Bio-Active Transition Metal Ions are Involved in the Formation of Equilibria and Thermodynamic Stability of Multinuclear Amino Acid and Pyrimidine Base Chelates
Received: 02-Nov-2022 / Manuscript No. ico-22-82574 / Editor assigned: 04-Nov-2022 / PreQC No. ico-22-82574(PQ) / Reviewed: 18-Nov-2022 / QC No. ico-22-82574 / Revised: 24-Nov-2022 / Manuscript No. ico-22-82574(R) / Published Date: 30-Nov-2022
Abstract
Coordination tendency of amino acid (valine) to form heterobinuclear complexes with some bi positive metal ions (CoII, NiII, CuII, ZnII) in presence of pyrimidine base (Uracil) have been explored potentiometricallyin aqueous medium. Formation constants of the complexes at 37±10 and at constant ionic strength 0.1 NaNO3 have been evaluated using SCOGS computer program. Solutions of Cobalt, Nickel, Copper, Zinc, metal ions (0.01M) were prepared and standardized by EDTA titration. The solution of uracil (0.01M) was prepared by dissolving the ligand into one equivalent of alkali sodium hydroxide, whereas valine solution (0.01M) wasprepared in double distilled water only. The speciation curves are finally sketched by running computer program ORGIN4.0. The overall stability constants of binary, ternary and multinuclear chelates follow Irving William Order. Titration and speciation distributioncurves werefound to be favourable for the complexation.
Keywords
Multinuclear Chelates; Amino acid (Valine); Pyrimidine base (Uracil); SCOGS
Introduction
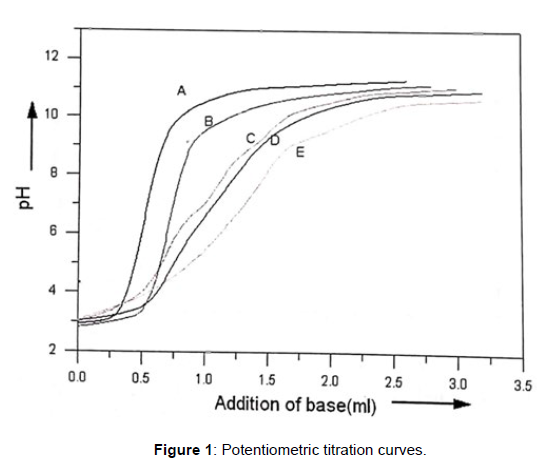
The Chelating agents in heavy metal intoxication treatment are a complex field were collaboration of different branches of science is required. The principle contribution of chemistry to this field is in determination of speciation constants and as coordination models of complexes between metal ions (viz. Co, Ni, Cu, Zn, Pb, Cd etc.) and chelating agents to compare the properties and strength of formed complexes. In the speciation process using in vivo chelation, it should be taken into account that there is always competition for the coordinating positions between essential and toxic metal ions such as Cu(II) , Zn(II), Mn(II), Mg(II), Ir(II)[1,2].Coordination chemistry in metallo protiens is dominated by amino acids [3,4]. Metal chelating functional and structural motifs plays an important role in the development of new model of complexes [5]. Mixed ligand and multimetal- multiligand complexes play important role in biological processes as exemplified by many instances in which enzymes are known to activate metal ions [6- 8]. Cobalt, Nickel, Copper, are an important trace elements for plants and animals and are involved in ternary complexation . Transition metals are involved in many redox processes requiring electron transfer . In continuation to our studies on mixed chelates, we report herein the speciation equilibria of multimetal – multiligand complexes, their overall formation constants and percentage distribution curves in accordance with the pH variations. The probable solution structures of metal complexes with said ligands and order of speciation constants of multinuclear chelates have been discussed (Figure 1).
Experimental
All the solutions were prepared in double distilled carbon dioxide free water. Metal nitrate solutions were standardized by ethylene diamine tetra acetic acid (complexometric titration). A digital pH meter century-model (CP901-S)with glass electrode at 37±10C, reading upto 0.01 units was used for measuring the pH of prepared solutions. The potentiometer was standardized using buffer solutions of 4.0, 7.0 and 9.2 .Ph. Calvin-Bjerrum pH titration technique as modified by Irving and Rossotti was used for potentiometric determination of proton ligand and metal ligand stability constants. Binary M:L1 / M:L2 (1:1) , Ternary M:L1:L2 (1:1:1) and Quaternary M1:M2:L1:L2 (1:1:1:1) metal ligand mixtures of following composition were prepared for titration, keeping total volume 50 ml in each case. Strength of metal and ligand =0.01 M and ionic strength I=0.1 M sodium nitrate (NaNO3). Solution A: 5ml NaNO3 (1.0M) + 5ml HNO3 (0.02M) + Water Solution B: 5 mlNaNO3(1.0M) + 5ml HNO3 (0.02M)+ 5ml L1 (0.01M) + water Solution C : 5ml NaNO3 (1.0M) + 5ml HNO3 (0.02M) + 5ml L1(0.01M) +5ml M1(II) (0.01M) + water Solution D: 5ml NaNO3 (1.0M) + 5mlHNO3(0.02M) + 5ml L1(0.01M) + 5ml M1(II) (0.01M) + 5ml L2(0.01M) + water Solution E : 5ml NaNO3 (1.0M) + 5ml HNO3(0.02M) + 5ml L1(0.01M) + 5ml M1 (II) (0.01M) + 5ml L2(0.01M) + 5 ml M2 (II) (0.01M) + water
Where M1 (II) and M2 (II) are Co/Ni/Cu and Zn, L1 = Primary ligand and L2=Secondary ligand. The pH meter readings with progressive addition of alkali to the titration mixtures were noted, once the reading gets stabilized. The titration was discontinued at the appearance of turbidity. The pH values were plotted against the volume of NaOH and titration curves were obtained. For evaluation of stability constants by the SCOGS computer program [30] in a system of the two different metal ion M1 and M2 and two different ligands L1and L2 in aqueous medium, speciation may be described according to equilibrium.
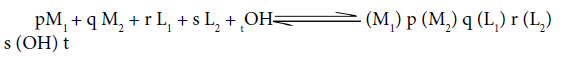
The overall stability constant ( βpqrst) is defined as:
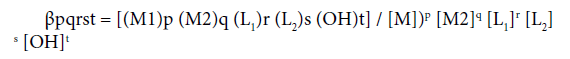
May be used to calculate the species distribution curves that provide the clues for the formation equilibria of the complexes. Values of constants were supplied to the computer as input data to obtain distribution curves of the complexes occurring at different pH. Ionic product of water (kW) and activity coefficient of hydrogen ion under the experimental conditions were obtained from literature.
Result and discussion
Valine is an aliphatic, nonpolar, hydrophobic amino acid. It contains two potentially ionisable functional groups (COOH and NH2) and behaves as a bidentate ligand. The side chain of valine is highly nonreactive, and is thus rarely directly involved in protein function. Particularly hydrophobic amino acids may be engaged in the binding or recognition of lipids and other hydrophobic ligands. The ionisation constants (pK) values correspond to the ionisation of a proton from amine and carboxylic groups.
Much interest has been shown in the chemistry ofβ-lactam in relation to their useful biological activities in recent years. Itis known that antibiotic activity is related to the ability of these compounds to form complexes with metal ions.
Uracil (a pyrimidine base) undergoes keto-enoltautomeric shift because of its resonating structures due to amine (NH2) and hydroxyl (OH) substrant.

Proton ionization from uracil in the strongly acidic medium has been reported based on spectroscopic data. Levene, Bass and Simmeobserved that uracil has two ionisable protons, yet they yield only one pK value i.e. 9.45, whereas srivastavas obtained its value 9.16 at 30 0C and 0.1 M (NaNO3) ionic strength.Protonation constants for the ligands have been determined by Irving- Rossotti titration technique are presented in the table given
Cu(II)-CO(II) –Valine-Uracil (1:1:1:1 ) system
Potentiometric titration curves and formation curves for [Cu (II) -Co (II)-Valine-Uracil ] system are presented in figure 1 and figure 2 respectively. pH titration curves it is clearly shows that the curve E (quaternary) set apart from curve D (ternary) at pH ≈4.2 ,indicating complex formation.Multimetal-Multiligand complex formation equilibria are found to incorporate quaternary complex species, H2L, HL (valine), HL (Uracil), binary, ternary complex species, free metal ions, and hydroxo species in present system. All the protonated ligand species are found to be decreasing with increase in the pH range ≈3.3- 8.5, which shows their involvement in the complex formation.The formation of metal ligandcomplexes is indicated by following.
The binary complexes [Cu(II)-L1] and [Cu(II)-L2H]+are present in the appreciable amount in the pH range≈3.2-9.0. The binary complexation starts in the beginning of titration and the mentioned complexes attain maximum concentration at ≈29% and≈19% respectively at pH ≈4.5. Whereas with further increase in pH, their concentrations decreases.
Mixed ligand complexes with Cu2+ (aq.) and Co2+ (aq.) is found to be remarkable speciesin the higher
pH range≈5.4-10.2 as follows.
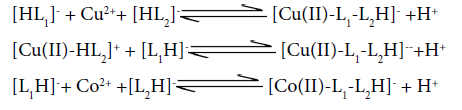
For quaternary system, the species distribution curves indicate the formation of heterobinuclear complex according to the following equilibrium:

Another form of multinuclear equilibrium can be written as:
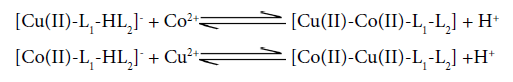
Speciation curve shows that there isa concomitant decline in the concentration of Cu2+ and Co2+ aqueous ions with the incline in the concentration of multimetal- multiligandcomplex species. Quaternary complex species is the predominant species in the present system. The complexation starts from the very beginning and the concentration of multinuclear species is increasing gradually with the gradual increase in pH attaining maximum value≈77% at ≈7.5. Metal hydroxo species Co(II)(OH)+ and Co(II)(OH)2 exit in the pH range≈7.4-10.2 involving the following equilibrium:

Ni(II)-Zn(II)-Valine-Uracil (1:1:1:1) system
Titration and speciation curves for [Ni(II)-Zn(II)-Valine-Uracil] system are presented in figure 3 and 4 respectively. Multinuclear species, protonated species; H2L,HL(Valine) , HL(Uracil), binary, ternary, free metal ions and hydroxo species exist in considerable amount throughout the entire pH range. It is clearly evident from the species distribution curves that the protonated ligand species H2L and HL of both the ligands are found to be decreasing with increase in pH range ≈3.2-7.5. The binary complex species [Ni(II)-Valine], [Zn(II)-Valine] are existing in the higher pH range ≈7.8-9.0,while [Ni(II)-Uracil], and [Zn(II)-Uracil] species are absent in the system. The complexation equilibria for binary complexes may be as follows:
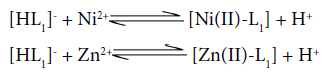
The formation of ternary complex species NiL1L2 and ZnL1L2 occurs in higher pH region ≈8.0-9.0. The concentration of mixed ligand complex inclines with increase in pH and attains maximum concentration ≈25% and 10% respectively, according to following equilibria:
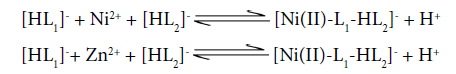
The alternative step-wise equilibria may be indicated as follows:
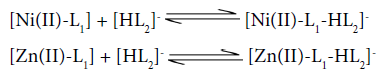
For multimetal-multiligand system formation curves indicate the complex formation equilibria as follows:

May be represented as:

Speciation curves clearly reveal that the concentration of free metal ions continuously fall with increase in pH , which shows involvement of free metal ions in the formation of multinuclearcomplexes.The complexation starts at very low pHregion and concentration of quaternary complexes with increase in pH attains maximum value≈85% at pH 7.6. The metal hydroxo species also exist in the system between the pH range ≈6.9-9.0, and attains maximum value ≈50%.
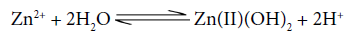
Refined values of binary,ternary and quaternary constants are listed in table 1.0, which are in good agreement with those in literature. The overall stability constants of mixed metal-mixed ligand [M1-M2-Valine- Uracil] systems have been found to follow the following order.
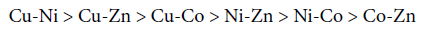
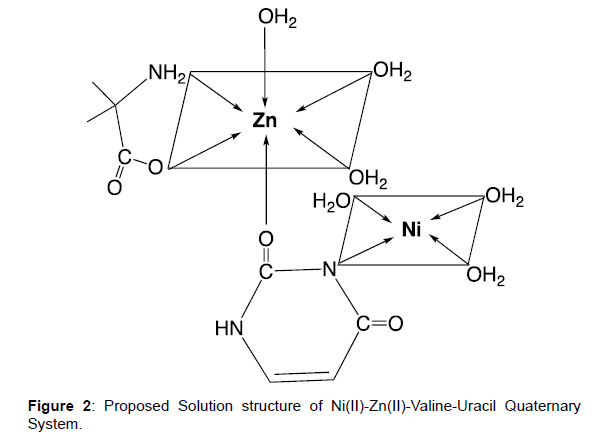
Solvated CO2+ ion is water coordinated from six molecules. Though only tetragonal coordination is feasible as two water molecules are situated at longer distance (Figure 2). Normally regular octahedral is the preferred configuration of hydrated nickel ion. However, strong field ligand may enforce tetragonal distortion leading to square planar configuration. Configuration of Zinc is regulated by nature of ligand bounded which can normally be converted to octahedral from tetrahedral geometry, Metal-ligand complex having the higher value of log β(ligand A) will be the first to attach, which further attaches to ligand B. Uncoordinated sites of ligands will then be occupied by another metal ion.
References
- Dunnick J K , Fowler BA , Seiler H G, Sigel A(Eds) (1988) Handbook on Toxicityof Inorganic Compounds . Marcel Dekker New York p-155
- Roat-Malone R M (2002) Bioinorganic Chemistry A short course john willey and sons Hoboken NJ wiley publisher united states.
- Zayed M A, Abdallan S M Spectrochim (2004) Acta part A Molecular and Bimolecular spectroscopy, 60: 2215.
- Sigel A, H Sigel (2001) Metal ions in biological system Marcel Dekker New York 1-38: 1971-2001.
- Mortal JM, Marhinez-Ferrer MJ, Jimnez, Donaire HR, Castells JJ (1992) Bioinorganic Chemistry In org Biochem 45: 231.
- Karlin KD, Tyekkr Z (1993) Bioinorganic Chemistry chapman hall New York.
- Chow ST, Mcavliffe CA (1975) Phosphine’s and metal phosphine complexes: relationship of chemistry to anticancer and other biological activity. Prog Inorg Chem19:51.
- H.M.Irving, R.J.P.Williams (1948) Nature (London) 76:162.
Citation: Shukla VP (2022) Bio-active transition metal ions are involved in the formation of equilibria and thermodynamic stability of multinuclear amino acid and pyrimidine base chelates. Ind Chem, 8: 209.
Copyright: © 2022 Shukla VP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2079
- [From(publication date): 0-2022 - Dec 01, 2025]
- Breakdown by view type
- HTML page views: 1608
- PDF downloads: 471