Bio Ecology of Aquatic and Semi-Aquatic Insects of Order Coleoptera in the World
Received: 24-Jan-2022 / Manuscript No. jmsrd-22-52231 / Editor assigned: 26-Jan-2022 / PreQC No. jmsrd-22-52231 / Reviewed: 17-Jun-2022 / QC No. jmsrd-22-52231 / Revised: 22-Jun-2022 / Manuscript No. jmsrd-22-52231 / Published Date: 29-Jun-2022 DOI: 10.4172/2155-9910.1000346
Abstract
Background: Aquatic Coleoptera plays a major role in freshwater ecosystems and is regarded as an effective bio indicator. At least 23 beetle families, from three of the four extant suborders, are predominantly aquatic as adults,larvae, or both. They play important role in ecosystem functioning, nutrient cycling primary production, decomposition,and materials translocation.
Methods: In this study, various databases including PubMed, Web of Knowledge, Scopus, Google Scholar, and science direct were used.
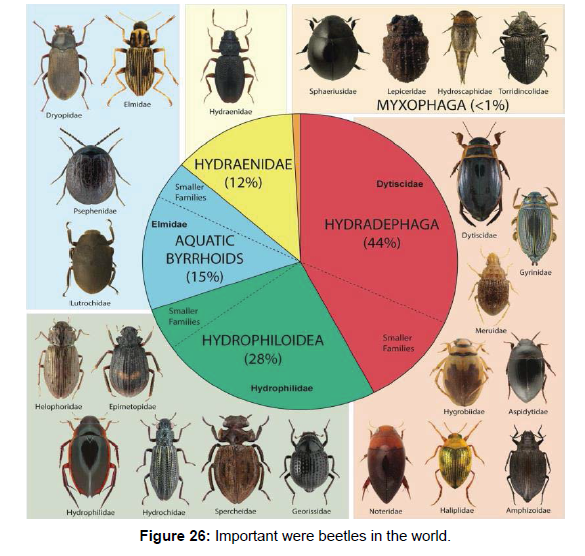
Results: Aquatic Coleoptera known as water beetles, with more than 13,000 described species, is one of the most abundant aquatic insects. Out of 4 suborders of Coleoptera, the suborder Myxophaga is truly aquatic whereas 8 of the 11 extant families of Adephaga are regarded as truly. As far as Polyphaga is concerned, the largest suborders of Coleoptera, only 13 of the 150 families are regarded as truly aquatic.
Discussion and Conclusion: Water beetles, although defined by their affinity for aquatic ways of life, occupy a broad array of habitats, and have shifted secondarily back to their terrestrial roots (either as adults, larvae, or both) on multiple occasions. This ecological variability coupled with repeated, parallel transitions has positioned water beetles as a premier study group for questions related to dispersal, ecological speciation, and diversification rates.The characteristics of a bioindicator are richness and diversity species, easy handling, ecological faithfulness, fragility to small environmental changes and good organism responses, and water beetles are very integral parts of any biotic component of any water bodies or wetlands. They are indicators of ecological diversity and habitat characteristics.
Keywords
Aquatic Coleoptera; Bioecology; Biodiversity; Habitat
Introduction
More species of insect have been described than of any other life form, and beetles are the greatest proportion of these. Beetles are an evolutionary success story par excellence, being by far the most species order of animals on earth. The Coleoptera are an old radiation, whose evolutionary origins may date back to the Permian or even Carboniferous [1, 2]. Water Beetles common name for any of numerous aquatic beetles including the true or diving water beetles, the whirligig beetles, and the water scavengers’ beetles. There are approximately 2000 species of true water beetle’s native to land areas throughout the world [3]. Water beetles are very integral parts of the biotic component of any water bodies or wetlands. They are indicator of ecological diversity and habitat characteristics [4]. It has been recognized as a vital component for an integrated assessment of water quality. Use of aquatic insects as bio monitor provides important insights into changes in water and habitat quality [5]. These changes are valuable in demonstrating the effects of anthropogenic disturbances in the aquatic ecosystems. Numerous taxa of aquatic insect’s groups have been used as biological parameter to know the ecological status of aquatic systems. Of these aquatic beetles provides all the requirements needed for the assessment that is, large diversity, various adaptations (tolerance and sensitivity), long life, and use of different types of habitats. All these qualities suit them perfectly to use as biological indicators. Slipinski et al. (2011) estimated that there are about 386500 species belonging to 29500 genera and 176 families under 4 suborders (Archostemata, Myxophaga, Adephaga, and Polyphaga), representing 40% of the total animal diversity of the world. [6]. Aquatic Coleoptera known as water beetles, with more than 13000 described species, is one of the most abundant aquatic insects [7]. They play an important role in freshwater ecosystems and are considered as a suitable bio indicator [8]. Grove etal. hypothesized that about 70–95% of the entire beetle species are yet to be discovered and described and emphasized that it would take 200 years to explore the entire beetle fauna of the world [9]. The beetles are found everywhere and in almost all ecosystems where animals can thrive with the exceptions of arctic snow and the sea water. Aquatic beetles or water beetles live in almost all kinds of aquatic habitats, such as rivers, springs, lakes, ditches, puddles, seepages, and ground water [10]. However, beetles do not inhabit the oceans, although numerous species live at their shores, where they can be found in hyper saline rock pools of the supralittoral, i.e., the spray (or splash) zone slightly above the intertidal zone. In contrast to other insects, water beetles prefer small, richly vegetated ditches. In larger lakes, they prefer the swampy margins, as for instance the reed belt of the Central Europe, where water beetle biomass is probably higher than anywhere on earth [11]. Water beetle can live in the water as larvae and as adults, but the pupae are generally on land (Some, such as Noterus of the Noteridae family, pupate underwater in air-filled cocoons). Nearly all aquatic Coleoptera go to land to pupate and then return to the water as adults. Water beetles, together with some Diptera and Heteroptera, are among the few insects able to tolerate hyper saline waters, tolerating concentrations of more than 200 g/L of total solutes. This tolerance has developed independently and recurrently in several lineages, mostly Hydrophilidae (e.g., Berosus, Enochrus, Paracymus), Hydraenidae (Ochthebius) and Dytiscidae (some species of Nebrioporus, Hygrotus, and in the Boreonectes group of genera). Coastal, most commonly saltmarshes or rock pools but they may also be found inland with saline streams forming one of the most unusual aquatic environments [12]. Although comprehensive water beetle surveys are still lacking for large parts of the Neotropical and Afrotropical Realms, it is estimated that the Palearctic region contains 3350 described species out 3900 total estimated, the Neotropical region 2510 out of 3900, and the Afrotropical Region contains 2700 out of 3750 total estimations, followed by the Oriental 2200 out of 3580 and the Australian/Pacific Realm 1340out of 2100 estimated species. Undoubtedly, the Nearctic 1420 out of 1550 species is by far the poorest region in terms of water beetle diversity. Within the Palearctic Region, the Mediterranean countries and Anatolia are to be regarded as biodiversity hot spots, at least for certain families. In the comparatively well-explored Oriental Region, Borneo was found to be a hot spot of paramount significance for many water beetle families [13].
Based on their association with aquatic life as of adults and of immature stages, the aquatic beetles can be classified into following six groups:
1. True Water Beetles,
2. False Water Beetles,
3. Phytophilous Water Beetles,
4. Parasitic Water Beetles,
5. Facultative Water Beetles,
6. Shore Beetles.
The adults of the true water beetles remain submerged for most of the time in water whereas their larvae and pupae may be aquatic or terrestrial. The species in this group are highly adapted to aquatic life showing important morphological adaptations such as swimming hairs on legs, divided eyes, plastron, large claws, and streamlined body form. In false water beetles, only the juvenile stages are aquatic, the adult stage is always predominantly terrestrial. Parasitic water beetles come in association with water only when their hosts are submerged whereas facultative water beetles and shore beetles are predominantly terrestrial beetle families with their juveniles live in very wet habitats [14].
Materials and methods
In this study, various databases including PubMed, Web of Knowledge, Scopus, Google Scholar and science direct were used and information was extracted based on keywords: Aquatic Coleoptera, bioecology, biodiversity, Habitat and categorized and analyzed based on content.
Results
Out of 4 suborders of Coleoptera, the suborder Myxophaga is truly aquatic whereas 8 of the 11 extant families of Adephaga are regarded as truly aquatic (Gyrinidae, Haliplidae, Meruidae, Noteridae, Amphizoidae, Aspidytidae, Hygrobiidae, Dytiscidae). As far as Polyphaga is concerned, the largest suborder of Coleoptera, only 13 of the 150 families are regarded as truly aquatic (Helophoridae, Epimetopidae, Hydrochidae, Spercheidae, Hydrophilidae, Hydraenidae, Scirtidae, Elmidae, Dryopidae, Lutrochidae, Psephenidae, Cneoglossidae, and Eulichadidae) [15].
Suborder Adephaga
0 species (18% aquatic) under 11 families globally [16]. The families, Gyrinidae, Haliplidae, Meruidae, Noteridae, Amphizoidae, Hygrobiidae, and Dytiscidae are truly aquatic in nature.
Family Gyrinidae (Whirligig Beetles): Gyrinidae with its worldwide distribution consists of about 1000 species fewer than 25 genera globally. The beetles in this family show peculiar swimming behavior where adults rapidly revolve around a fixed point on the surface of the water. In static or moderately running water and preferably live in the habitats with rich oxygen contents. The beetles are commonly known as whirligig beetles and can be distinguished from the other Adephagan families by following characters: compound eyes divided completely, so placed with upper pair on the dorsal surface of the head, remains above the water line and the lower pair on the ventral surface of the head, remains below the water line when the beetle swims; antennae short with a broad, cup-shaped scape, sub triangular pedicel and elongate but compact flagellum, and meso and metathoracic legs broadly expanded and fringed with setae for swimming. All stages except for the pupa are aquatic, with the adults spending the major part of their live on the water surface, being half submerged; the larvae, in contrast, are always fully submerged. Larvae creep about on the vegetation or bottom substrate. They can also swim by up- and downward undulation of the body. They often form huge aggregations of individuals. Interspecific swarms of up to eight species may occur [17]. In ponds and lakes, gyrinids are often found between stands of emergent plants such as reed, and between Pandanus stems. Many species, especially in the tropics are however, rheobiontic and will be found in different kinds of streams, often hiding in undercut river banks or in crevices of submerged wood during daytime. Several species are attracted to light traps [18] (Figure 1).
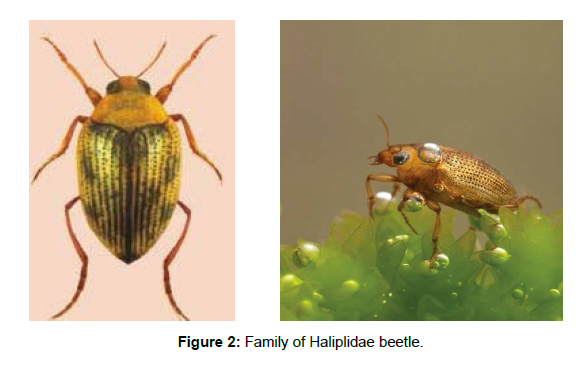
Family Haliplidae (Crawling Water Beetles): Haliplidae consists of 220 species fewer than 5 genera: Algophilus, Apteraliplus, Brychius, Peltodytes, and Haliplus [19]. The genus Haliplus is widely distributed in Australian, Afrotropical, Nearctic, Neotropical, Oriental and Palaearctic regions. The family Haliplidae is a comparatively small group of inconspicuous, small water-dwelling beetles, which are commonly termed as crawling water beetles. The features that distinguish these beetles from other families are: extremely large meta-coxal plates which cover most of the abdominal ventrites, tarsal formula 5-5-5 and body form adapted for aquatic life [20]. All stages except for the pupa are aquatic. Larvae cannot swim, rather they can be observed creeping over the substrate or on water plants. Adults move rather quickly in the water by alternately moving their hind legs. This produces the impression of water walking. Larvae do not need to access atmospheric air; they breathe via the integument and their gills [21]. Adults carry an air supply beneath the elytra and under the coxal plates; this supply has to be renewed at the surface from time to time (Figure 2).
Family Meruidae: Meruidae is a family of aquatic beetles in the suborder Adephaga, with only one genus and species, Meru phyllisae. The term meru comes from the local indigenous population’s (the Pemon people) word for ―waterfall. Appropriately so, this beetle is only found in the natural rock water slide in El Tobogán, Las Amazonas and Venezuela. These beetles are morphologically highly distinct and at a body length of 0.8 mm represent the perhaps smallest individuals of Adephaga A survey of aquatic beetles of Venezuela indicated that Meru is most common during the wet season, when larger areas of granitic rock surface are covered with water film, which the adult beetles as well as the larvae inhabit [22] (Figure 3).
Family Noteridae (Burrowing Water Beetles): Globally the family includes 258 species belonging to 16 genera under 3 sub families: Noterinae (240 species), Notomicrinae (11 species), and Phreatodytinae (7 species) [23]. The members of this family are called as burrowing water beetles. They are usually found in large numbers in sunny and shallow lentic habitats, and generally avoid the situations where they need to burrow [24]. The larvae cannot swim, but crawl rapidly on the substrate or among water plants, and larvae of some species (Noterus) are active burrowers [25]. Adults and larvae are aquatic; Noterus pupates under water in air-filled cocoons. Noteridae are commonly found in stagnant water between roots of water plants. Most species inhabit stagnant or slowly flowing water rich in emergent and floating vegetation. The dense networks of roots latter are often inhabited by numerous noterids [26] (Figure 4).
Family Amphizoidae (Trout Stream Beetle): Monogeneric family with five described species, known only from North America and China. Larvae and adults of all species are aquatic living in rather fast flowing rivers. The member of this family lives in North America and China. Larvae and adults of all species are aquatic. Both larvae and adults live in fast-flowing mountain and foothill streams where they feed primarily on stonefly (Plecoptera) naiads. They may be abundant on driftwood and floating trash or along undercut banks among roots and accumulations of Pine needles. Both larvae and adults live in fast flowing mountain and foothill streams where they feed primarily on stonefly (Plecoptera) naiads. Amphizoids live at the interface between aquatic and terrestrial environments and seem at home in both. Larvae remain just out of the water on twigs or other wood. They enter the water to seize prey, but quickly return to the twig to eat the victim [27] (Figure 5).
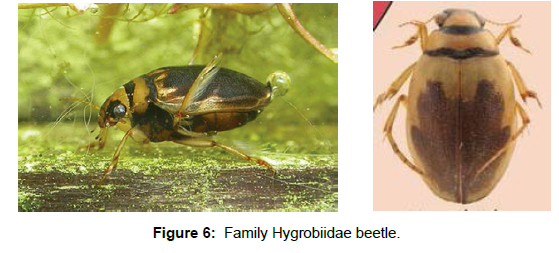
Family Hygrobiidae: Monogeneric family with six described species, occurring in Europe, China, and Australia. Larvae and adults of all species are aquatic lives in stagnant water [28]. Their size is moderate (8.5-10 mm). They are most common in stagnant water, where they walk or swim; they do not dive. They obtain their oxygen from the air collected and stored under the elytra [29] (Figure 6).
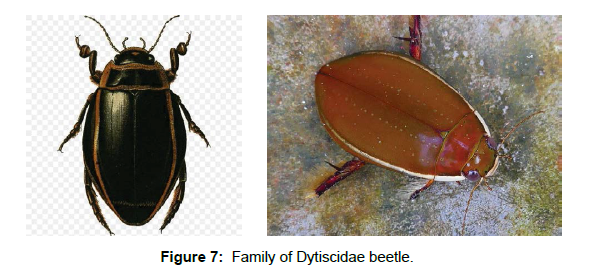
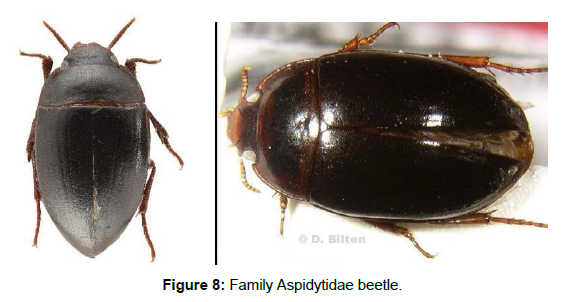
Family Dytiscidae (Diving Beetles): This family is worldwide in distribution with about 4,303 species belonging to 190 genera so far described globally [30]. The beetles in this family are adapted well to aquatic life. The adults and larvae live in water and can be found in any aquatic ecosystems. They mostly feed on aquatic invertebrates and fish eggs. This is why they are commonly called as predacious diving beetles‘. Eggs, larvae and adults are aquatic. However, adults of a few species from India, Australia and New Caledonia were described as terrestrial. Adult Dytiscidae produce antimicrobial secretions with their pygidial glands. These secretions are applied to the body on land, where the beetle cleans and brushes his body with the legs [31] (Figure 7).Family Aspidytidae (cliff water beetles): Aspidytidae is a family of aquatic beetles of the suborder Adephaga, described in 2002 from specimens in South Africa and China [32]. There are only two known species in the family and these were originally described in the genus Aspidytes [33]. But later the new genus Sinaspidytes was erected for the species found in China [34]. Larvae and adults of both A. niobe and S. wrasei are found in or in close proximity of hygropetric habitats. Meaning they require a rock surface covered by a thin layer of water. Both adults and larvae are likely predacious. Eggs and pupa are yet unknown (Figure 8).
Suborder Myxophaga
Myxophaga includes 84 species under 4 families from the world: Lepiceridae (2 species), Torridincolidae (60 species), Hydroscaphidae (22 species), and Sphaeriusidae (23 species). The two known species of Lepiceridae are reported from Mexico and Venezuala, whereas Torridincolidae occurs in South America, Africa and with few species from Asia.
Family Lepiceridae: Monogeneric family with two New World species. Lepicerus inaequalis and L. bufo both usually found on sand banks very close to streams but assumed they might get submerged under water [35]. In 2010 Lepicerus pichilingue new species (type locality: Quevedo, Los Ríos, Ecuador) is described from leaf litter in mixed plantings of plantain and cacao in western Ecuador. L. pichilingue is very similar to L. inaequalis but differs distinctively in the structure of the aedeagus [36] (Figure 9).
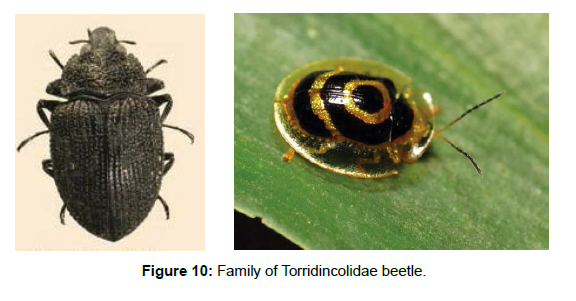
Family Torridincolidae: Torridincolidae has about 40 species distributed in seven genera, but only three are found on Neotropics. Torridincolidae are usually dark colored, some of which bearing iridescent scales, with smooth surface or finely sculptured with punctuation and carinae [37]. The species are found in mountain streams [38] (Figure 10).
Family Hydroscaphidae (Skiff Beetles): The world fauna of this family includes about 22 species under 3 genera: Scaphydra (3 species), Yara (2 species), and Hydroscapha (17 species) globally [39]. The beetles in this family are small sized and commonly called as skiff beetles. They can be distinguished by the presence of a distinct not pleural suture and of their aquatic life [40]. Larvae, pupa, and adults are aquatic, which is probably also true for the egg. They are found on algal mats, over which a thin water film flow [41]. Adults are Hydro scapha able to move very quickly on water films; it seems, however, that they cannot swim freely. Because of its relatively large size, only one egg matures at a time. Larvae and adults feed on algae. Members of this family are found on hygrometric rocks and in the interstitial of gravelly river margins and residual pools. They are also reported from hot springs up to a temperature of 46°C [42] (Figure 11).
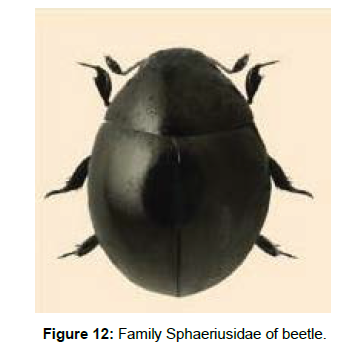
Family Sphaeriusidae: The beetles in this family are called as minute bog beetles which can be distinguished from other families of the uborder by their comparatively large and prominent head, capitate antennae, design of meso and metasternum, large posterior coxal plates and unequal length of visible abdominal sternites. They are found in mud, under stones, or on algae along the edge of streams and rivers, among the roots of plants, in mosses associated bogs or inhabiting moist leaf litter further away from the bodies of water. This monogeneric family is widely distributed, and includes 23 species belonging to the genus, Sphaerius from the world (Figure 12).
Suborder Polyphaga
Only 13 of the 150 recognized families of the large suborder Polyphaga are regarded as predominantly aquatic. Family Helophoridae: The Helophoridae is a relatively small hydrophiloid family with ca. 190 known species classified into a single genus, Helophorus Fabricius, 1775. Adults of the genus are easily recognizable by the elongate body and five longitudinal furrows on the pronotum. The majority of species occur mostly in Palaearctic and Nearctic regions and only a few species are known from the Afrotropical and Oriental regions [43, 44]. Adult beetles are saprophagous and usually inhabit shallow standing water bodies, only a few species are terrestrial. In contrast, larvae are terrestrial, living in moist habitats near water, preying on various small invertebrates. A few species have herbivorous larvae feeding on various Brassicaceae or Poaeceae [45] (Figure 13).
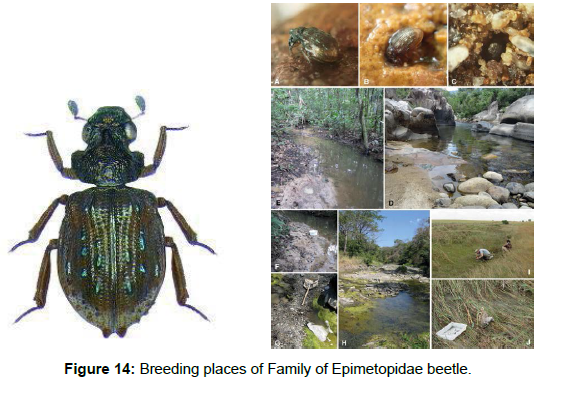
Family Epimetopidae: Epimetopidae are a small beetle family of the superfamily Hydrophiloidea, comprising 72 described species in three genera: the American Epimetopus Lacordaire, 1854 (56 species), Asian Eumetopus Balfour-Browne, 1949 (eight species) and African Eupotemus Ji &Jäch, 1998 (eight species, of which six are described as new here) [46]. Eggs, larvae and adults are aquatic. Eggs are carried by the female in an egg case underneath the abdomen (brood care); these cases contain up to 17 eggs as far as is known [47]. Besides that, hardly anything appears to be known about the biology of Epimetopidae. Most species of Epimetopidae live in similar habitats: wet sand or gravel at sides of various types of streams and rivers, occasionally also with algal mats or accumulations of plant debris. The observation of living specimens of Eumetopus acutimontis from Vietnam indicate that the specimens were diging and hiding in the wet sandy or gravelly substrate at the margin of a small river. In contrast, some Epimetopus (e.g., E. venezuelensis Perkins, 2012) were collected in standing water, typically well vegetated shallow marshes (Figure 14).
Family Hydrochidae (water scavenger beetle): Monogeneric family with about 180 species; hydrochids occur on all continents. All species are truly aquatic, living in well-vegetated stagnant water and/or at the edges of very slowly flowing water [48]. All inhabit well vegetated marginal lentic habitats where both the larvae and adults are aquatic but do not swim; they crawl on vegetation where the adults are herbivores and detritivores. The larval feeding habits are not known. Adults are generally slowed moving and cryptic and will often remain motionless for some time in the water net and so may be overlooked (Figure 15).
Family Spercheidae: Monogeneric family with 18 species. The genus occurs on all continents. Larvae and adults generally live in stagnant water. Most of them from the Ethiopian and Oriental zoogeographic regions [49]. They occur in stagnant water with rich vegetation, often in the mud on the edge of small ponds. Both adults and larvae are among the few coleopteran filter-feeders, feeding on detritus or possibly small crustaceans and insect larvae (Figure 16).
Family Hydrophilidae (water scavenger beetle): There are a total of 1740 species in 66 genera, on all continents, adults and larvae of most species are living in stagnant water, running water, in phytotelmata or seepages, numerous species are reportedly riparian or terrestrial (humicolous) (Figure 17).
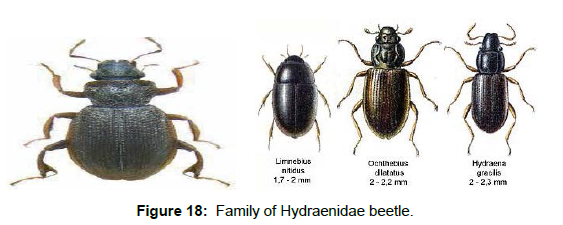
Family Hydraenidae (Minute Moss Beetles): Hydraenidae is a cosmopolitan family of tiny beetles, ranging in size from 0.5 to less than 4.0 mm in length. The majority of species are adapted for marginal life, mainly in the freshwater aquatic world [50, 51].The family includes more than 1500 species, but several hundred await formal description [52]. The family is currently divided into 4 subfamilies: Orchymontiinae Perkins, Prosthetopinae Perkins, Ochthebiinae Thomson, and Hydraeninae Mulsant [53, 54]. Globally, Hydraenidae includes 1600 species under 42 genera, occurring on all continents. Eggs and adults of most species are aquatic; there is however several strictly terrestrial and riparian genera. Larvae appear to be terrestrial or riparian throughout, few of them (e.g. Enicocerus) seem to be amphibious (facultatively aquatic); they usually inhabit damp sites close to the water [55]. Adult hydraenids cannot swim well. They crawl on the substrate and if one washes them loose, they float to the surface, where they usually cling upside down to the underside of the water surface trying to reach the next stone, leaf and the like to search for foothold and shelter. Certain adults, especially those living in well oxygenated streams use an incompressible gas gill (or microplastron), which enables them to stay permanently submerged. The aquatic species may be encountered in a variety of habitats e.g: saline coastal rock pools, swamps, streams, seepage water. Often, numerous beetles can be encountered in leaf packs, between roots or any kind of wood in lentic and lotic situations [56] (Figure 18).
Family Scirtidae (marsh beetles): These beetles are commonly referred to as marsh beetles, as the larvae are typically associated with stagnant water, but can be found in flowing water. Adults prefer decomposing plant material near the water’s edge [57].About 900 species in 30 genera. Scirtids occur on all continents. Larvae are usually aquatic, although there are reports about scirtid larvae found in wet soil and on rotten logs. Imagos are generally terrestrial, but adults of Hydrocyphon are occasionally collected under water; pupae of Hydrocyphon are also reported to be aquatic.Scirtid larvae are found in running water (about 20%), in stagnant water, phytotelmata, and in groundwater [58] (Figure 19).
Family Elmidae: The beetles in this family are commonly called as riffle beetles and they can be both aquatic and semi-aquatic in nature. Till date, 1498 species belonging to 147 genera under 2 subfamilies Elminae and Larainae have been described from the world [59]. The adults and juvenile stages of the subfamily Elminae live totally aquatic habitats and on occasions leave water. The subfamily Larainae is centered in Afrotropical and Neotropical regions with lower diversity in the Oriental region [60] (Figure 20).
Figure 20: Family Elmidae beetle adults, habitus in dorsal view. A) Neocylloepus boeseli Brown; Neoelmis sp.; (C) Neolimnius palpalis Hinton; (D) Notelmis sp.; (E) Onychelmis sp.; (F) Pagelmis amazoˆnica Spangler; (G) Pilielmis sara Hinton; (H) Portelmis nevermanni Sanderson; (I) Stethelmis kaszabi Hinton; (J) Stegoelmis sp.; (K) Stenhelmoides rufulus-group; (L) Tolmerelmis pubipes (Hinton); (M) Tyletelmis sp.; (N) Xenelmis sp.
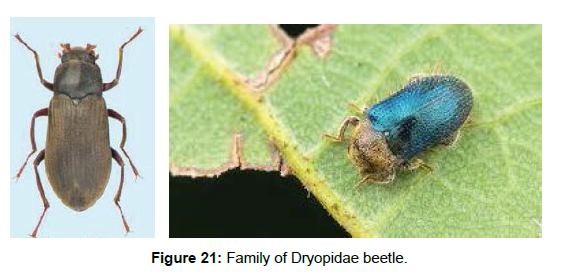
Family Dryopidae (Long-toed water beetle): Dryopidae is an ecologically diverse family, with some members being aquatic, while others are completely terrestrial or semiaquatic. Only five aquatic genera are recognized in the Neotropical Region. They are found on the sandy banks of lotic habitats in soft stream substrate, while others occur on emergent vegetation and sticks protruding from streams. They are often collected near lights at night; for these the habitat is unknown. In the terrestrial dryopids, adults and larvae can be found in forest leaf litter. This family contains about 300 species in 33 genera worldwide. Dryopids occur in all biogeographic regions, but they are absent from the Australian continent. Larvae are generally riparian or terrestrial; adults of about 75% of the species are regarded as aquatic (lotic and lentic habitats), the remaining ones are riparian or terrestrial [61] (Figure 21).
Family Lutrochidae (Travertine Beetles): About 15 species all confined to the New World. Larvae and adults are reported to be aquatic in lotic habitats (riparian gravel, emergent rocks, or submerged wood) [62] (Figure 22).
Family Psephenidae (Water-penny beetle): About 272 species in 35 genera. Psephenids occur on all continents. They are comprised of four subfamilies: Eubrianacinae, Eubriinae, Psepheninae, Psephenoidinae.
Larvae are always aquatic, almost exclusively occurring in running water, with few exceptions, adults and pupae are strictly terrestrial [63] (Figure 23).
Family Cneoglossidae: This family contains ten species in a single genus. Small beetles 3-6 mm long. Larvae of Cneoglossa edsoni were collected in submerged rotting brushwood in shallow flowing streams [64] (Figure 24).
The main species are : Cneoglossa brevis Champion, 1897, Cneoglossa collaris Guérin-Méneville, 1849, Cneoglossa elongata Pic, 1916, Cneoglossa gournellei Pic, 1916, Cneoglossa lampyroides Champion, 1897, Cneoglossa longipennis Pic, 1915, Cneoglossa peruviana Pic, 1916, Cneoglossa rufifrons Pic, 1916, Cneoglossa testacericollis Pic, 1916
Family Eulichadidae (Forest Stream Beetles): The small elateriform family Eulichadidae comprises of two extant genera: the monotypic Californian genus Stenocolus LeConte, 1853, and the predominately Oriental genus Eulichas Jacobson, 1913, with 30 described and more than 10 undescribed species [65, 66]. The larvae of both genera are aquatic, while the adults live on vegetation near water, and especially the genus Eulichas is often attracted at light. These five genera belong to the family Eulichadidae: Cretasyne Yan, Wang & Zhang, 2013, Eulichas Jacobson, 1913, Mesaplus Hong, 1983, Mesodascilla Martynov, 1926, Stenocolus Le Conte, 1853 (Figure 25).
Eulichas genus of family Eulichadidae. E. alesbezdeki s, E. bertiae, E. dudgeon, E. jendeki, E. siamensis sp., E. similis sp., E. uniformis, E. baeri, E. baeri, E. gigantea, E. incisicollis, E. incisicollis, E. oborili sp, E. robusta, E. sausai, E. serricornis, E. sikkimensis, E. sundaensis, E. sundaensis, E. wewalkai, E. fasciolata, E. jakli, E. subocellata, E. villosa, Gunung Emas .
Discussion
Water beetles, although defined by their affinity for aquatic ways of life, occupy a broad array of habitats, and have shifted secondarily back to their terrestrial roots (either as adults, larvae, or both) on multiple occasions. This ecological variability coupled with repeated, parallel transitions has positioned water beetles as a premier study group for questions related to dispersal, ecological speciation, and diversification rates [67, 68]. The development of comprehensive online specimen and fieldwork databases also has helped anchor our knowledge of water beetle. Many important surveys that focus in total or in part on water beetles have been carried out in the last quarter of a century. Ecologically, focusing on under sampled sampled habitats such as seepages, underground waters and, to a lesser degree, the margins of river and streams will likely yield new discoveries regardless of their geographical location [69]. Although regional research and generic revisions exist to a certain extent for some groups, the recent publication of comprehensive resources on aquatic coleoptera biology and identification put into sharp relief the varying degrees to which water beetle identification is feasible [70]. Of the 10 water beetle families with more than three described genera, modern global genus level keys now exist for just half: Dytiscidae, Noteridae, Gyrinidae, Hydroscaphidae and Psephenidae. The diversity assessment and preparation of the water beetle inventories are considered an essential tasks now a day, due to the importance of wetlands in the of conservation planning and endeavours. Some species of water beetles have aquatic larvae and terrestrial adults. Water beetles from family Gyrinidae, Haliplidae, Noteridae, Amphizoidae, Dytiscidae and Hydroscaphidae are aquatic in all life stages. The adult water beetles from family Hydroscaphidae, Hydrophilidae, Lutrochidae, Dyropidae, Elmidae, Eulichadidae, Heteroceridae, Limnichidae, Psephenidae, Ptilodactylidae and Sphaeriusidae are not aquatic. The characteristics of a bioindicator are richness and diversity species, easy handling, ecological faithfulness, fragility to small environmental changes and good organism responses and water beetles are very integral parts of any biotic component of any water bodies or wetlands. They are indicators of ecological diversity and habitat characteristics (Figure 26).
References
- Toussaint EFA, Seidel M, Arriaga-Varela E, HÁJEK J, KRÁL D, et al. (2017) The peril of dating beetles. Systematic Entomology 42:1-10.
- Zhang S-Q, Che L-H, Li Y, Liang D, Pang H, et al. (2018) Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat Commun 9:205.
- Sharma S, Pandey P, Dave V (2013) Role of aquatic beetles for water quality assessment. Int j.recent sci res 4: 1673-1676.
- Sanchez-Fernandez D, Abellan P, Velasco J, Millan A (2004) Selecting areas to protect the biodiversity of aquatic ecosystems in a semiarid Mediterranean region using water beetles. Aquat Conserv Mar Freshw Ecosyst 14: 465-479.
- Rosenberg DM, Resh VH (1993) Freshwater Biomonitoring and benthic Invertebrates. Chapman and hall, New York.
- Slipinski SA, Leschen RAB, Lawrence JF (2011) Order Coleoptera Linnaeus, 1758. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148: 1-237.
- Short AEZ (2018) Systematics of aquatic beetles (Coleoptera): current state and future directions. Syst Entomol 43:1-18.
- Dong B, Geng Ch, Cai Y, Ji L (2014) Aquatic Coleoptera response to environmental factors of Freshwater Ecosystems in Changbai Mountain, northeast China. Aquat Ecosyst Health Manag 17:171-178.
- Grove S J & Stork N E (2001) An inordinate fondness for beetles. Invert Tax 14: 733-739.
- Yule CM, Yong HS (2004) Freshwater Invertebrates of the Malaysian Region. Academy of Sciences Malaysia, Kuala Lumpur Malay.
- Biswas S, Mukhoupadhyay P, Saha SK (1995) Insecta: Coeloptera: Adephaga, family dystiscidae. In: State fauna Series 5. Fauna of West Bengal ZSI Calcutta 5: 77-120.
- Mill´an A, Velasco J, Guti´errez-C´anovas C, Arribas P, Picazo F, et al. (2011) Mediterranean saline streams in southeast Spain: What do we know? J Arid Environ 75:1352-1359.
- Balian EV, Lévêque C, Segers H, Martens K (2008) Fresh water animal diversity assessment. Hydrobiologia 595: 419-442.
- Jäch MA (1998) Annotated check list of aquatic and riparian/littoral beetle families of the world (Coleoptera). Wien 2: 25-42.
- Jäch Mam, Balke M (2008) Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia595: 419-442.
- Arnett RH, Thomas MC (2001) American Beetles, Volume I Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. London, New York.
- Stehr FW (1991) Immature Insects. Kendall Hunt Dubuque 2: 319-320.
- Catherine M Yule, Hoi Sen Yong (2004) Fresh water invertebrates of the Malaysian region. Academy of Sciences, Malaysia Kuala Lumpur.
- Nilsson AN, Vondel BJ van (2005) World Catalogue of Insects volume7, Amphizoidae, Aspidytidae, Haliplidae, Noteridae and Paelobiidae (Coleoptera, Adephaga).
- Nilsson AN (1996) Coleoptera Haliplidae, Crawling Water Beetles. Aquatic Insects of North Europe, A Taxonomic Handbook, Stenstrup, Apollo Books, 130-138.
- Spangler PJ, Warren E, Steiner JR (2005) A new aquatic beetle family, Meruidae, from Venezuela (Coleoptera: Adephaga). Systematic Entomology 30: 339-357.
- Nilsson AN (2011) A World Catalogue of the Family Noteridae. Version 16:54.
- Miller KB (2009) On the systematics of Noteridae (Coleoptera: Adephaga: Hydradephaga): phylogeny, description of a new tribe, genus and species, and survey of female genital morphology. Syst and Biodiv 7: 191-214.
- Dettner K (1997). Insecta: Coleoptera: Noteridae. In: Schwoerbel, J. & Zwick, P. (eds), Süßwasserfauna von Mitteleuropa. 20(2, 3 and 4): 98-126.
- Sharma S, Sharma G, Pir FA (2019) Diversity and habitat selection of aquatic beetles (Coleoptera). Int j pharm biol sci 14: 31-37.
- Nilsson AN, van Vondel B J (2005) Amphizoidae, Aspidytidae, Haliplidae, Noteridae and Paelobiidae (Coleoptera, Adephaga). World Catalogue of Insects, Stenstrup, Apollo Books.
- Nilsson AN, van Vondel B J (2005) Family Paelobiidae (Coleoptera, Adephaga). In Nilsson, A. N. & van B. J. Vondel, 2005(eds), Amphizoidae, Aspidytidae, Haliplidae, Noteridae and Paelobiidae (Coleoptera, Adephaga). World Catalogue of Insects, Stenstrup, Apollo Books, Vol. 7: 154–163.
- https://digitalcommons.mtu.edu/bryophyte-ecology2/
- http://www.waterbeetles.eu/documents/W_CAT_Dytiscidae_2015.pdfhttp://www2.emg.umu.se/projects/biginst/andersn/
- Kovac D, Maschwitz U (1990) Secretion-grooming in aquatic beetles (Hydradephaga): a chemical protection against contamination of the hydrofuge respiratory region. Chemoecology 1: 131-138.
- Ribera I, Beutel RG, Balke M, Vogler AP (2002) Discovery of Aspidytidae, a new family of aquatic Coleoptera. Proc Biol Sci 269: 2351-2356.
- Beutel RG, Balke M & Ribera I (2016) 7.7. Aspidytidae Ribera,Beutel, Balke and Vogler, 2002. In: Beutel, R.G. & Leschen, R.A.B. (eds.): "Handbook of Zoology, Arthropoda: Insecta. Coleoptera, Beetles. Vol. 1: Morphology and Systematics" (Archostemata, Adephaga, Myxophaga, Polyphaga partim). 2nd Edition. Walter de Gruyter, Berlin. pp. 141-149.
- Toussaint EFA, Beutel RG, Morinière J, Jia F, Xu S, et al. (2016) Molecular phylogeny of the highly disjunct cliff water beetles from South Africa and China (Coleoptera: Aspidytidae). Zool J Linn Soc 176: 537-546.
- Reichardt H (1976b) Revision of the Lepiceridae (Coleoptera, Myxophaga). Pape´isAvulsos de Zoologia 30: 35-42.
- Flowers RW, Shepard WD, Mera RT (2021) A new species of Lepicerus (Coleoptera: Lepiceridae) from Ecuador. Zootaxa 2639: 35-39.
- Hamada N, Thorp J, Rogers DC (2018) Thorp and Covich's Freshwater Invertebrates. Academic Press 3: 519-525.
- Jach M A (1998). Torridincolidae: I. First record of Torridincolidae from China (Coleoptera). In Ja¨ch, M. A. & L. Ji (eds), Water Beetles of China, Vol. II. Zoologisch- Botanische Gesellschaft in O¨ sterreich and Wiener Coleopterologenverein, Wien: 51–52.
- Fikáček M, Šípková H (2009) New Asian Hydroscapha, with comments on male-female association of co-occuring species (Coleoptera, Myxophaga, Hydroscaphidae). Zootaxa 2286: 31-48.
- Arnett RH, Thomas MC (2001) American beetles, Volume I: Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. CRC Press LLC, Boca Raton, Florida.
- Vanin SA, Beutel RG, Arce-Pérez R (2005) Coleoptera, Beetles. Vol. 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim) (Eds. R.G. Beutel & R.B. Leschen). Berlin, New York 4: 49-52.
- MerrittRW, Cummins KW (1996) An introduction to the aquatic insects of North America. Dubuque Kendall Hunt 3: 399-473.
- Hansen M (1999) World Catalogue of Insects. Volume 2: Hydrophiloidea (s.str.) (Coleoptera). Apollo Books, Stenstrup.
- Short AEZ, Hebauer F (2006) World Catalogue of Hydrophiloidea – additions and corrections, 1 (1999-2005). Koleopterologische Rundschau, 76: 315-359.
- Angus RB (1992). Insecta Coleoptera Hydrophilidae Helophorinae. In: J. Schwoerbel, & P.Zwick (eds): Süsswasserfauna von Mitteleropa, 20, part 10 (2), Gustav Fischer Verlag, Stuttgart, Jena, New York, xi + 144 pp.
- Fikáček M, Matsumoto K, Perkins P, Prokin A, Sazhnev A, et al. (2021) The family Epimetopidae (Coleoptera: Hydrophiloidea): review of current knowledge, genus-level phylogeny, and taxonomic revision of Eupotemus. Acta Entomol Mus Natl Pragae 61: 1-34.
- Archangelsky M (1997) Studies on the biology, ecology, & systematics of the immature stages of New World Hydrophiloidea (Coleoptera: Staphyliniformia). Bulletin of the Ohio biological survey N S 12: ix + 207.
- Darilmaz MC, Kiyak S (2011) A study of the family Spercheidae (Coleoptera) from Turkey. Turk J Zool 35: 441-444.
- Hansen M (1998) World catalogue of insects, Volume 1: Hydraenidae (Coleoptera). Apollo Books 1: 1-168.
- Perkins PD (1997) Life on the effective bubble: Exocrine secretion delivery systems (ESDS) and the evolution and classification of beetles in the family Hydraenidae (Insecta: Coleoptera). Ann. Carnegie Mus 66: 89-207.
- Jäch MA, Diaz (2016) New and little known Palearctic species of the genus Hydraena (s.l.) XI (Hydraenidae). Koleopterologische Rundschau 86: 61-81.
- Jäch M (2004) Catalogue of Palaearctic Coleoptera Vol. 2. Hydrophiloidea-Histeroidea-Staphylinoidea. Apollo Books, Stenstrup.
- Beutel RG\, Anton E, Jäch MA (2003) On the evolution of adult head structures and the phylogeny of Hydraenidae (Coleoptera, Staphyliniformia). J Zoolog Syst Evol 41: 256-275.
- Hansen M (1997) Evolutionary trends in "staphyliniform" beetles (Coleoptera). Steenstrupia 23: 1-52.
- Jach MA, Beutel RG, Diaz JA, Kodada J (2000) Subgeneric classification, description of head structures, and world check list of HydraenaKugelann (Insecta: Coleoptera: Hydraenidae). Ann Naturhist Mus Wien102B: 177-258.
- Epler JH (2010) The Water Beetles of Florida – an identification manual for the families Chrysomelidae, Curculionidae, Dryopidae, Dytiscidae, Elmidae, Gyrinidae, Haliplidae, Helophoridae, Hydraenidae, Hydrochidae, Hydrophilidae, Noteridae, Psephenidae, Ptilodactylidae and Scirtidae. Florida Department of Environmental Protection.
- Lawrence JF (2005) Scirtidae Fleming, 1821. In Beutel, R. G. & R. A. B. Leschen (eds), Handbook of Zoology, Vol. IV (Part 38), Coleoptera, Beetles, Vol. 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphagapartim). Walter de Gruyter, Berlin, 443-450.
- Jäch MA, Kodada J, Brojer M, Shepard W, Čiampor F (2016) World Catalogue of Insects, volume 14: Coleoptera: Elmidae and Protelmidae. E J Brill 14:318.
- Shepard WD (2002) Dryopidae Billberg, 1820. In: American beetles, Volume 2: Polyphaga: Scarabaeoidea through Curculionoidea (Eds. Arnett, R. H. Jr.& Thomas, M.C.). CRC Press, Boca Raton, London, New York, Washington D C.
- Kodada J & Ja¨ch M A (2005). Elmidae Curtis, 1830. In Beutel, R. G., & R. A. B. Leschen (eds), Handbook of Zoology, Vol. IV (Part 38), Coleoptera, Beetles, Vol. 1 Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphagapartim). Walter de Gruyter, Berlin: 471–496.
- Ide S, Costa C & Vanin S A (2005). LutrochidaeKasap&Crowson, 1975. In Beutel, R. G. & R. A. B. Leschen (eds), Handbook of Zoology, Vol. IV (Part 38), Coleoptera, Beetles, Vol. I: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphagapartim). Walter de Gruyter, Berlin: 508–512.
- Lee C F, Ja¨ch M A & Beutel R G (2005). Psephenidae Lacordaire, 1854. In Beutel, R. G. & R. A. B. Leschen (eds), Handbook of Zoology, Vol. IV (Part 38), Coleoptera, Beetles, Vol.: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphagapartim). Walter de Gruyter, Berlin: 521–533.
- Costa C, Vanin SA, Ide S (1999) Systematics and bionomics of Cneoglossidae with a cladistic analysis of Byrrhoidea sensu Lawrence & Newton (1995) ( Coleoptera , Elateriformia ). Arquivos de Zoologia 35S: 231-300.
- Hájek J (2007) Revision of the genus Eulichas Jacobson, 1913 (Coleoptera: Eulichadidae) I. Introduction, morphology of adults, key to subgenera and species groups, and taxonomy of E. funebris species group. Zootaxa 1620: 1-35.
- Ribera I, Vogler A, Balke M (2008) Molecular phylogeny and diversification of diving beetles (Coleoptera, Dytiscidae). Cladistics 24: 563-590.
- Bloom DD, Fikáˇcek M, Short AEZ (2014) Clade age and diversification rate variation determine species richness patterns in aquatic beetle lineages. PLoS One 9: e98430.
- Short AEZ (2018) Systematics of aquatic beetles (Coleoptera): current state and future directions. Syst Entomol 43: 1-18.
- Miller KB, Bergsten J (2016) Diving Beetles of the World. Johns Hopkins University Press, Baltimore, Maryland.
- Miller KB, Bergsten J (2012) Phylogeny and classification of whirligig beetles (Coleoptera: Gyrinidae): relaxed-clock model outperforms parsimony and time-free Bayesian analyses. Syst Entomol 37: 706-746.
- Short AEZ, Joly LJ, García M, Wild A, Bloom DD, Maddison DR (2015) Molecular phylogeny of the Hydroscaphidae (Coleoptera: Myxophaga) with description of a remarkable new lineage from the Guiana shield. Syst Entomol 40: 214-229.
- Lee C -F, Sato M, Shepard WD, Jäch MA (2007) Phylogeny of Psephenidae (Coleoptera: Byrroidea) based on larval, pupal, and adult characters. Syst Entomol 32: 502-538.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ramin K, Hassan V, Ghazal T, Ali J (2022) Bio Ecology of Aquatic and Semi-Aquatic Insects of Order Coleoptera in the World. J Marine Sci Res Dev 12: 346. DOI: 10.4172/2155-9910.1000346
Copyright: © 2022 Ramin K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4315
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 3580
- PDF downloads: 735