6]. These hydroxyl groups can form bridge-type hydrogen bonds within the side chains of hydroxyl, carboxyl, amino or amide groups of the collagen molecules [6]. The formation of these hydrogen bonds is the reason of the stability of chitosan-collagen or chitosan: collagen: cyclodextrin interaction [6]. By positioning itself between collagen molecules, host: guest complex formed by chitosan: therapeutic agent (such as naproxen, ibuprofen and/or aspirin) can potentially also form ionic bonds, as well as covalent bonds with collagen fibrils [16]. Furthermore, in the process of formation of hydrogen bonds host: guest complex, molecules can replace the water molecules bound to collagen in the extra-complex compartments [16]. This study has shown that newly developed hydrogels are capable of improving the modulus of elasticity of demineralised dentine.
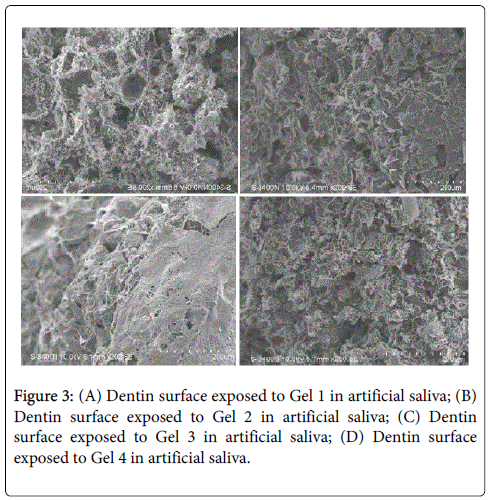
SEM images of the dentin surface exposed to BIOF-IPNs Gels 1-4 for 2 weeks in artificial saliva. The surface of the dentin has been significantly influenced by the treatment with Gels 1-4, illustrating previously reported unique properties of the chitosan in promoting formation of the hydroxyapatite crystals as demonstrated in figures 4A-4D.
Interestingly, the surface of the dentin has been significantly influenced by the treatment with Gels 1-4, confirming previously reported unique properties of the chitosan in promoting a formation of the hydroxyapatite crystals, as well as prevent demineralization of enamel and dentin, which has been reported previously [16] (Figures 3A-3C). The potential applications of chitosan and cyclodextrin in dentistry include strengthening of the collagen matrix, increasing resin–dentine bond strength, inactivation of collagen-bound proteases and remineralization of root caries [17]. The detailed investigation of the potential mechanism is currently being researched in our laboratory.
Release of therapeutic agents
The controlled release of naproxen depends on pH value of the media. The slower release kinetics at the lower pH conditions have been attributed to amino groups of chitosan protonation, which results in a soluble and positively charged polysaccharide leading to faster swelling in the acidic media. The cumulative release is represented as the “fractional release data” and it follows Higuchi model [18,19]. The plot reveals that there are 2 stages for the release of the naproxen from the Gel 3. The first stage of release was initially rapid (burst release), probably resulting from the rapid diffusion of naproxen from the chitosan: β-cyclodextrin BIOF-IPNs, and the initial swelling. The second stage of release demonstrates a slow, controlled release.
The burst release has an advantage in reaching a biologically relevant concentration much faster, where as a slow release will control the sustainable concentration of naproxen in plasma. The release of the corresponding BIOF-IPNs containing ibuprofen as a potential therapeutic agent prototype remained stable in the early hours of the experiment, allowing a more constant release, which would ensure an effective and prolonged anti-inflammatory activity when applied clinically. This property would make the system a suitable candidate for further development as a functional dual action restorative material. The release pattern for BIOF-IPNs containing aspirin is similar to the BIOF-IPNs containing ibuprofen, and it is correlated to the swelling ability of the corresponding hydrogel. Figure 4C shows the relationship between the release time and the percentage of aspirin released. The release of aspirin decreases gradually and finally reaches the equilibrium state where the aspirin is no longer released. The advantage of the chitosan: β-cyclodextrin system in BIOF-IPNs is the increase of the time of the release of therapeutic agents, as well as protecting the active ingredient from any type of free-radical damage produced in and around the active site.
Cytotoxicity investigations
It is well documented that dentin and dentinal fluid can act as a peroxide and oxygen reservoir [20] which may be present at the bonding interface, inhibiting the polymerization reaction and reducing bond strength. Thus, an increase in the antioxidant strength of the application, which may vary according to the antioxidant agent, and/or an increase in the concentration used for dentin, may be indicated in an endeavor to neutralize the free oxygen present in a higher amount in dentin than in enamel. Antioxidant release occurs through the pores of the low polymer concentration of the network structure.
The positive properties of BIOF-IPNs systems lead to the question of their cytotoxicity and their effect on pulpal cells. In this study, each cell-test survival rate determination was done simultaneously with the control, to standardize the results. To calculate the % viable cells between the controls and the test samples, each raw test value was divided by the median of the corresponding control survival values [5,6]. Normally, the cell survival rate will depend on a number of factors: type of substance to be tested, the concentration of the substance, the type of cell-line used and the time of cell exposure to the substance to be test-ed. All gel systems utilized in this study (1 mg of chitosan: therapeutic agent/ml growth medium over a 24 hour period) were tested for their survival rates. This can be considered as a high concentration and well in the top range of concentrations normally tested for other products. This is similar to the highest concentration used (1000 μg/ml) to test the cytotoxic effect of medicinal plants [6,21,22]. An exposure period of 24 hours used in this study can also be accepted as a long exposure period (in the top range) [6,21,22]. The release of the therapeutic agents (from the anti-oxidant chitosan) such as naproxen and ibuprofen from their chitosan gels showed a positive influence on the survival rate in comparison to the therapeutic agents alone (Figure 2E). However, aspirin (active ingredient acetylsalicylic acid and at a pH of 8.0 a salt) alone showed a value of 102% which is not expected but can be explained by the fact that this organic acid becomes neutralized in the growth medium where the pH is kept at 8.0. When the sequence of the survival rates (Figure 2E) of the chitosan gels and the 3 therapeutic agents are considered it can be expected that aspirin should have a cell survival rate of less than its chitosan gel (88%) when not neutralized. We previously reported [6] that the pH of the growth medium alone played a very important role on the cell survival rate and the lower the pH of the growth medium the lower the cell survival rate.
The present results demonstrate the capability of the newly designed hydrogel system (BIOF-IPNs) to play an important role as a “build in” free radical defense mechanism, and acting as a “proof of concept” for the functional multi-dimensional restorative repair materials. The present findings suggest that the new material would definitely find applications in functional dental composites and regenerative dentistry.
Acknowledgements
The authors would like to acknowledge the financial contribution of VTPCHEM PTY LTD and the University of Western Cape. Authors would like to declare that there is none conflict of interest associated with the research presented.
References
- Roy I, Gupta M (2003) Smart polymeric materials: Emerging biochemical applications. Chemistry & Biology 10: 1161-1171.
- Patel JK (2006) Bio adhesive microspheres: a review.Pharmaceutical Reviews 4: 6-18.
- Chen J, Park K (2000) Synthesis and characterization of superporous hydrogel composites.Journal of Controlled Release 65: 73–82.
- Van Dijk-Wolthuis WNE, Tsang SKY, Hennink JJ (1997) A new class of polymerizable dextrans with hydrolyzable groups: hydroxyethyl methacrylated dextran with and without oligolactate spacer.Polymer 38: 6235–6242.
- Grobler SR, Olivier A, Moodley D, Kotze TJW (2004) Cytotoxicity of two concentrations of a dentine bonding agent on mouse 3T3 and human pulp fibroblast cell-lines. SA Dent J 59: 368-372.
- Grobler SR, Olivier A, Moodley DS, W Kotze TJ (2008) Cytotoxicity of recent dentin bonding agents on mouse fibroblast cells. Quintessence Int 39: 511-516.
- Moodley D, Grobler SR, Olivier A (2004) Cytotoxicity of a dentine bonding agent on four different cell-lines. SA Dent J 60: 101.
- Han B, Jaurequi J, Tang BW, Nimni ME (2003) Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. Journal of Biomedical Materials Research Part A 65: 118–124.
- Aasen SM (1990) History of dentinal bonding. Esthet Dent Update 1: 43-46.
- Schmalz, Gottfried, Arenholt Bindslev, Dorthe (2009) Biocompatibility of dental materials. Heidelberg: Springer.
- Heymann HO, Bayne SC (1993) Current concepts in dentin bonding: Focusing on dentinal adhesion factors. The Journal of the American Dental Association 124: 27-36.
- Perchyonok TV, Grobler S, Olivier A, Zhang S, Oberholzer T (2013) Insights into chitosan hydrogels on dentine bond strength and cytotoxicity. Open Journal of Stomatology 3: 75-82.
- Tang Y, Singh J (2008) Controlled delivery of aspirin: effect of aspirin on polymer degradation and in vitro release from PLGA based phase sensitive systems. Int J Pharm 357: 119–25.
- Titley KV, Smith DC, Chernecky R, Maric B, Chan A (1995) An SEM examination of etched dentin and the structure of the hybrid layer. Journal of Canadian Dental Association 61: 887-894.
- Tjaderhane L, NascimentoFd, Breschi L, Mazzoni A, Tersariol Il, et al. (2013) Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dental Materials 29: 116–135.
- Yamagishi Y, Onuma K, Suzuki T, Okada F, Tagami J, et al. (2005) A synthetic enamel for rapid tooth repair. Nature 433: 819.
- Van Nieuwenhuysen JP, D’Hoore W, Carvalho J, Qvist V (2005) Long-term evaluation of extensive restorations in permanent teeth. Journal of Dentistry 31: 395–405.
- Higuchi T (1961) Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci 50: 874–875.
- Castellan CS, Bedran-Russo AK, Karol S, Pereira PNR (2011) Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. Journal of the Mechanical Behavior of Biomedical Materials 4: 1343–1350.
- Liu Y, Tjaderhane L, Breschi L (2011) Limitations in bonding ¨ to dentin and experimental strategies to prevent bond degradation. Journal of Dental Research 90: 953–968.
- Hashimoto M (2010) A review—micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. Journal of Biomedical Materials Research Part B Appl Biomater 92: 268–280.
- Li L, Pan H, Tao J, Xu X, Mao C, et al. (2008) Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem 18: 4079–4084.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 14537
- [From(publication date):
February-2015 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 9893
- PDF downloads : 4644