Research Article Open Access
Bioactive Sol Gel Coatings Applied Using the Pneumatic Spray Technique on AISI 316L Stainless Steel
Rendón J1, Toro A1 and Garcia C2*1Tribology and surfaces Group, Universidad Nacional de Colombia, Medellín-Colombia
2Claudia Garcia, Physics school. Universidad Nacional de Colombia sede Medellín, Colombia
- Corresponding Author:
- Claudia Garcia
Physics school, Universidad Nacional de Colombia sede Medellín
Calle 59A # 63-20 Medellín, Colombia
Tel: 574430984
Fax: 5744309327
E-mail: cpgarcia@unal.edu.co
Received date: December 17, 2014, Accepted date:: March 30, 2015, Published date:: April 06, 2015
Citation: Rendón J, Toro A and Garcia C (2015) Bioactive Sol Gel Coatings Applied Using the Pneumatic Spray Technique on AISI 316L Stainless Steel. J Biotechnol Biomater 5:174. doi:10.4172/2155-952X.1000174
Copyright: © 2015 Claudia G et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Bioactive coatings were obtained via the sol gel method and deposited on stainless steel AISI 316L using a pneumatic spray. TEOS and MTES were used as precursors, obtaining a hybrid sol; wollastonite bioactive particles were dispersed inside the sol. The corrosion resistance of the coatings was tested with potentiodynamic curves after immersion of the coatings in SBF for 7 and 40 days. It was possible to obtain coatings with a thickness of 47.7 ± 8 μm. In comparison with other methods of deposition, it was possible to obtain thicker coatings with the pneumatic spray deposition technique, giving the substrate a better protection factor against corrosion. After immersion in SBF (simulated physiologic fluid), the formation of an appetite film on the surface and inside the pores was observed. Deposition of the apatite film helps to promote Osseo integration, and the product formation inside the pores helps to improve corrosion resistance of the substrate.
Keywords
Bioactive coating; Bioactivity; Corrosion resistance; Pneumatic spray; Sol gel; Wollastonite
Introduction
The most common causes of failure of an orthopedic implant are biological incompatibility and degradation of the piece. Metals are used due to their mechanical properties, but a risk of metallic debris produced by wear and ion release produced by chemical degradation exists, which could lead to early removal of the implant [1-5]. Also, most metals are not able to create a natural bond with the mineralized bone. One way to avoid ion release by the metal of the implant is to coat the metallic implant with a protective film, which in turn could be functionalized with the addition of bioactive particles. That way, the coating acts as a barrier, preventing the release of metal ions and aiding the formation of a bond with the old bone through formation of hydroxyapatite (HAp) [6-8]. Wollastonite particles have generated much interest due to its promise as a potential implantable material because it can form bonds with bone tissue through the development of a biological apatite layer on the surface [9,10]. The formation of apatite on wollastonite ceramics is faster than that on other bioglass and glass-ceramics in Simulated Body fluids (SBF) [11]. Sol gel coatings have been successfully used on diverse substrates and have improved their oxidation and corrosion resistance; also sol gel technique is versatile and allows the synthesis of many oxides using high-purity precursors [12-14]. Dip coating and pneumatic spray are two techniques that can be used to deposit bioactive coatings on metal substrates. With the dip coating technique, very uniform and thin coatings (<4μm) can be obtained [14-16]. Regarding to the spray technique it has been found good results with bioactive coatings applied by airbrush, with thickness in the 50-500 μm range and with adhesion strength of 25 MPa [17]. As well as in others electronic and industry applications, the spray technique has been used, and the main advantage of this technique is that the access to very irregular zones is possible, making pneumatic spraying an affordable and versatile technique that can be used in many applications [17-22]. The main goals of this study were to synthetize bioactive coatings via the sol gel method, obtain coatings with significant thickness by pneumatic spray, and compare the electrochemical behavior of an stainless steel 316L substrate using two different techniques: spray and dip coating.
Materials and Methods
The sol was prepared using TEOS (Tetraethyl orthosilicate, Merck) and MTES (Methyltrietoxysilane, Merck) as silica precursors, absolute ethanol (J.T Baker 99.7%) as a solvent, and acetic acid and 0.1 N nitric acid as catalysts. The molar ratio of TEOS/MTES was 40:60, H2O:SiO2 was 2:1, and H2O:acetic acid was 10:1. The sol was prepared by mixing the alkoxides and the ethanol at room temperature inside a balloon glass. A magnetic mixer was used during the synthesis process. Then water and acetic acid were added. Once the reactants were mixed, the balloon glass was placed in a thermostatic bath for 3 hours at 40°C, obtaining a transparent sol with a pH of 6. For the suspensions, an ester phosphate dispersant was added to the solution and stirred using a high-shear rotor-stator (Silverson L2R) for 3 minutes; the proportion of dispersant was 3% by weight with respect to wollastonite particles. Finally, Wollastonite (10% by weight) particles were added to the solution and stirred using the high rotator for 3 minutes. Mirrorpolished metal plates with a size of 2.5 x 2.5 x 1 (mm) of 316L alloy were used as substrates. Samples were degreased, hand washed with distilled water, and rinsed in ethanol. Coatings were obtained via pneumatic spray using a spraying distance of 60 cm, a nozzle diameter of 1.5 mm and air pressure of 50 psi. After each deposition, the coatings were treated at 450°C for 30 minutes. After ten depositions, the coating’s thickness was measured by viewing a cross-section scanning electron microscope (SEM) image of the sample. The electrochemical behavior was tested using an electrochemical unit (GAMRY 600) in a Simulated Body Fluid (SBF) at pH 7.3 after 7 and 40 days of immersion at 37°C. The bioactivity of the coatings was tested according to the capacity of the coated materials to induce apatite films on their surface after different periods of immersion in SBF [21,22]. The analysis of the surface and the determination of the presence of the apatitic phases were carried out by means of a scanning electron microscope (JEOL JSM5910LV) equipped with a microanalysis EDS and WDS system, used to detect the composition of the apatitic phase.
Results
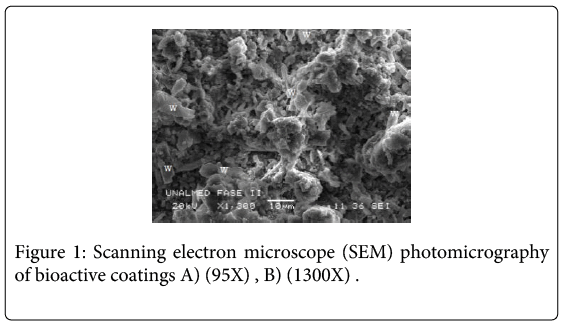
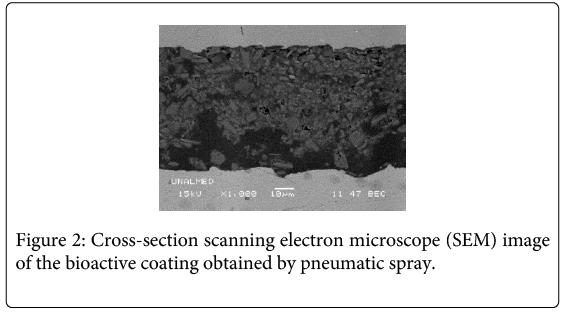
Wollastonite particles (46.15% CaO and 51.60% SiO2) exhibited an acicular shape and were randomly dispersed in the sol silicon matrix. The surface coating composition is mostly based on amorphous SiO2 which belongs to the TEOS–MTES, having wollastonite particles homogeneously distributed in the film. Microscopic images of the coatings showed a uniform and crack-free film (Figure 1). Average thickness measured in the images was approximately 47.7 ± 8 μm (Figure 2). In past investigations [23], the dip coating technique was used to obtain TEOS-MTES with wollastonite particles, obtaining coatings with a homogeneous distribution of the wollastonite particles in the sol matrix, without flaws or cracks on the surface or around the wollastonite coatings. The thickness obtained with the dip coating technique was 1.10 ± 0.10 μm, four orders of magnitude lower than the thickness obtained through pneumatic spray.
The sol gel method was introduced to generate nanoscale coatings with appropriate pore size and structures for improving osseointegration [24]. Some deposition methods used with the sol technique to obtain bioactive coatings are: dip coating [25,26], spin coating [27], and reactive magnetron sputtering [28]. All of these methods are used to obtain nanoscale coatings. However, with the pneumatic spray technique it is possible to obtain thicker coatings without any cracks.
Table 1 shows the parameters extracted from curves of polarization corresponding to the coatings obtained with immersion-extraction [29] and pneumatic spray after 7 and 40 days of immersion in SBF. After 7 days of immersion in SBF, the passivation current density was three orders of magnitude lower with coatings obtained by means of spray (10-8) compared with immersion coatings (10-5). After 40 days of immersion in SBF, the electrochemical behavior of the coatings obtained via pneumatic spray remains invariant. The average thickness was 1.10 ± 0.1 μm for immersion coatings and 47.7 ± 8.7 μm for the spray technique. The thickness increased when the spray technique was used, and consequently the corrosion resistance of the substrate also improved.
| Coating Technique (coating´s thickness) |
Polarizations currents and voltages | Bare metal | Bioactive coatings after inmersion in SBF | |
| 7 days 40 days | ||||
| 1. Pneumatic spray (47.7 ± 8,7 µm) |
ipass (A/cm2) Ecorr (V) | 1,16x10-2 -0,18 | 1,81 x10-8 -0,21 | 1,67 x 10-8 -0,17 |
| 2. Inmersion-extraction (1.10 ± 0.10 μm) [21] | ipass (A/cm2) Ecorr (V) | 2x10-2 -0,15 | 7,2x10-5 -0,15 | 2,2x10-5 -0,27 |
Table 1: Main results of polarization curves of coatings obtained through pneumatic spray and immersion-extraction
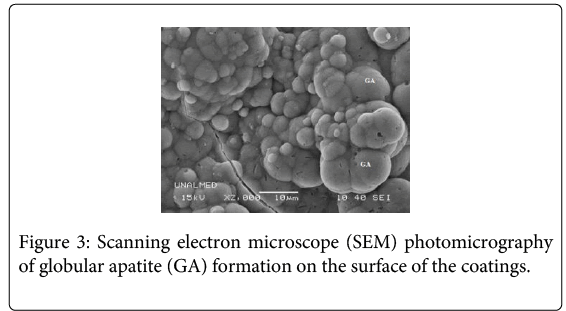
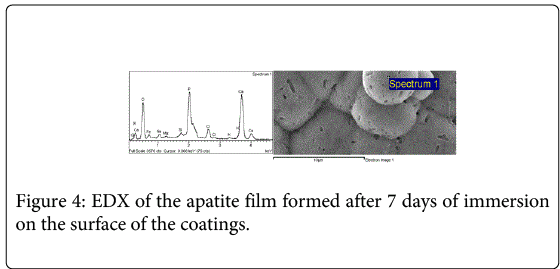
All the coatings formed an apatite film (Figure 3) on the surface after 7 and 40 days of immersion in simulated physiological fluid (SBF). EDX analysis registered the presence of calcium phosphates on the surface of the coatings (Figure 4).
Discussion
In the present study, the pneumatic spray technique was used for coating a stainless steel 316L used in implants and hip prosthesis. This technique allows multiple depositions (ten depositions in our case). During the spray deposition, ethanol evaporation took place before the solution reached the substrate; this helped to minimize internal stresses during the drying and sintering stages. Also, the sprayed solution reached the substrate with high velocity and moderate pressure, which was enough to overcome the superficial tension of the substrate. Residual stresses generated during deposition and sintering were not significant; thus a porous coating without any cracks was obtained. A free crack and thicker bioactive coating was obtained with the pneumatic spray deposition technique. This was very different to what happens with the thickness of the coating obtained by dip technique; although the dip coating technique allows the fabrication of very uniform and crack-free sol gel bioactive coatings, there is a restriction with respect to the thickness; the thickness obtained in previous investigations was not greater than 2 μm because the dip coating technique has a critical thickness, due to the evaporation of the solvents [23].
The thickness of the coating obtained with airbrush spraying and pneumatic spray method is only dependent on the number of spraying cycles; Francesco Baino et al. prepared Wollastonite coatings with thickness in the 50-500 μm range and conclude that this technique is suitable to manufacture homogeneous and continuous coatings on model curved ceramic surfaces [15]. Other authors have developed coatings on metals including particles as hydroxyapatite, zircon, bioglass, etc. [23,24]. However, coatings including wollastonite particles, not only show better bioactivity than others do [25,26], but also have interesting mechanical properties because the particles form [27] and distribution of the bioactive particles on the surface of the coating.
The electrochemical in vitro assays revealed that the presence of the coating improved the corrosion resistance of the stainless steel substrate after immersion in SBF, indicating that the coating acts as a barrier to prevent the electrolyte from reaching the substrate at 7 and 40 days of immersion in SBF. All the potentiodynamic data showed a decrease of the passivation current density (ipass), which is the current density at which the metal remains passive, and a shift to positive potentials of the breakdown potential compared to the bare material. Coatings obtained through dip coating had less corrosion resistance compared with coatings obtained through pneumatic spray. The difference between thicknesses could be responsible for the improvement in resistance.
Electrochemical tests showed an interesting protective behavior of the bioactive coatings applied via spray. Corrosion resistance was proportional to the immersion time of the coatings in SBF. This behavior could indicate that although coatings exhibited pores and defects after immersion in SBF, these defects could be blocked by corrosion products from the metal or by degradation of the particles present in the coating [13,26,30].
The presence of Mg and Na in the EDX spectra of the apatite film formed after 7 days immersed in SBF can be attributed to the SBF where the samples were immersed. The EDX results indicate that the chemical composition of the deposited materials on the wollastonite surface consists of P, Ca and a small quantity of Si, which suggests that the deposited material is a type of apatite. The presence of apatitic phases indicates that the tested coatings are potentially bioactive [31-33]. Previous in vitro studies have also reported that materials containing calcium oxide (CaO) and SiO2 (silicon dioxide) are bioactive and the formation of apatite is faster than on other bioglasses or glass-ceramics in the SBF solution [11].
An in vivo study about bioactive coatings using wollastonite has been demonstrated that the bioactive sol–gel/wollastonite coating on surgical grade stainless steel promotes formation and growth of a new bone in the periphery of the implant, both in contact with the old bone (remodellation zone) and the marrow [23].
Conclusion
The pneumatic spray technique is a versatile and economic technique for use in applying bioactive coatings on metal substrates; this method was used to obtain thicker bioactive coatings synthetized by the sol gel process. With this technique, it is possible to obtain crack-free coatings. Since the thickness of the coatings is higher, the protective behavior of the coatings obtained using the pneumatic spray technique is better than the coatings obtained through dip coating. Wollastonite dispersed in the sol matrix is compatible with the physiological environment and the composition of SiO and CaO helps to accelerate the osteointegration forming an apatite layer in a short period. This fast bioactive response allows to the implant to have a good fixation to the bond.
Acknowledgements
This paper was supported by the Universidad Nacional of Colombia sede Medellín.
References
- Lohmann CH, Schwartz Z, Köster G, Jahn U, Buchhorn GH, et al. (2000) Phagocytosis of wear debris by osteoblasts affects differentiation and local factor production in a manner dependent on particle composition. Biomaterials 21: 551-561.
- KeRen, AnandDusad, Yijia Zhang, Dong Wang (2013) Therapeutic intervention for wear debris-induced aseptic implant loosening. ActaPharmaceuticaSinica B 3: 76-85.
- Geringer J, Pellier J, Cleymand F, Forest B (2012) Atomic force microscopy investigations on pits and debris related to fretting-corrosion between 316L SS and PMMA. Wear 292: 207-217.
- Bozic KJ, Ries MD (2005) Wear and Osteolysis in Total Hip Arthroplasty. Seminars in Arthroplasty 16: 142-152.
- Wang ML1, Sharkey PF, Tuan RS (2004) Particle bioreactivity and wear-mediated osteolysis. J Arthroplasty 19: 1028-1038.
- Fathi MH, Doost MA (2008) Preparation and characterization of sol–gel bioactive glass coating for improvement of biocompatibility of human body implant. Materials Science and Engineering: A 474: 128-133.
- Liu X, Morra M, Carpi A, Li B (2008) Bioactive calcium silicate ceramics and coatings. Biomed Pharmacother 62: 526-529.
- Gopi D, Ramya S, Rajeswari D, Kavitha L (2013) Corrosion protection performance of porous strontium hydroxyapatite coating on polypyrrole coated 316L stainless steel. Colloids Surf B Biointerfaces 107: 130-136.
- Salahinejad E, Hadianfard MJ, Macdonald DD, Mozafari M, Vashaee D, et al. (2013) A new double-layer sol–gel coating to improve the corrosion resistance of a medical-grade stainless steel in a simulated body fluid. Materials Letters 97: 162-165.
- Liu X, Ding C, Chu PK (2004) Mechanism of apatite formation on wollastonite coatings in simulated body fluids. Biomaterials 25: 1755-1761.
- Liu X, Ding C (2002) Morphology of apatite formed on surface of wollastonite coating soaked in simulate body fluid. Materials Letters 57: 652-655.
- Rahimi H, Mozaffarinia R, HojjatiNajafabadi A (2013) Corrosion and Wear Resistance Characterization of Environmentally Friendly Sol–gel Hybrid Nanocomposite Coating on AA5083. Journal of Materials Science & Technology 29: 603-608.
- Wang D, Bierwagen GP (2009) Sol–gel coatings on metals for corrosion protection. Progress in Organic Coatings 64: 327-338.
- García C, Ceré S, Durán A (2004) Bioactive coatings prepared by sol–gel on stainless steel 316L. Journal of Non-Crystalline Solids 348: 218-224.
- Ballarre J, López DA, Schreiner WH, Durán A, Ceré SM (2007) Protective hybrid sol–gel coatings containing bioactive particles on surgical grade stainless steel: Surface characterization. Applied Surface Science 253: 7260-7264.
- Tan ALK, Soutar AM, Annergren IF, Liu YN (2005) Multilayer sol–gel coatings for corrosion protection of magnesium. Surface and Coatings Technology 198: 478-482.
- Kobayashi M, Olding TR, Sayer M, Jen CK (2002) Piezoelectric thick film ultrasonic transducers fabricated by a sol-gel spray technique. Ultrasonics 39: 675-680.
- Kang YJ, Lim K, Jung S, Kim DG, Kim JK, et al. (2012) Spray-coated ZnO electron transport layer for air-stable inverted organic solar cells. Solar Energy Materials and Solar Cells 96: 137-140.
- Puetz J, Gasparro G, Aegerter MA (2003) Liquid film spray deposition of transparent conducting oxide coatings. Thin Solid Films 442,: 40-43.
- Parkhill RL, Knobbe ET, Donley MS (2001) Application and evaluation of environmentally compliant spray-coated ormosil films as corrosion resistant treatments for aluminum 2024-T3. Progress in Organic Coatings 41: 261-265.
- Pan H, Zhao X, Darvell BW, Lu WW (2010) Apatite-formation ability--predictor of "bioactivity"? ActaBiomater 6: 4181-4188.
- Bohner M, Lemaitre J (2009) Can bioactivity be tested in vitro with SBF solution? Biomaterials 30: 2175-2179.
- Karamian E, KalantarMotamedi MR, Khandan A, Soltani P, Maghsoudi S (2014) An in vitro evaluation of novel NHA/zircon plasma coating on 316L stainless steel dental implant. Progress in Natural Science: Materials International 24: 150-156.
- Gomez-Vega JM, Saiz E, Tomsia AP, Marshall GW, Marshall SJ (2000) Bioactive glass coatings with hydroxyapatite and Bioglass particles on Ti-based implants. 1. Processing. Biomaterials 21: 105-111.
- Xue W, Liu X, Zheng X, Ding C (2005) In vivo evaluation of plasma-sprayed wollastonite coating. Biomaterials 26: 3455-3460.
- Liu X, Ding C (2002) Plasma sprayed wollastonite/TiO2 composite coatings on titanium alloys. Biomaterials 23: 4065-4077.
- Lin K, Zhai W, Ni S, Chang J, Zeng Y, et al. (2005) Study of the mechanical property and in vitro biocompatibility of CaSiO3 ceramics. Ceramics International 31: 323-326.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14412
- [From(publication date):
April-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 9860
- PDF downloads : 4552