Review Article Open Access
Bimoclomol and Arimoclomol: HSP-co-Inducers for the Treatment of Protein Misfolding Disorders, Neuropathy and Neuropathic Pain
Keppel Hesselink JM*
Department of Molecular Pharmacology, Pain Specialist, Faculty of Health, University of Witten, Germany
- *Corresponding Author:
- Keppel Hesselink JM
Faculty of Health, University of Witten/Herdecke, Germany
Tel: 06-51700527
E-mail: jan@neuropathie.nu
Received date: November 21, 2016; Accepted date: December 23, 2016; Published date: December 27, 2016
Citation: Keppel Hesselink JM (2016) Bimoclomol and Arimoclomol: HSP-co-Inducers for the Treatment of Protein Misfolding Disorders, Neuropathy and Neuropathic Pain. J Pain Relief 6:279. doi:10.4172/2167-0846.1000279
Copyright: © 2016 Keppel Hesselink JM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Bimoclomol and arimoclomol are small new chemical entities which have been synthesized during the end of last century by a Hungarian pharmaceutical company, Biorex. Both compounds consistently increase Heat Shock Protein (HSP) expression and reduce functional as well as structural pathology in a series of animal models. The development of bimoclomol stagnated, most probably due the short half life time of the compound. Arimoclomol is currently in development for orphan disorders such as amyotrophic lateral sclerosis, the sphingolipidose Niemann- Pick type C and inclusion body myositis. Arimoclomol has high oral availability and good CNS penetration, without inducing troublesome CNS side effects. It might also be a promising compound for the treatment of diabetic neuropathy and neuropathic pain in (diabetic) neuropathy, due to its neuroprotective and analgesic properties.
Keywords
Heat shock protein; Heat shock protein inducers; Niemann-Pick; Amyotrophic lateral sclerosis; amyotrophic lateral sclerosis; Inclusion body myositis
Introduction
New effective and safe treatments for complications of diabetes are clearly needed, and we will discuss in this paper a special group of compounds which stimulate the innate repair and protective mechanisms of our body, via co-inducing Heat Shock Proteins (HSPs) [1]. These are the hydroxylamine compounds bimoclomol and arimoclomol which have been consistently found to increase the expression of the molecular chaperones, the HSPs, in cells exposed to a physiological stress. The hydroxylamines are characterized as coinducers of HSPs, because they up-regulate HSP production only in situations of cellular stress responses, for instance induced by diabetes or ischemia, first described in a patent filed in 1996 [2].
In this review, we will discuss some findings supporting the use of such compounds to treat complications of diabetes, such as neuropathy and neuropathic pain, with an emphasis on arimoclomol, as this compound is still in clinical development, while the development of its parent compound seems have come to stand still by the end of last century. However, as both compounds have a similar mechanism of action, we will also review data related to bimoclomol.
Bimoclomol and arimoclomol both targets stressed cells in general, and neurons as well as non-neuronal cells such as glia can be indirectly protected against cellular stressors, via induced HSPs. HSPs have been demonstrated to consistently protect a great variety of cells against stress (ischemia, endo- and exotoxic factors) [3]. HSP co-inducers have been recognized as useful in the treatment of chronic diabetic complications, especially retinopathy, neuropathy and nephropathy [4-6].
Clinical development of a first lead compound within the class of hydroxylamines, bimoclomol, started around 1995-1997 in the field of neuroprotection of cells in diabetes, the first patent in this field dates from 1996. A new pharmacological superior derivative with a 4 times longer half life time, arimoclomol, was developed soon. Currently the development focus of arimoclomol is on orphan disorders such as amyotrophic lateral sclerosis (ALS), Niemann-Pick and inclusion body myositis. Clinical development up to phase IIa in these disorders supported its use as safe treatment, with hints of efficacy; the compound has an uncomplicated pharmacokinetic profile, and penetrates the CNS easily.
To Following where Nature Leads
To treat diseases by inducing cellular repair and protective mechanisms and making use of existing corrective pathways is unique. Such has been foreseen as a new inroad for drug development of the future by the famous neuroscientist Professor Erminio Costa, who coined a phrase for this approach already in the 80s of last century: ‘to following where nature leads’. This principle was given shape in the 90s of last century, by the Nobel laureate, the late professor Rita Levi- Montalcini, whose work put a new emphasis on innate regulatory pathways to maintain a homeostatic balance, when organs or tissues were challenged by stress and injury [7]. The principle of heat shock protein co-inducers has been identified in the last century and can be traced back to as early as 1996 [8]. Therapies activating innate repair and protective mechanisms are rare in medicine would be very much welcomed!
Overexpression studies of HSPs such as Hsp70, Hsp40, and Hsp27 demonstrated protective effects of HSPs in several animal models of neurodegenerative diseases, characterized by protein misfolding [9,10]. HSP-co-inducers could indeed have broad therapeutic relevance for Alzheimer’s disease, Parkinson’s disease, ALS and many other neuro- (and retina-) degenerative disorders, as well as in ischemic neurological disorders and the various complications of diabetes.
Bimoclomol
N-[2-hydroxy-3-(1-piperidinyl)propoxy]-3-pyridinecarboximidoyl- chloride (bimoclomol) was proven to be a HSP coinducer in the last century [11]. It has been created and patented by researchers from the Biorex Research and Development Company, a pharmaceutical research and development company in Hungary, founded in 1988 [12].
Soon the compound In animal models was found to be a retina- and neuroprotectant, and more generally a cytoprotectant, especially related to diabetic complications [13-14]. Bimoclomol was reported to be in phase II clinical development for the treatment of diabetic complications in different papers, published between 1997-2002 [15]. However, one of the required properties for further drug development at that time was that the compound should reduce insulin resistance, which it could not, although it clearly decreased complications of diabetes [14]. In 1997 bimoclomol was reported by Biorex to be a vasoprotective drug and under (‘advanced’) phase II clinical development for the treatment of diabetic complications. First indications that the molecule acted via HSP co-induction were published in 1996 [16]. Bimoclomol and its analogues act as coinducers of heat shock proteins, in particular HSP60, HSP70, HSP90 and CRP94 [15]. Drug development as a cellular protective agent in indications such as diabetic complications however went too slow, and the absence of bimoclomol’s effect on insulin resistance was regarded as a no-go. Abbott Laboratories acquired the rights on bimoclomol in diabetes by paying Biorex $28 million in 1998 [17]. However, no further development milestones in its development have been reported since. In 2000 a new derivative was identified, surprisingly with an improved pharmacological and pharmacokinetic profile, arimoclomol.
Arimoclomol: An Improved Bimoclomol Derivative
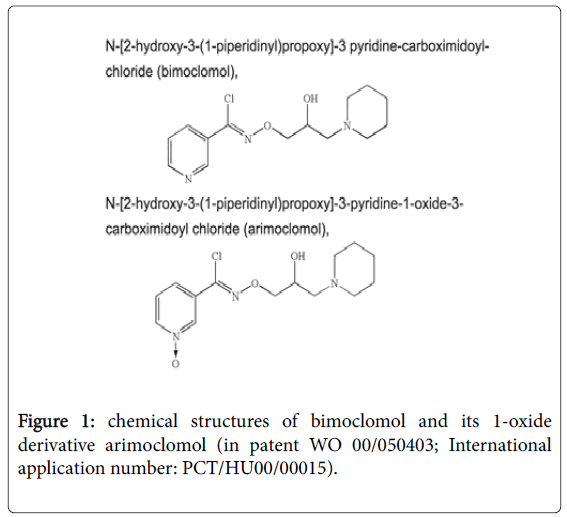
N-[(2R)-2-Hydroxy-3-(1-piperidinyl)propoxy]-3- pyridinecarboximidoyl chloride 1-oxide is the IUPAC Name for arimoclomol, which can exist as a maleate salt (code Biorex name BRX-220) or a citrate salt (BRX-345). The compound was synthesized by Biorex researchers as a N-oxide derivative of bimoclomol (Figure 1) to create a superior follow-up compound for bimoclomol around 1999. First studies focused on improvements of its effect in diabetes (insulin resistance and diabetic neuropathy) over bimoclomol, described in detail in a patent [18].
Biorex, the parent company, was acquired by CytRx Corporation in 2005, and with it the development of arimoclomol came into new hands. In 2005 CytRx focused on arimoclomol in models of diabetes, in line with previous indications for bimoclomol [19]. However, probably due to strategic reasons, the company refocused the development of arimoclomol and started in 2006 in amyotrophic lateral sclerosis and stroke. In 2011 the worldwide rights to arimoclomol (as well as bimoclomol) were transferred to a Danish biotech company specialized in orphan indications, Orphazyme ApS. This company proceeded by developing arimoclomol in orphan indications such as Niemann-Pick type. Both the European Medicines Agency (EMA) and U.S. Food & Drug Administration (FDA) granted orphan drug designation to arimoclomol as a potential treatment for Niemann-Pick type C in 2014 and 2015 respectively. Its value as a cytoprotectant in diabetes and as a treatment for neuropathic pain was lost from the radar, reason to write this short review and commentary.
Arimoclomol is claimed to be a pharmacologically improved analog of bimoclomol, with superior pharmacokinetic properties, a longer half-life time, four times longer than that of bimoclomol (1 hour) without relevant serum protein binding [19], and is referred to as an orally available drug with a broad therapeutic potential, penetrating the CNS easily [20]. The last property is remarkable, given the polar molecule.
Arimoclomol has been shown to consistently amplify heat shock protein (HSP) gene expression in a number of models [21], and thus further elevates the HSP levels already induced by physical or metabolic stressors. Its mechanism of action seems to be based on the prolonged activation of Heat Shock Factor-1 (HSF1)[22]: arimoclomol acts as a co-inducer of HSPs that prolongs the binding of activated HSF1 to heat shock elements (HSEs) in the promoter regions of heat shock genes.
Cellular and neuroprotection in different models
We will discuss a number of studies supporting arimoclomol’s therapeutic value in (diabetic) neuropathy and neuropathic pain.
In a rat pancreatitis model 20 mg/kg arimoclomol was administered intragastrically, followed by a pancreatitis inducer, 75 mg/kg CCK [23]. The expressions of pancreatic HSP60 and HSP72 were significantly decreased in control animals with pancreatitis, while in treated animals both HSP60 and HSP72 were significantly increased. BRX-220 treatment also significantly decreased signs of inflammation: the pancreatic leukocyte infiltration and adherence and the vacuolization, necrosis and apoptosis of the acinar cells all were diminished.
Arimoclomol was tested in a rat sciatic nerve crush model [24]. Arimoclomol was administered (10 mg/kg BW, ip.) following a nerve crush, up to a maximum of 3 weeks versus control. 10 weeks after injury 42% (n=6) of motor neurons survived in the treated group compared to only 15% (n=6) in the saline-treated group. (p<0.02). The same significant improvement was detected in the electrophysiological read-out based on active motor units. Spinal cords of animals treated with arimoclomol contained significantly higher levels of HSP70 and HSP90 (p<0.01).
In a one-month preventive treatment study arimoclomol 2.5, 5, 10, and 20 mg/kg per os dose-dependently improved diabetes-related deficits [19]. Arimoclomol dose-dependently improved both the motor and sensory functions and in the highest dose achieved an almost complete normalization. Its effects were more pronounced compared to bimoclomol.
The potential neuroprotective and analgesic effect of arimoclomol was further tested in a model of sciatic nerve axotomy [25]. Evaluated was whether a number of important surrogates could be influenced by arimoclomol: the expression of calcitonin gene-related peptide (CGRP), the expression of substance P and the binding of IB4 (parameters are known to down-regulate following nerve injury). Animals were treated once daily by gavage (10 mg/kg BW) for up to 4 weeks. Arimoclomol improved CGRP staining pattern in the dorsal horn at 4 weeks: the pattern was significantly different from that of vehicle-treated animals (p<0.05): 4 weeks after injury 9% of cells were strongly CGRP positive compared to only 4.5% in vehicle-treated dorsal root ganglia cells. Treatment also prevented the reduction in IB4 binding in the dorsal horn, and SP release was 1.5 times greater than basal levels, whereas in vehicle-treated rats, SP release did not reach basal levels and was only 0.9 times the basal outflow. Hand in hand with these neuroprotective effects, treated spinal cords contained markedly higher levels of HSP70. 15-25 days after the nerve injury mechanical and cold allodynia behavior improved significantly compared to vehicle-treated animals. The authors were not clear about the mechanism of analgesia, substance P might play a role, or alternatively they suggested that the compound mobilized a rather unspecific recovery mechanism through the increased induction of HSP expression rather than targeting specific receptors.
Following these experiments, a series was published in models of neurodegenerative disorders, such as ALS [26-30]. The compound also protected brain cells against hypoxic stress, and could protect the retinal cells in a model of retinitis pigmentosa [31,32]. In a human neuronal cell line neuroprotective effects were documented, while HSPs increased after the application of arimoclomol [33].
A series of experiments evaluating the role of arimoclomol in stroke models have been published in a separate patent [34]. Rats were given arimoclomol, p.o., starting at one hour after the occlusion at 200 mg/kg/d once daily for 3 days, then 50 mg/kg/d once daily for a total of 29 days. Neurological recovery in the arimoclomol group was superior to the vehicle group (p<0.05), although the infarct volume was not different between groups. This was interpreted as that arimoclomol might accelerate the repair of neurological damage caused by stroke. In a follow up experiment, neurological recovery of the group that received arimoclomol 48 hours after occlusion was indistinguishable from those that received arimoclomol 6 hours after occlusion for time points up to and including 21 days after occlusion. Neuroprotective in cerebro effects were detected for a derivative of arimoclomol. In a different in vitro paradigm, rescue of rat cortical neurons after oxygen/ glucose deprivation arimoclomol added one hour after insult to the wells at 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, and 50 μM concentrations, saved the neurons. CytRx pointed out in 2007 that the preclinical results were so compelling that the company decided to evaluate the clinical benefit of arimoclomol in stroke recovery. In 2009 the company presented further preclinical data confirming the first favorable preclinical results, based on an embolic stroke model, indicating that arimoclomol leads to better functional recovery, even given 24 hours after the onset of stroke and thus has the potential to of use in stroke rehabilitation programs. To date however, no clinical trials in stroke have commenced and none are registered in ClinicalTrials.gov.
Arimoclomol in Clinical Trials
Meanwhile arimoclomol has been tested in a number of phase I studies in healthy volunteers and two Phase II studies, one in patients with ALS, and one in sporadic Inclusion Body Myositis. Studies in Nieman-Pick C and SOD1 Positive Familial Amyotrophic Lateral Sclerosis are ongoing.
In 2008 results were presented of a number of phase I studies as well as a pilot trial in ALS [35]. Observed were dose-related increases in serum concentrations of arimoclomol at all oral doses in the ALS study (75, 150, and 300 mg/day). Sixty-nine patients from the Phase 2a double-blind study were treated with 300 mg/day of arimoclomol for up to 6 months, without significant side effects. Some serious adverse events, such as pulmonary embolism and respiratory failure were related to the illness as such.
There were no adverse events in these first studies more common with arimoclomol as compared with placebo. There were no clinical meaningful chances in laboratory values. Arimoclomol treatment resulted in a small (10%), but statistically significant, increase in serum creatinine and a decrease in creatinine clearance, which was not regarded to be clinical meaningful, as the effects fell within the normal range.
Arimoclomol (400 mg t.u.d.) administered to volunteers penetrates the human blood-brain barrier as cerebrospinal fluid (CSF) levels at 3 and 6 hours after oral administration were detectable. The concentration arimoclomol in CSF rose dose-dependently. There were no data in the paper presented on clinical endpoints within the doubleblind phase of the study. However, the ALS rating scale total change from baseline in the open-label compared to historical control data, supported a therapeutic effect of arimoclomol.
The results of the pilot trial in sporadic IBM has been hidden in a preclinical paper, published in 2016 [36]. The aim of the study was to evaluate the safety and tolerability of arimoclomol, and to explore hints for efficacy. The study followed a randomized, double-blind, placebocontrolled design and patients were treated for 4 months, and followup was 8 months; dose arimoclomol 200 mg tablets, t.i.d. Side effects were mostly gastrointestinal and transient. Other transient adverse events were two cases of hyponatremia, one case of hematuria and one case of high thyroxine levels. There were some positive trends on the functional ratings scale and physical function and muscle strength decline rates were higher in the placebo group compared to the arimoclomol group.
Further trials in rare disorders are under way.
Recently arimoclomol was discussed in detail and the conclusion was that the drug “has several advantages relative to other investigational drugs such as oral formulation, daily dosing, safe preclinical and clinical profile, CSF penetration, and dose- dependent drug kinetics ” [37].
Human studies in patients as described in ClinicalTrials.gov are:
1. Completed: A Multicenter, Dose Ranging Safety and Pharmacokinetics Study of Arimoclomol in 80 ALS patients: safety and tolerability of arimoclomol in ALS patients following 90 days of dosing. Dose ranging study with pharmacokinetics and vital capacity.
2. Completed: Arimoclomol in 24 patients suffering from Sporadic Inclusion Body Myositis: safety, muscle strength, biopsy and functional scale.
3. Active study: Phase II/III Randomized, Placebo-controlled Trial of Arimoclomol in SOD1 Positive Familial Amyotrophic Lateral Sclerosis: 100 or 200 mg three times daily in 30 patients with SOD1 positive familial ALS. Safety and Rate of decline of ALSFRS-R (ALS functional rating scale-revised) over a period of up to 12 months.
4. Active study: Arimoclomol Prospective Study in 46 Patients Diagnosed with NiemannPick Disease Type C, Change in NPC disease severity score.
5. Active study: A Prospective Non-therapeutic Study in 46 Patients Diagnosed With Niemann-Pick Disease Type C, NP-C clinical disease severity.
6. Planned study: start date January 2017: Study of 200 mg arimoclomol t.i.d. in Inclusion Body Myositis (IBM): Decline in Inclusion body myositis functional rating scale (IBMFRS).
Conclusion
Arimoclomol is a new bimoclomol-1-oxide derivative. The compound has clear and consistent cellular and neuroprotective properties, just as bimoclomol, and possesses a more favorable pharmacokinetic profile, with a longer half life time. There is consistency in literature that arimoclomol upregulates HSPs production. In a number of animal models for neuroprotection and neuropathic pain arimoclomol tested positive as a neuroprotective and analgesic compound. Arimoclomol currently is developed solely in a number of orphan indications such as the sphingolipidose Niemann- Pick C, most probably due to strategic focus. However, it is recommended to further study the value of arimoclomol in diabetic neuropathy and neuropathic pain, as this molecule activates innate repair and defense mechanisms via the heat shock proteins and has analgesic activity.
References
- Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, et al. (2009) Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci 10: 85-95.
- Biorex patent (1995) Hydroxylamine derivatives useful for enhancing the molecular chaperon production and the preparation thereof. EP 0801649 B1.
- Aufricht C (2005) Heat-shock protein 70: molecular supertool? Pediatr Nephrol 20: 707-713.
- Ishii Y, Kwong JM, Caprioli J (2003) Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci 44: 1982-1992.
- Ma J, Farmer KL, Pan P, Urban MJ, Zhao H, et al. (2014) Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J Pharmacol Exp Ther 348: 281-292.
- Crul T, Toth N, Piotto S, Literati-Nagy P, Tory K, et al. (2013) Hydroximic acid derivatives: pleiotropic HSP co-inducers restoring homeostasis and robustness. Curr Pharm Des 19: 309-346.
- Keppel HJM (2016) Palmitoylethanolamide: Research Synergy between Academia and Industry, Based on Insights and Work of Nobel Laureate Rita Levi-Montalcini. J Clin Trials Pat 1: 1-3.
- Rossi A, Elia G, Santoro MG (1996) 2-Cyclopenten-1-one, a new inducer of heat shock protein 70 with antiviral activity. J Biol Chem 271: 32192-32196.
- Muchowsk PJ, Walker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci 6: 11–22.
- Magrane J, Smith RC, Walsh K, Querfurth HW (2004) Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci 24: 1700-1706.
- Vígh L, Literáti PN, Horváth I, Torok Z, Balogh G, et al. (1997) Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects, Nat. Med 3: 1150-1154.
- Nagy PL, Balazs B, Boross M, Szilbereky J, Zsila G, et al. (1988) Novel o-(3-amino-2-hydroxypropyl)-hydroximic acid halides and process for preparing the same. WO 1990004584 A1.
- Bíró K, Pálhalmi J, Tóth AJ, Kukorelli T, Juhász G (1998) Bimoclomol improves early electrophysiological signs of retinopathy in diabetic rats. Neuroreport 9: 2029–2033.
- Bíró K, Jednákovits A, Kukorelli T, Hegedüs E, Korányi L (1997) Bimoclomol (BRLP-42) ameliorates peripheral neuropathy in streptozotocin-induced diabetic rats. Brain Res Bull 44: 259-263.
- Visy J, Fitos I, Mády G, Urge L, Krajcsi P, et al. (2002) Enantioselective plasma protein binding of bimoclomol. Chirality 14: 638-642.
- Vigh L, Litera ?ti NP, Horva ?th I (1996) BRLP-42, a new generation of drugs acting as a chaperone inducer. In: Molecular chaperones and the heat shock response.
- NN (1998) Hot-shot proteins. The cell’s chaperones may provide novel cures for a number of diseases.
- Biorex Patent (2000) N-[2-hydroxy-3-(1-piperidinyl)propoxy]pyridine-1-oxide-3-carboximidoyl chloride and its use in the treatment of insulin resistance. EP 1163224 B1.
- Kürthy M, Mogyorósi T, Nagy K, Kukorelli T, Jednákovits A, et al. (2002) Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann N Y Acad Sci 967: 482-489.
- Cudkowicz ME, Shefner JM, Simpson E, Grasso D, Yu H, et al. (2008) Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle nerve 38: 837-844.
- Bloomer SA, Cernak I, Haak JL, Kregel KC (2010) Arimoclomol® enhances hepatic stress protein accumulation after an acute bout of heat stress. The FASEB Journal 24: 1000-1008
- Hargitai J, Lewis H, Boros I, Rácz T, Fiser A, et al. (2003) Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem Biophys Res Commun 307: 689-695.
- Rakonczay Z, Iványi B, Varga I, Boros I, Jednákovits A, et al. (2002) Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats. Free Radic Biol Med 32: 1283-1292.
- Kalmar B, Burnstock G, Vrbova G, Urbanics R, Csermely P, et al. (2002) Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats. Exp Neurol 176: 87-97.
- Kalmar B, Greensmith L, Malcangio M, McMahon SB, Csermely P, et al. (2003) The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury. Experimental neurology 184: 636-647.
- Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, et al. (2004) Treatment with arimoclomol, a co-inducer of heat shock proteins, delays disease progression in ALS mice. Nat Med 10: 402-405.
- Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, et al. (2008) Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1G93A mouse model of ALS. J Neurochem 107: 339-350.
- Kalmar B, Greensmith L (2009) Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects. Cell Mol Biol Lett 14: 319-335.
- Kalmar B, Edet-Amana E, Greensmith L (2012) Treatment with a coinducer of the heat shock response delays muscle denervation in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Amyotroph Lateral Scler 13: 378-392.
- Malik B, Nirmalananthan N, Gray AL, La Spada AR, Hanna MG, et al. (2013) Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: implications for therapy. Brain 136: 926-943.
- Xu K, Sun X, Erokwu BO, Cernak I, LaManna JC (2011) A heat-shock protein co-inducer treatment improves behavioral performance in rats exposed to hypoxia. Adv Exp Med Biol 701: 313-318.
- Parfitt DA, Aguila M, McCulley CH, Bevilacqua D, Mendes HF, et al. (2014) The heat-shock response co-inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death and Disease 5: e1236.
- Deane CA, Brown IR (2016) Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol. Cell Stress Chaperones 21: 837-48.
- Cytrx Patent (2006) Hydroxylamine derivatives for the treatment of stroke WO 2008070010.
- Lanka V, Wieland S, Barber J, Cudkowicz M (2009) Arimoclomol: a potential therapy under development for ALS. Expert opinion on investigational drugs 18: 1907-1918.
- Ahmed M, Machado PM, Miller A, Spicer C, Herbelin L, et al. (2016) Targeting protein homeostasis in sporadic inclusion body myositis. Science translational medicine 8: 331-341.
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 6321
- [From(publication date):
January-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 5346
- PDF downloads : 975