Bio Fertilizers an Emerging Cure to Increasing Plastic Contaminations; an Experimental Study on Cereal Crops in Asia
Received: 01-Dec-2023 / Manuscript No. acst-23-123734 / Editor assigned: 04-Dec-2023 / PreQC No. acst-23-123734 / Reviewed: 18-Dec-2023 / QC No. acst-23-123734 / Revised: 22-Dec-2023 / Manuscript No. acst-23-123734 / Published Date: 29-Dec-2023
Abstract
Microplastics (MPs) in soils can deleteriously impact soil biodiversity and function. Soil additives can boost the quality of MP-contaminated soil and its functions and processes. However, nothing has been discovered about how soil additives enhance MP-contaminated soil quality. Thus, the current work is conducted to investigate the impacts of two types of biochar; activated carbon biochar (ACB) and corn cob biochar (CCB) at 2.5% and 5% concentrations each on growth, physiological, biochemical, antioxidant, and ionic contents of wheat. Surface-sterilized wheat seeds were sown in pots in two groups: non-contaminated and MP-contaminated. Data were collected for further analysis from healthy harvested plants. The results demonstrate that biochar changed plant vegetative, photosynthetic, osmolytes, oxidative stress indicators and ionic contents to varying degrees depending on the biochar type. The application of ACB and CCB ameliorated the toxic effects of MPs in wheat at both concentrations. Still, better vegetative growth physiological and biochemical responses were noticed with their higher concentrations, i.e., at 5%. A considerable reduction in MP effects on wheat was noticed in MP-contaminated soil due to ACB and CCB amendments. Moreover, by increasing their concentrations from 2.5% to 5% had considerable positive effects on all studied parameters, both in non-contaminated and MP-contaminated soils. It can be concluded that using ACB and CCB provides an effective innovative technique to reduce MP vegetative, physiological, biochemical, ionic and oxidative disturbances in wheat
Keywords
Wheat; Biochar; Growth; Toxicity; Yield
Introduction
Microplastics in soils have been discovered in various terrestrial ecosystems [1] when deposited to the soil surface by diversity of input ways [2] comprising biological activity contributing to the merging of macroplastic components into soil [3]. Microplastics (MPs) decomposition ratio in soil is however presently unidentified and it is assumed that this material is tenacious and hence keeps accumulating [4]. Microplastic is capable of changing soil biophysical properties including aggregation of soil, bulk density and soil water holding capability [5]. Global population has reached up to 7.7 billion individuals as per April 2019 that revealed tremendous increase [6]. This increase has brought an increase in waste products generated by people along with it along. On the-go lifestyles require effortless disposable items, like soda cans or bottles of water, but accretion of these products has resulted in enhanced bulks of plastic pollution worldwide. Plastic is basically an organic polymer derived out of fossil feed stocks like natural gas, oil or coal etc. Polluting plastic contaminants are usually biochemically inert owing to their larger molecular sizes and can possibly result in harmful impacts to the environment. Large plastic (macroplastic) debris in the environment faces purification by natural mechanisms to give out tiny plastic fabrics called microplastics. Microplastics can be categorized as plastic substances of micro size dimension (often between range of 1 μm-5 mm) [7].
The occurence of MPs in the environment is continuously flourishing notably in oceans as detected by upsurge in frequency as well as bulk quantity of plastic gulped by seabirds [8]. One of the primary environmental constraints linked with microplastics is that they are omnipresent and always available for effecting marine life [9] soil organisms and plants relying on microplastic contaminated soils (Li et al., 2019) and their activities are also reported in in plants like wheat [10]. When these are accumulated in a terrestrial environment, some crops may ultimately wilt down in the ground water system [11]. This relocation of microplastics into groundwater streams may be harmful for plants, animals, microbes, human health as well as the environment. There is need to mitigate the devastating effect of MPs in terrestrial ecosystem especially with agricultural lands to overcome the lost yield of cereals and staple food crops such as wheat. Biochar is biological remains yielded by thermal and/or chemical conversions without oxygen.
Inclusion of biochar to soil can aid in refining organo-mineral complexes along with soil formations that rectifies heavy metals [12]. And organic pollutants like absorbable organic halides, polychlorinated dibenzofurans, polychlorinated dibenzodioxins, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons [13 ] and to minimize fluctuations by sequestering the carbon in multi-contaminated soil [14]. Sewage sludge (SS) is a heterogeneous mixture usually including microorganisms, animal and plants based residues, fibers, parasites and pathogens [15]. There is a quick need to improvise metholodigies and actions for restricting of MPs volume and soil perilous materials in sewage wastewaters [16]. Plastic will be tainted and disintegrated into minor plastics using biological, physical and chemical means i.e., weathering, oxidative degradation, water erosion, and decomposition by microorganisms and agriculture cultivation [17]. Micro-plastics (MPs) are diversly abundant, bulk in quantities yet smaller in size and may be intake by soil organisms that threatens their life cycle.
Variations in soil physicochemical characteristics as well as microbial functions activated by MPs and fluctuations in MPResearch affected soil due to diverse water bodies and organic treatments are yet comparatively indistinct. Soil interventions, like animal manure, biochar, composting and plant residues are commonly employed to boost soil quality [18]. Amongst them, biochar amendment has been categorized as a valuable method owing to its particular features for refining soil physio-chemical as well as biological characteristics [19]. Hence, it can be hypothesized that diminished soil quality produced by MPs might be rectified by biochar application to MP-disintegrated soils. Besides, different forms of into biochar like activated carbon biochar and corn cob biochar as a source of it could be examined and subsequent inclusion in soil can be an effectual/66management approach and a sustainable agricultural practice that can help minimize issues associated MP contamination of soil. In this evaluation, two forms of biochar were formed using activated carbon and corn cob will be observed in both contaminated and non-contaminated soils. Wheat, cultivated for staple food production [20] using the variety “Anaaj” may be useful to manage challenges of handling microplastics. Potential interactive impact of two different types of biochar in soil in various biochar amendments and MP contaminated soil were investigated in this trial. Objectives of this study were to evaluate the combined application of biochar mitigating microplastic contamination of soil and improving the yield percentage of modern wheat cultivar. Perhaps, this is foremost primary report concentrating on influence of combined biochar on chemical along with biological features of MP contaminated soils.
Materials and Methods
Experimental site and design
Wheat seeds of variety Anaaj was raised in pots in University trial area (at 30° to 31.5° N; 73° to 74° E and 184.4 m above sea level). Experimental design followed was randomized complete block design (RCBD) and was tri-replicated.
Soil characteristics
Soil was obtained (9-13 inches deep) from University’s agricultural trial site, air-desiccated, and sieved using a 2 mm sieve. Soil was clay to sandy loam in texture with E Ce=1.4 dS m−1, pH=7.4, organic matter contents=0.6%, total N=0.06%, extractable K=167 mg kg−1 and available P=6.5 mg kg−1.
Collection of seeds, sterilization and sowing
The seeds were sterilized with 95 percent ethanol for 1 minute and then with 70 percent sodium hypochlorite solution (NaOHCl) for 10 minutes to prevent microbial infection. The seeds were then rinsed six times in distilled water. Firstly, 10 seeds were sown in each plastic pot (30 cm height x 25 cm diameter) with 8 kg soil, and after emergence was finished, seedlings were shrunk to five seedlings per pot.
Treatments
The treatments (two sets viz MP contaminated and noncontaminated soil) were: 1) control [untreated], 2) ACB 1 [activated carbon biochar at 2.5% w/w of soil], 3) ACB 2 [activated carbon biochar at 5% w/w of soil], 4) CCB 1 [corn cob biochar at 2.5% w/w of soil] and 5) CCB 2 [corn cob biochar at 5% w/w of soil].
Soil contamination and biochar amendment in soil
DPE (low-density polyethylene) was used as the MP contamination of soil as it is often employed as a mulching film in agricultural soil, resulting in widespread contamination [21]. LDPE (MP) pieces were formed by cry milling. The LDPE mulching films were crushed using liquid nitrogen and ground using an ultra-centrifugal mill and then sieved via 5 mm ring sieve. The particles were kept at 25°C after drying. MP concentration of 1% (w w-1) was chosen for this study to replicate average MP contaminated concentrations in the soil.
Corncob was colected from a local farmer .Corncob is an excellent source of active carbon [22]. The feedstock was first air-dried. Corncob was converted to biochar using a slow pyrolizer at 700°C under low oxygen conditions (Liu et al., 2014). The temperature was raised at a rate of 5°C per minute until the needed temperature of 700°C was reached, and then this peak temperature was retained for 2 hours. Biochar was then gently crushed and sieved to a powder through a 0.15 mm sieve and it was referred to as CCB.
Wheat straw biochar was made by pyrolysis (at 700°C in an inert atmosphere) and activated carbon biochar (ACB) was created in oxidizing atmosphere (O2) at higher temperatures (900°C) utilizing NaOH alkali (Nanda et al., 2016). The desired dose of biochar (2.5% and 5% w/w of pot soil) was delivered to each 8 kg soil in pot. Biochar was put to pot soil in a single session and allowed to settle for a month to achieve equilibrium.
Harvesting and measurement of growth attributes
Wheat was harvested 60 days after seed sowing. Shortly after they were collected, the length of the roots and shoots were measured. For further examination, fresh samples were enclosed in aluminum foil, kept in plastic zipper bags, and stored at-30°C in a biomedical refrigerator. Three samples out of each treatment were oven-desiccated for three days at 65°C to conduct ion analyses.
Chlorophyll contents
Dere et al. (1998) protocol was followed to evaluate chlorophyll concentrations in wheat leaves. 0.2 g leaf samples were extracted in 10 ml of 96% methanol for 1 minute at 1000 rpm using a homogenizer. The homogenate was filtered before being centrifuged for 10 minutes at 2500 rpm. This supernatant liquid was separated and employed to find chlorophyll by spectrophotometer (Model SM1200; Randolph, NJ, USA) at wavelengths of 666, 653, 470 nm for chlorophyll a, b, and total carotenoids estimation.
Leaf biochemical analysis
Fresh preserved leaf samples were used from each replicate and the biochemical experiment was performed. For this, 50 mg of leaf samples were inserted in 10 mL of 80% ethanol and then filtered. To evaluate non-enzymatic antioxidant components, this solution was re-extracted in 10 mL ethanol with a final volume of 20 mL and from this, phenolics [23], proline [24], soluble sugars [25] and proteins [26] contents were quantified using spectrophotometer (Model SM1200; Randolph, NJ, USA). Glycine betaine was found by procedure as given by Holmstrom et al. (2000).
Measurement of malondialdehyde, electrolyte leakage and H2O2 contents
Heath and Packer [27] technique, that estimates malondialdehyde (MDA) contents after thiobarbituric acid reaction, was used to assess lipid peroxidation in chloroplasts. Electrolyte leakage (EL) was assessed using a procedure developed by [28]. H2O2 contents was estimated using Mukherjee and Chaudhari’s protocol (1983). 0.1 g of leaf samples were extracted in 10 mL of cold acetone and centrifuged at 10,000 rpm for this experiment. 5 mL of strong ammonium solution and 4 mL of titanium reagent was then included in the solution. After centrifuging at 10,000 rpm for another 5 minutes, the resulting precipitate was left to dissolve in 10 mL 2N H2SO4. This mixture was centrifuged again to remove any suspended particles, and the optical density was measured using a spectrophotometer (UV-2550; Shimadzu, Kyoto, Japan).
Determination of ionic contents
The dried plant materials were digested in an acid mixture of HNO3 and HCIO4 on a hot-plate oven until a clear liquid was formed, then filtered and diluted with distilled water (Chapman and Pratt, 1961). A digested sample aliquot was used to determine potassium (K) ions using a flame-photometer [29]. The molybdate/ascorbic acid blue approach was used to determine the phosphorus (P) content [30]. Atomic Absorption Spectrum was used to analyze the calcium (Ca) and sodium (Na) ions (AAS; Shimadzu instruments, Inc., Spectra AA-220, and Kyoto, Japan).
Statistical analysis
Data was interpreted by two-way ANOVA in completely randomized block design. LSD test was used for the comparison of means at 5% significance level (P <0.05). Logarithmic data transformations were employed to define near-normal distributions before analysis.
Results
Fresh weight and length
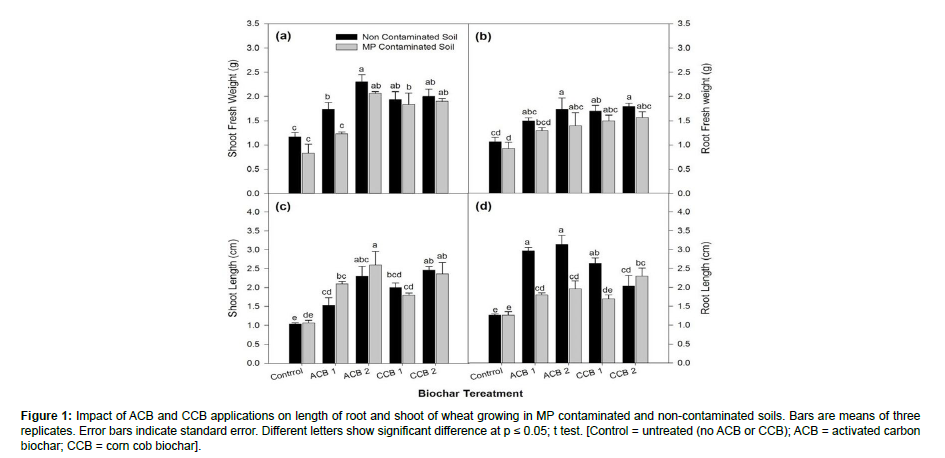
Results from Figure 1 indicate that the influence of ammendments was significant on root and shoot fresh weight and lengths. MP contamination in soil significantly reduced plant fresh weight and length of both root and shoot compared to non-contaminated plants. The applied treatments had significant positive effects in ameliorating MP toxicity. Results showed that ACB 2 and then CCB 2 improved the shoot fresh weight in MP contaminated and non-contaminated soils. Considering root fresh weight, both ACB 2 and CCB 2 had equal efficiencies in increasing shoot fresh weight. ACB 2 and CCB 2 differed significantly better over ACB 1, CCB 1 and control in MP contamination for shoot length with ACB 2 having more profound effects. ACB 2 significantly improved root length in comparison with other treatments in MP stressed plants.
Figure 1: Impact of ACB and CCB applications on length of root and shoot of wheat growing in MP contaminated and non-contaminated soils. Bars are means of three replicates. Error bars indicate standard error. Different letters show significant difference at p ≤ 0.05; t test. [Control = untreated (no ACB or CCB); ACB = activated carbon biochar; CCB = corn cob biochar].
Photosynthetic pigments
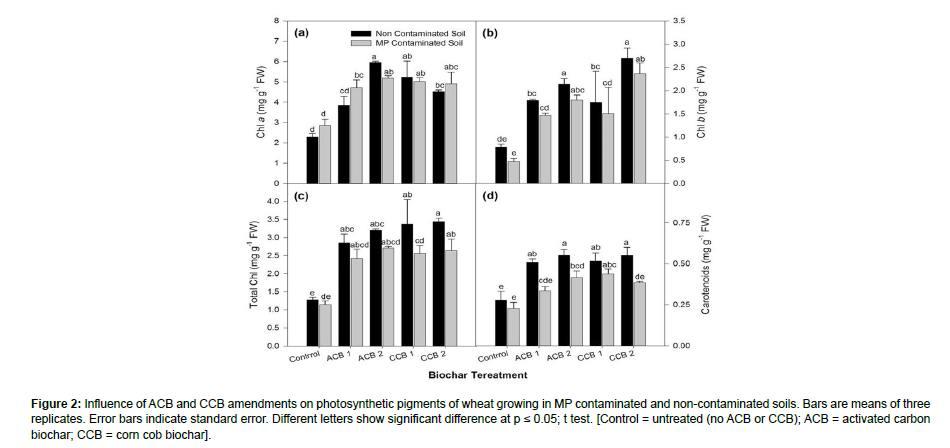
Results showed that the effect of all given amendments was significant on photosynthetic pigments i.e., chlorophyll a, chlorophyll b, total chlorophyll as well as carotenoid contents (Figure 2). A significant negative effect was observed on all studied photosynthetic pigments in MP contaminated soil as compared to non-contaminated plants. There was a positive and significant effect of ACB and CCB in ameliorating MP toxicity. Results showed that ACB 2 and then CCB 1 improved chlorophyll a in both MP-contaminated and non-contaminated soils. Considering chlorophyll b, CCB 2 had efficiently increased chlorophyll b pigment contents. ACB 2 and CCB 2 differed significantly better over ACB 1 and CCB 1 respectively over control in MP contamination for total chlorophyll with CCB 2 having more profound effects. ACB 2 and CCB 2 significant effect in comparison with other treatments for an increase in carotenoid contents in MP-contaminated plants.
Figure 2: Influence of ACB and CCB amendments on photosynthetic pigments of wheat growing in MP contaminated and non-contaminated soils. Bars are means of three replicates. Error bars indicate standard error. Different letters show significant difference at p ≤ 0.05; t test. [Control = untreated (no ACB or CCB); ACB = activated carbon biochar; CCB = corn cob biochar].
Osmolytes
It is obvious from Figure 2 that the ACB and CCB amendments significantly affected on total soluble protein as well as sugars contents of wheat plants. MP contamination in soil significantly reduced both studied osmolytes i.e., total soluble protein and total soluble sugar contents as compared to non-contaminated control plants. The applied treatments had significant positive effects in ameliorating MP toxicity. Results showed that ACB 2 and CCB 2 increased the amount of total soluble proteins in both MP contaminated and non-contaminated plants.
Oxidative stress indicators and ionic content Results showed that the effect of all given amendments was significant on hydrogen peroxide (H2O2), malondialdehyde (MDA), proline, phenolics, glycine betaine, electrolyte leakage, sodium (Na), calcium (Ca), potassium (K) and phosphate (P) contents (Table 1). A significant positive correlation was observed on all studied oxidative stress indicators and Na contents in MP contaminated plants as compared to non-contaminated ones. There was a significant positive effect of ACB and CCB in ameliorating MP toxicity.
| Main Effect of Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| H2O2 (μmol/g fwt) | MDA (μmol g-1 FW) | Proline (µg/g f.wt) | Phenolics (mg/g dwt) | Glycine Betaine | Electrolyte leakage (%) | Sodium | Calcium (mg g-1 DW) | Potassium (mg g-1 DW) | Phosphate (mg/L) |
| (µg/g f.wt) | (mg g-1 DW) | ||||||||
| 3.36±0.3ab | 1.98±0.33b | 8.88±2b | 84±15.56ab | 70.97±56.14a | 26.17±4.31ab | 17.75±2.91ab | 2.57±0.78a | 2.43±0.48bc | 4.64±1.15a |
| 3.11±0.4b | 1.77±0.27b | 8.8±1.77b | 82.26±14.01ab | 65.09±42.89a | 23.17±2.32bc | 15±5.51b | 3.13±0.7a | 3.67±0.82a | 5.29±0.89a |
| 3.41±0.23ab | 1.95±0.34b | 11.4±1.95ab | 88.95±15.3ab | 61.02±34.73a | 21.5±4.28bc | 17.17±2.21ab | 2.7±1.22a | 2.68±0.4bc | 4.65±1.48a |
| 3.19±0.15b | 1.92±0.24b | 8.06±2.26b | 75.63±19.69b | 71.31±30.7a | 20.17±4.17c | 16.08±1.96ab | 3.12±0.62a | 3.02±0.74ab | 5.55±1.92a |
| 3.77±0.62a | 2.64±1.07a | 14.79±6.02a | 102.03±16.49a | 90.68±44.65a | 30±5.18a | 19.92±3.67a | 2.33±0.82a | 2.12±0.55c | 2.57±1.6b |
| Main Effect of Varity | |||||||||
| 3.12±0.22b | 1.72±0.25b | 8.06±1.71b | 74.83±10.9b | 38.87±21.51b | 21.2±4.16b | 14.93±3.53b | 3.32±0.68a | 3.14±0.83a | 4.47±1.49a |
| 3.62±0.43a | 2.39±0.66a | 12.72±4.15a | 98.32±15.11a | 104.76±26.38a | 27.2±4.57a | 19.43±2.09a | 2.22±0.62b | 2.43±0.57b | 4.62±1.98a |
| 3.37±0.42 | 2.05±0.6 | 10.39±3.92 | 86.57±17.61 | 71.81±41.01 | 24.2±5.27 | 17.18±3.66 | 2.77±0.85 | 2.78±0.79 | 4.54±1.72 |
| 12.45 | 28.98 | 37.71 | 20.35 | 57.11 | 21.77 | 21.28 | 30.8 | 28.3 | 37.85 |
| All values are means of three replicates ± SD. Different labels represent significant different alphabets by LSD test. [Control = untreated (no ACB or CCB); ACB = activated carbon biochar; CCB = corn cob biochar; hydrogen peroxide = H2O2 and malondialdehyde = MDA] | |||||||||
Table. 1: Oxidative stress indicators and ionic components of wheat grown in MP contaminated and non-contaminated soils.
All values are means of three replicates ± SD. Different labels represent significant different alphabets by LSD test. [Control = untreated (no ACB or CCB); ACB = activated carbon biochar; CCB = corn cob biochar; hydrogen peroxide = H2O2 and malondialdehyde = MDA]
Discussion
Wheat crop was cultivated to check effect of biochar mitigating the effect of microplastic contaminated soil where TSP, TSS, shoot fresh weight, shoot length, root fresh weight, root length, chlorophyll a, b and carotenoids and total chlorophyll contents were observed better in non-contaminated soil as compared to MP contaminated one under given treatment of biochar, that might be accredited to volatilization of organic compounds that yield vacuums inside biochar structures and improved pyrolysis temperature [31]. Comparatively, a low pyrolysis temperature might shrink organic volatiles, empowering pore clogging and declining surface area of biochar [32].
Generally, biochar based on plant straw (e.g., rice, wheat, flax, and rapeseed straw) possesses higher proportions of mineral components (e.g., Na, K, Mg, and Ca) than other plant derived Biochar [33]. Availability of these elements is indicatory of elevated pH, EC, and ash contents in biochar and particularly, salts of Na, K, Mg, Ca, as well as carbonates depict elevated biochar EC. These results are in agreement with those of Kloss and coworkers [34]. The available H2O2, MDA, Proline Conc. Phenolics, Glycine betaine, Electrolyte (EC) linkage were greater in when observed in controlled condition in contaminated soils but, Na, Ca, K, and P were greater at higher levels of ACB and CCB as combined biochar added under noncontaminated soil. This might be ascribed to boosted P mineralization in high soil moisture presence [35].
Microbial availability, bulking, and their activities enhance in higher soil moisture levels [36,37] and were revealed that accretion of MPs in soil boosted soil microbial and enzyme actions by time, thus promoting recycling of soil C, N, and P, hence elevating naturally available C, N, and P in soil. Comparatively, inclusion of 0.2% polyethylene MPs do not influence available soil minerals, like available P, affirming that environmentally linked MP proportions might not discourage soil nutrient availability. Furthermore, 2% biodegradable polylactic acid MPs amendment does not affect soil mineral profile, including inorganic phosphorus. These findings suggest that amassed MPs possibly promote a soil enzymatic activity that flourishes organic C, N, and P dissolution in soil also enhancing their natural availability to plants. Moreover, using biochar on MP affected soils regulated microbial as well as enzyme actions that speed up availability of minerals into soil [38]. In biochar-amended soils yield enhanced total exchangeable basic cations (TECs). TEC of soil enhances by 49.4% and 64.8% after using ACB and CCB, under non contaminated condition. In MP Devasted soils ACB and CCB boosted soil TEC only by 45.1% and 44.4%, which added to higher exchangeable cations in soil [39]. Basic cations like Na+, K+ and Ca2+ are fundamental plant nutrients.
Our findings depict a notable upgradation in soil fertility and wheat plant growth after applying combined biochar, which might prove profitable in terms of plant growth even in MP contaminated soil even. Also, pyrolysis and ensuing alterations of biochar to soils could probably be a recycling method for agricultural waste like ACB and CCB, as they exhibit zero toxic components and are a good source of biochar to combat microplastic contamination [40].
Conclusion
In conclusion, the application of either of activated carbon biochar (ACB) or corn cob biochar (CCB) can alleviate MP soil contamination. However, the higher concentrations of ACB and CCB (ACB 2 and CCB 2) can effectively enhanced wheat growth attribute and nutrients uptake under MP contaminated soil conditions. The supplementation of ACB and CCB had both significant and positive effects when their application rates were increased. Thus, either ACB or CCB was efficient in improvement of chlorophyll contents of wheat under as well as increasing its nutritional constituents. There is a dire demand for further inquiries at field level while bearing in mind other climatic constraints to authenticate the effectiveness of ACB and CCB against MP contaminated soil.
Acknowledgements
The Author extend his appreciation to the deanship of scientific research for funding this article by Taif University Researching supporting project Taif University Saudi Arabia.
References
- Chen J, Liu X, Zheng J, Zhang B (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71: 33-44.
- Blasing M, Amelung W (2018) Plastics in soil: Analytical methods and possible sources. Sci Total Environ 612: 422-435.
- Lwanga, Esperanza Huerta (2017) Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environmental Pollution 220: 523-531.
- Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil. Environ Sci Technol 46: 6453-6454.
- Rillig MC, Ingraffia (2017) Microplastic incorporation into soil in agroecosystems. Front Plant Sci 8, 1805.
- Palansooriya KN, Sang MK (2022) Biochar alters chemical and microbial properties of microplastic-contaminated soil. Environ Res 209: 112807.
- Ebere EC, Wirnkor VA (2019) Uptake of microplastics by plant: a reason to worry or to be happy. World Sci News 131: 256267.
- Desforges JPW (2014) Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar Pollut Bull 79: 94-99.
- Ju H, Zhu D (2019) Effects of polyethylene microplastics on the gut microbial community, reproduction and avoidance behaviors of the soil springtail, Folsomia candida. Environ Pollut 247: 890-897.
- Uheida, Abdusalam (2021) Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. Journal of hazardous materials 406: 124299.
- Wanner P (2021) Plastic in agricultural soils--a global risk for groundwater systems and drinking water supplies. A review Chemosphere 264: 128453.
- Yaseen S, Amjad SF, Mansoora N, Kausar S (2021) Supplemental effects of biochar and foliar application of ascorbic acid on physio-biochemical attributes of barley (Hordeum vulgare L.) under cadmium-contaminated soil. Sustainability 13: 9128.
- Quan G (2020) Characteristics of organo-mineral complexes in contaminated soils with long-term biochar application. J Hazard Mater 384: 121265.
- Rehman, Sidra (2022) Associative effects of activated carbon biochar and arbuscular mycorrhizal fungi on wheat for reducing nickel food chain bioavailability. Environmental Technology & Innovation 26: 102539.
- Mansoora N, Kausar S (2021) Application of sewage sludge combined with thiourea improves the growth and yield attributes of wheat (Triticum aestivum L.) genotypes under arsenic-contaminated soil. PLoS One 16:0259289.
- Masto, Reginald E (2012) Evaluation of the co-application of fly ash and sewage sludge on soil biological and biochemical quality. Environmental technology 8: 897-905.
- Palansooriya, Kumuduni Niroshika (2019) Response of microbial communities to biochar-amended soils: a critical review. Biochar 1: 3-22.
- Palansooriya, Kumuduni Niroshika (2020) Soil amendments for immobilization of potentially toxic elements in contaminated soils. A critical review Environment international 134: 105046.
- Ameen M, Zamri NM (2022) Effect of acid catalysts on hydrothermal carbonization of Malaysian oil palm residues (leaves, fronds, and shells) for hydrochar production. Biomass Convers Biorefinery 12: 103-114.
- Amjad SF, Mansoora N (2021) Application of zinc fertilizer and mycorrhizal inoculation on physio-biochemical parameters of wheat grown under water stressed condition. Sustainability 13: 11007.
- Rong L, Zhao L (2021) LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 773: 145640.
- Ioannidou OA (2008) Use of biogenic solids for activated carbon via pyrolysis: the case of corn cob. High Temp. Mater Process 27: 355-360.
- Bray HG, Thorpe WV (1954) Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal 1: 27-52.
- Bates LS, Waldren RP (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205-207.
- Dubois M, Gilles KA (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem 28: 350-356.
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72. 248-254.
- Heath RL, Packer L (1968) Photo peroxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125: 189-198.
- Anjum SA, Tanveer M (2015) Cadmium toxicity in Maize (Zea mays L.): consequences on.
- Donald AH, and Hanson D (1998) Determination of potassium and sodium by flame emission spectrophotometry, in: Kalra, Y. (Ed.), Handbook of Reference Methods for Plant Analysis. CRC Press Washington 153-155.
- Coutinho J (1996) The molybdate/ascorbic acid blue method for the phosphorus determination in very dilute and colored extracts by segmented flow analysis. Commun Soil Sci Plant Anal 27: 1363-1375.
- Melia PM, Busquets R (2019) Driving forces and barriers in the removal of phosphorus from water using crop residue, wood and sewage sludge derived biochar. Sci Total Environ 675: 623-631.
- Yadav A, Ansari KB, Simha (2016). Vacuum pyrolysed biochar for soil amendment. Resour. Technol. 2: 177-185.
- Kloss, Stefanie (2012) Characterization of slow pyrolysis biochars: effects of feedstocks and pyrolysis temperature on biochar properties. Journal of environmental quality 41.4: 990-1000.
- Liu, Yang (2018) Negative impacts of biochars on urease activity: high pH, heavy metals, polycyclic aromatic hydrocarbons, or free radicals. Environmental science & technology 52.21: 12740-12747.
- Li S, and Chen G. (2018) Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag 78: 198-207.
- Ng, Ee-Ling (2018) An overview of microplastic and nanoplastic pollution in agroecosystems. Science of the total environment 627: 1377-1388.
- Arenberg M.R and Arai Y (2019) Uncertainties in soil physicochemical factors controlling phosphorus mineralization and immobilization processes. Adv Agron 154: 153-200.
- Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci Technol 50: 5774-5780.
- Liu H, Yang X, Liu (2017) Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 185: 907-917.
- Lalarukh I, Naeem Z, (2022) Integral effects of brassinosteroids and timber waste biochar enhances the drought tolerance capacity of wheat plant. Sci Rep 12: 12842.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Dhumri SA (2023) Bio Fertilizers an Emerging Cure to Increasing PlasticContaminations; an Experimental Study on Cereal Crops in Asia. Adv Crop Sci Tech11: 653.
Copyright: © 2023 Dhumri SA. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2084
- [From(publication date): 0-2024 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 1759
- PDF downloads: 325