Biliary and Pancreatic Stricturoplasty for Recurrent Stricture after Hepaticojejunostomy and Pancreaticojejunostomy
Received: 03-Sep-2020 / Accepted Date: 21-Oct-2020 / Published Date: 28-Oct-2020 DOI: 10.4172/2161-069X.1000632
Abstract
Purpose: To evaluate the safety and success of a novel biliary and pancreatic stricturoplasty (BPS) technique in relieving resistant biliary-enteric anastomotic stricture.
Materials and Methods: A retrospective review of patients with hepaticojejunal stricture (HJS) and/or pancreaticojejunal stricture (PJS) treated with the novel technique was performed. Strictures were approached through enterotomy, manually divided, and marsupialized in a manner similar to transdoudenal sphincteroplasty for Sphincter of Oddi Dysfunction. Success was defined by: no increased risk of life-threatening complications, no evidence of restricturing or evidence of complications attributable to restricturing on long-term follow-up. Clinical, radiologic, and laboratory parameters were reviewed in all patients until last clinical encounter.
Results: Seven patients with a mean age of 49.7 ± 12 years underwent eight BPS procedures at Albany Medical Center between 2008 and 2018 after failing previous non-operative approaches when possible. A single episode of cholangitis was noted on short term follow up of HJS stricture, but no long-term complications were noted. Pancreatitis recurred in two PJS patients during long term follow up, but no short-term complications were noted. These complications could not be attributed to recurring stricture or procedure failure.
Conclusion: The BPS technique offers a safe alternative to repeat hepaticojejunostomy or pancreatojejunostomy for resistant biliary-enteric stricture.
Keywords: Pancreaticojejunostomy, Chronic disease, Biliary surgery, Stricture
Introduction
Stricture after biliary-enteric anastomosis occurs in 5-15% of patients followed from 13.6 to 96 months after surgery [1-6]. Stricture rates as high as 30% have been reported in liver transplant recipients [7]. When considering the incidence of hepaticojejunostomy stricture (HJS), the literature would suggest 9-15% occurrence outside of liver transplantation [3-6]. Conversely, this rate drops to 2-11% in pancreaticojejunostomy stricture (PJS) [8]. Stricture constitutes a major burden for both patient and hospital, particularly in benign pathologies where the objective remains cure. Patients are subjected to multiple hospitalizations, imaging studies, and interventions often in the most productive phase of life [6,8,9]. Although these strictures are difficult to correct, general consensus exists for the management of HJS and PJS. In benign biliary stricture, access to the biliary tree is first attempted endoscopically, then via percutaneous transhepatic approach [6]. Once the stricture is accessed, a variety of interventions - balloon dilation, cutting balloon, endostent, or covered metal stent - are employed in its treatment [6]. Unfortunately, these conservative measures have a significant failure rate. 13-27% of strictures recur after endoscopic intervention [6,10] and 8-20% recur after transhepatic interventions [7,11-14]. Surgery is offered as a curative option after failure of the other methods [9,10,15-18]. Endoscopic approaches have been described as a first line treatment in PJS without success [19]. Consequentially, authors have suggested surgery as a first line treatment in PJS [8,20].
Surgical intervention for stricture relief is limited to re-doing the HJ or PJ anastomosis [8,16,18-21]. Redo-hepaticojejunostomy subjects the patient to the inherent risks of these procedures and the extant 5-15% stricture recurrence risk [1-6]. Furthermore, redo-hepaticojejunostomy predisposes the patient to increased risk of restricture, which rises with each operation [22]. The data for PJS anastomotic takedown and revision is limited and associated with significant complications. To our knowledge, there remains a paucity of surgical alternatives. We found only one report of such an alternative, proposed by Elbir et al in their 2012 paper [16]. After performing a Heinke-Mikulicz stricturoplasty in HJS, they observed no complications in one 58 year old male after 18 months of follow-up [16].
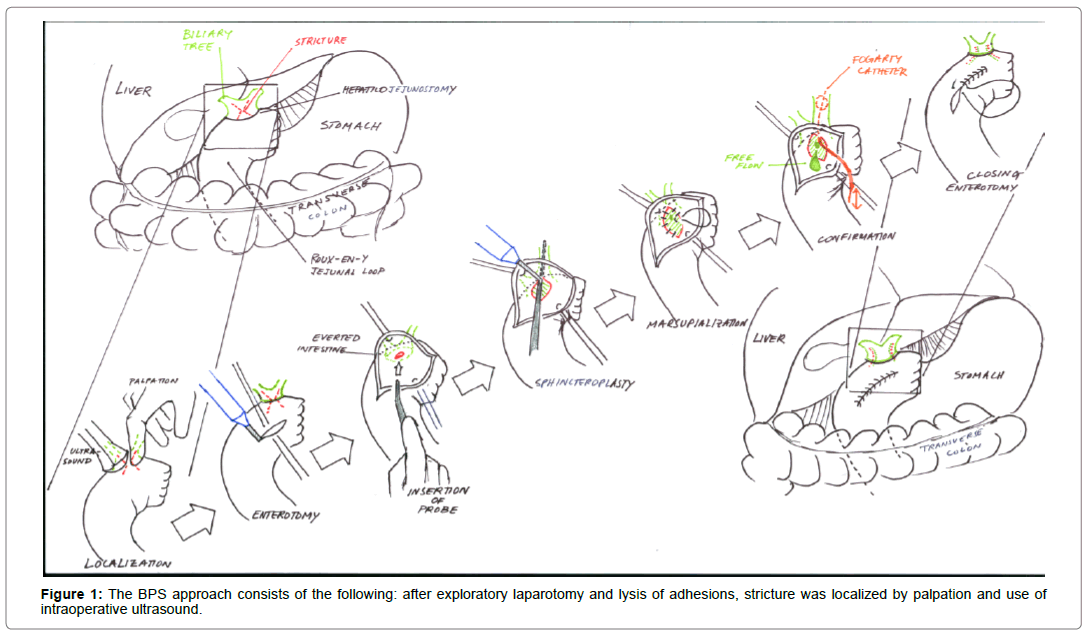
Here, we describe a novel biliary and pancreatic stricturoplasty (BPS) technique, modeled after the transduodenal sphincteroplasty for Sphincter of Oddi dysfunction (Figure 1) [23]. Additionally, we review our experience with the BPS for relief of symptoms and prevention of HJS and PJS in 8 patients.
Materials and Methods
Patients studied
From 2008 to 2018, seven patients underwent eight BPS procedures performed by a single surgeon at Albany Medical Center, a highvolume tertiary care center. IRB approval was obtained (number 5125). All of the patients developed benign HJS and/or PJS after Whipple procedure which proved refractory to conservative therapy. Diagnosis was obtained based on clinical presentation and records. The diagnosis was radiologically verified by reviewing available imaging for ductal dilatation and stricture. Time to development of the stricture was considered to be from the Whipple procedure to the first mention of or first imaging study revealing presence of a stricture.
Procedure
As illustrated in Figure 1, the BPS approach consists of the following: after exploratory laparotomy and lysis of adhesions, stricture was localized by palpation and use of intraoperative ultrasound (Figure 1). A longitudinal enterotomy was made opposite the stricture and the intestine was everted to visualize the anastomosis. By passage of a lacrimal probe across the connection, its location and that of the stricture were confirmed by intraoperative ultrasound, direct visualization and palpation. If needed, choledochoscopy was performed. Debris was cleared by passing a fogarty catheter. With the probe in place, the mucosa and stricture were divided with hot cautery at 12 o’clock, exposing the probe. If needed, a fogarty catheter was introduced again to clear debris. Any transhepatic biliary stents were left in place. From the apex of the incision in both directions, mucosa was sewn to mucosa in such a manner that the marsupialization widened the stricture. Two steps were taken to confirm patency: first, an inflated fogarty catheter was passed; and second, the free flow of biliary or pancreatic juices was appreciated. The enterotomy was closed and an abdominal drain was placed next to the operative site.
Follow-up
Patient presentation and management were reviewed in both electronic medical record systems employed by our institution. Demographic information, presentation, and type of stricture were obtained. One patient developed both HJS and PJS and was counted in both categories (Table 1, patient G). The initial operation predisposing the patient to stricture and indication for the operation was noted. Pathology leading to the Whipple procedure was noted. A comprehensive history of stent placement was obtained, noting whether any stents were placed at any time, the type of stents, and whether there were stents deliberately left in place preoperatively. If a stent was placed at any time point, then we recorded its duration from the first to last mention in the medical record. Preoperative stent duration was considered to be from the time of placement to the operative date.
| Preop | Preoperative | Immediate postop | |||||||||||||||
| History of CP | Stricture | Liver Pathology | Pre-op Stent left | Type of Preop stent | Notable | Bacteremia | Cholangitis | AST | ALT | ALP | |||||||
| 1 | PJS | 0 | 0 | 0 | psychiatric comorbidities | 0 | 0 | N | N | N | |||||||
| 1 | PJS | 1 | 1 | pancreatic | irretrievable pane stent liver Bipos | 0 | 0 | 51 | 73 | 63 | |||||||
| 0 | HJS | 0 | 1 | biliary | ductal dilation | 0 | 0 | 27 | 26 | 164 | |||||||
| 1 | PJS | 0 | 0 | 0 | 0 | 0 | 31 | 50 | 98 | ||||||||
| 0 | HJS | 0 | 1 | biliary | ductal dilation | 1 | 1 | 82 | 72 | 217 | |||||||
| 0 | PJS | 0 | 0 | 0 | pancreatic manipulation | 0 | 0 | 29 | 29 | 63 | |||||||
| 1 | PJS, HJS | 0 | 0 | 0 | refused stent foreign body | 0 | 0 | 19 | 19 | 245 | |||||||
| Continued. | |||||||||||||||||
| Immediate postop | Long term postop | ||||||||||||||||
| Lipase | Amylase | T billi | WBC | Pancreatitis | isolated AST/ALT | elevated lip/amy with bout | normal lip/amy with bout | radiologic evidence | |||||||||
| N | N | N | 14.5 | 1 | 0 | 1 | 1 | 0 | |||||||||
| N | 0.9 | 13.4 | 1 | 1 | 0 | 1 | 0 | ||||||||||
| 11 | 22 | 0.7 | 21.5 | N | N | N | N | N | |||||||||
| 12 | 57 | 0.6 | 16.1 | 0 | 0 | 0 | 0 | 0 | |||||||||
| 16 | N | 1.6 | 11.2 | N | N | N | N | N | |||||||||
| 206 | 151 | 1.1 | 15 | 0 | 0 | 0 | 0 | 0 | |||||||||
| N | 23 | 0.4 | 16.5 | N | N | N | N | 0 | |||||||||
Table 1: Case specific details. Patients are presented in a randomized fashion, along with curated preoperative, perioperative, and postoperative findings.
Investigations, imaging studies, and management of any complications were recorded. Laboratory values from postoperative days 0 – 10 were considered immediate postoperative lab values. If no labs could be retrieved from that time frame, then we assumed the patient lacked immediate postoperative lab values. Any notes and blood work thereafter to the last known encounter in the electronic medical record were carefully reviewed for complications indicative of stricture. This included but was not limited to: explicit mention in a radiology report, ductal dilatation, liver function test abnormalities, amylase or lipase elevations, or clinical presentation concerning for restricturing. Standard cutoffs for abnormal laboratory values were used.
Follow-up time was defined as the date of surgery to the last documented control time (patient encounter). If no further communication from the patient, return appointment, lab draws, or any other indication of an encounter beyond the last control time was discovered, then we made the assumption the patient was still doing well.
Discussion
Long term outcomes of the pancreaticojejunal and hepaticojejunal anastomosis are of particular interest, given increasing numbers of these procedures and their importance in patient outcomes when performed for benign stricture [8,9,24]. Our best option in treating resistant biliary-enteric anastomotic stricture remains re-doing the anastomosis, which has been suggested to predispose patients to significant complications [22,24,25]. At the very least, their risk of restricture is equivalent to that of the original procedure.
Our lack of restrictures in our small group of patients is not in keeping with prior reports of the success of redo HJS or PJS [1,2,4- 6,8,23]. Though we examine more patients over a longer period – eight patients over 1811 ± 1000 days - our rate is comparable to Elbir’s case report of HM principle in benign HJS stricture (Table 2) [16]. Ischemia is known to predispose to various anastomotic failures. For example, Strasburg and colleagues found inadequate pancreatic blood supply risks pancreatic fistula [26]. It follows that ischemia to an anastomotic site could also predispose to increased fibrosis and stricture. An existent anastomosis already has a pattern of blood supply, adapted to a new surgical anatomy. Re-doing that anastomosis disrupts the blood supply and restarts the process of generating another set of supporting vessels. Elbir and our results would suggest that preserving the extant anastomosis and revising it preserves much of this blood supply, and may contribute to the lower complication rate. The principle holds true in other anastomotic sites [27].
| Postoperative Period | |||||||
| Overall | HJS | PJS | |||||
| Median Time to Follow-up (days) | 1811 ± 1000 | 1660 ± 1283 | 1583 ± 1184 | ||||
| Immediate (POD 0 - 10) | Drain | 6 | 85.7% | 3 | 100.0% | 4 | 80% |
| Stent left in place | 3 | 42.9% | 2 | 66.7% | 1 | 20% | |
| Bacteremia | 1 | 14.3% | 1 | 33.3% | 0 | 0% | |
| Leukocytosis | 7 | 100.0% | 3 | 100.0% | 5 | 100% | |
| Total Bilirubin | 1 | 14.3% | 1 | 33.3% | 0 | 0% | |
| ALP | 4 | 57.1% | 3 | 100.0% | 1 | 20% | |
| AST/ALT | 2 | 28.6% | 1 | 33.3% | 1 | 20% | |
| Lipase/Amylase | 1 | 14.3% | 0 | 0.0% | 1 | 20% | |
| Cholangitis | 1 | 14.3% | 1 | 33.3% | 0 | 0% | |
| Fistula | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Biliary Leak | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| SSI | 0 | 0.0% | 0 | 0.0% | 0 | 20% | |
| Hemorrhage | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Biloma | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Bile Effusion | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Hemobilia | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Pancreatitis | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Long term (3 mo, 6 mo, 9 mos, 1 yr, 2 yr, 3 yr) | Pancreatitis | 2 | 28.6% | 0 | 0.0% | 2 | 40% |
| Fistula | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Bile Leak | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Infection | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| ALP | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| AST/ALT | 1 | 14.3% | 0 | 0.0% | 1 | 33% | |
| Lipase/Amylase | 1 | 14.3% | 0 | 0.0% | 1 | 33% | |
| Cholestasis | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Cholangitis | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Lithiasis | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
| Radiologic evidence | 0 | 0.0% | 0 | 0.0% | 0 | 0% | |
Table 2: Incidence of postoperative complications and time to follow-up for the entire study cohort and PJS, HJS groups individually. Complications are highlighted.
Given that any instance of restricture would be found on longer term follow up, we focused on attention on clinical presentations and any available imaging that would suggest restricture. Key clinical signs were determined by literature review and largely inspired by those Zhu and colleagues used [1,8,13,25]. Other authors report MRCP or secretin stimulated MRCP as a primary modality in assessing [24]. As our study was retrospective, a similar standard is not easily attained. However, we were able to look for ductal dilatation which remains consistent regardless of the type of scan obtained. As Ghazanfar and colleagues comment, this is not always a reliable sign. [8] For this reason we also looked for any stricture itself on the scan. Our series did reveal two episodes of pancreatitis and one episode of isolated AST/ALT elevations, concerning for restricture (Table 2). However, both patients (A, B) presenting with pancreatitis on long term follow up had prior history of pancreatitis, CT scans from the episodes failed to reveal stricture, and their bouts occurred in the setting of unremarkable amylase and lipase levels (Table 1). We believe this is consistent with the phenomenon of chronic pain from pancreatitis as described by Cioffi et al and Morgan et al. [20,24]. Patient A did present with a bout of pancreatitis in the setting of elevated amylase and lipase. CT at the time demonstrated no restricture, therefore we feel the isolated episode was secondary to lifestyle as opposed to our intervention. Additionally, Patient B experienced an isolated rise in AST/ALT elevations secondary to independent liver pathology diagnosed before the BPS intervention. Therefore, the complications on long-term follow-up do not appear attributable to the BPS procedure or the result of a restructure. Although chronic pancreatitis is a risk factor for repeat stenosis, we did not find this in our patients undergoing BPD [24]. Given numerous confounders we must consider the possibility that patient A’s pancreatitis was secondary to stricture. In this theoretic case, our restricture rate would be 1 in 7 (14%) which is comparable to the reported rate of 5-15% that modern surgical approaches report [1-6].
The immediate follow-up was notable only for altered laboratory parameters reflective of surgical intervention such as leukocytosis (Table 1) [28]. One PJS patient (F) developed isolated elevated amylase and lipase secondary to pancreatic manipulation. Four patients (B, C, E, G) with various insults to the biliary tree experienced ALP elevation [29]. Patients B and E went on to develop concomitant total bilirubin/ALP/AST/ALT elevations secondary to liver wedge biopsy and cholangitis, respectively. Although patient E’s cholangitis raises concern for BPS technique related infection patient E experienced preoperative instrumentation of the biliary tract, which is a known risk factor for development of bacteremia [30].
We defined success of the BPS procedure as (1) no increased risk of life-threatening complication and (2) no evidence of restricturing or complications attributable to restricturing in long-term follow-up. To the best of our knowledge, no postoperative surgical, percutaneous or endoscopic interventions were needed on any patient and no complication required intervention other than antibiotics and supportive care. We believe our complications were non-specific manifestations of well described hepatobiliary surgical complications. Additionally, the cohort was subdivided into HJS and PJS groups to better understand if stricture locale has any bearing on outcomes. The groups are comparable, but not perfectly matched in overall demographic and preoperative risk factors (Tables 3 and 4). Charlson Comorbidity Index differed significantly due to an outlier of 13 in the dataset. When accounted for, the scores dropped to 1 and 1.83 in the HJS and PJS groups, thereby reflecting the four patients in the PJS group with preoperative chronic pancreatitis. Analysis of the patient’s histories and preoperative risk showed immediate rises in ALP, AST, ALT attributable to preoperative stenting and intraoperative maneuvers, which was constant across PJS and HJS groups. Cholangitis, however, was not constant across groups and could not be definitely ruled out as a complication of our surgical intervention in the HJS group. Because neither group saw long term complications, stricture locale did not appear to influence the results of BPS.
| General Characteristics | |||||||
| Overall | HJS | PJS | |||||
| Age | 49.7 ± 12.5 | 54.3 ± 17.8 | 50.4 ± 11.6 | ||||
| Race | Caucasian | 6 | 85.7% | 3 | 100% | 4 | 80% |
| African American | 1 | 14.3% | 0 | 0% | 1 | 20% | |
| Gender | Male | 3 | 42.9% | 1 | 33% | 2 | 40% |
| Female | 4 | 57.1% | 2 | 67% | 3 | 60% | |
| Time to Stricture (days) | 1435 ± 1119 | 2288.7 ±1306.2 | 1373±1321 | ||||
| Initial Pathology (leading to operation, then stricture) | Pancreatitis | 4 | 57.1% | 1 | 0.3333 | 4 | 80% |
| Choledochal Cyst | 1 | 14.3% | 0 | 0 | 1 | 20% | |
| Ampullary Carcinoma | 1 | 14.3% | 1 | 0.3333 | 0 | 0% | |
| Papillary Mucinous Adenoma | 1 | 14.3% | 0 | 0 | 1 | 20% | |
| Benign Head Mass | 1 | 14.3% | 1 | 0.3333 | 0 | 0% | |
Table 3: General characteristics of our entire study cohort and PJS, HJS groups individually.
Outside of notably reducing restructure rate, our procedure has several advantages. Due to the open nature of BPS, it can be employed in surgically altered anatomy that is difficult to navigate for even the most experienced endoscopist [5]. BPS can be used in situations where re-do HJ or PJ is not possible whether this is due to increased fibrosis or other operative difficulties. BPS is easier to perform in comparison to redoing a biliary or pancreatic anastomosis and dissecting out the previous anastomosis. By offering an alternative to re-anastomosis BPS also appears to decrease the known risk of restricture which increases with every re-do hepaticojejunostomy [22]. Theoretical risks of the procedure are similar to redoing the anastomoses.
Our study is subject to several limitations. As a retrospective chart review, we lack controls and are limited in our data collection ability. We assumed that if the patient did not follow-up, have scans, or labs updated in the computer they had no restricture, which is not a certainty. As we are limited only to our records, we cannot guarantee these patients received interventions or imaging proving restricture at other institutions. Other notable limitations include our small sample size, inability to comment on location of the stricture in the biliary tree, and limitation to only benign HJS, PJS.
In summary, we report the novel surgical approach to benign HJS and PJS with significant advantages over standard surgical corrections of these complicated problems. Though our study has limitations, our scope was to report a technique we hope becomes a valuable tool in the hepatobiliary surgeon’s arsenal. Future, prospective studies are warranted in order confirms the advantages of our technique over standards currently used by surgeons.
Results
Mean age of our study cohort was 49.7 ± 12.5 years old with 6 Caucasians and one African American (Table 1) of seven total patients, there were 4 females and 3 males. Average time to stricture was 1435 ± 1119 days (Table 4). All patients experienced HJS and/or PJS secondary to a Whipple procedure. Initial pathology leading up to the Whipple procedure was chronic pancreatitis in 4 patients; one choledochal cyst, one ampullary carcinoma, one papillary mucinous adenoma, and one benign pancreatic head mass (Table 3). One patient (G) with chronic pancreatitis alone experienced both HJS and PJS and counted in both categories. 5 of 7 (71%) patients had a stent sometime before their operation with an average duration of 508 days (Table 4). Only 3 of 7 (43%) patients had a stent at the time of operation, 2 of 3 were instances where their stent was left in place (Table 4). Patient G declined planned biliary stent placement. 1 of 3 preoperative stents occurred in patient B, a PJS patient with an irretrievable pancreatic stent. Notably, patient a likely had stenting and prior interventions in her history. Due to limited records from an outside hospital, we lack the evidence to substantiate that claim. The average Charlson Comorbidity Index was 3.4, indicating relatively high comorbidity. However, if an outlier value of 13 was eliminated the average dropped to 1.83. Smoking was the most prevalent preoperative risk factor, with 3 of 7 (43%) patients being smokers (Table 4). Patients were followed 1811 ± 1000 days after surgery (Table 2). 6 of 7 (85%) had an intraabdominal blake drain placed. We expect the remaining patient (A) also had this, however, were unable to definitively affirm the suspicion for the aforementioned reason. In the immediate postoperative period, 7 of 7 (100%) of patients showed mild leukocytosis though only 3 of 7 (43%) patients (B, C, E) had ALP elevations (Table 1 and 2). Patient E experienced cholangitis, and by extension, bacteremia, elevated total bilirubin, AST, ALT, ALP. Patient F experienced elevated lipase and amylase, while 2 of 7 (28.5%) patients (C, E) experienced elevated AST, ALT and ALP. Long term, 2 of 7 (28.5%) patients experienced pancreatitis. On closer examination of these two patients, patient A experienced pancreatitis confirmed by lipase and amylase as well as bouts of chronic pancreatitis occurring the setting of unremarkable lipase and amylase (Table 1). Patient B experienced only chronic pancreatitis in the setting of unremarkable lipase and amylase levels. No postoperative surgical, percutaneous or endoscopic interventions were needed on any patient. No patient experienced complications requiring intervention other than antibiotics and supportive care.
| Preoperative Risk Factors | ||||||
| Overall | HJS | PJS | ||||
| Diabetes | 2 | 28.6% | 1 | 33.3% | 1 | 20.0% |
| Smoking | 3 | 42.9% | 1 | 33.3% | 2 | 40.0% |
| Heart Disease | 2 | 28.6% | 1 | 33.3% | 1 | 20.0% |
| BMI >35 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Charlson Comorbidity Index | 3.43 | 5.00 | 1.83 | |||
| Benign Tumor | 2 | 28.6% | 1 | 33.3% | 1 | 20.0% |
| Albumin | 3.5 | 3.27 | 3.62 | |||
| Elevated LFT | 5 | 71.4% | 3 | 100.0% | 4 | 80.0% |
| Preoperative Jaundice | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Elevated CA 19-9 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Biliary Duct >6mm | 2 | 28.6% | 2 | 66.7% | 1 | 20.0% |
| Preoperative Stent | 3 | 42.9% | 2 | 66.7% | 1 | 20.0% |
| Preoperative Stent duration | 508 | 172.5 | 843.5 | |||
| Previous Intervention | 5 | 71.4% | 3 | 100.0% | 3 | 60.0% |
| PTBD | 2 | 28.6% | 2 | 66.7% | 1 | 20.0% |
| Endoscopic dilation | 1 | 14.3% | 1 | 33.3% | 2 | 40.0% |
| Any stent | 5 | 71.4% | 3 | 100.0% | 3 | 60.0% |
Table 4: Incidence of perioperative risk factors entire study cohort and PJS, HJS groups individually
HJS group
Three patients (C, E, G), two Caucasian females and one Caucasian male compose the HJS group (Tables 1 & 3). Average age was 54.3 ± 17.8 years old while time to stricture was 2288.7 ± 1306.2 days (Table 3). Initial pathologies included ampullary carcinoma, chronic pancreatitis, and benign pancreatic head mass. No patient experienced more than one initial pathology. 3 of 3(100%) patients had some prior intervention to the biliary tree (Tables 1 & 4). Only 2 of 3 (67%) were stented at the time of operation as patient G declined any non-surgical intervention (Table 1). The average Charlson Comorbidity Index of 5 was higher than our overall cohort average. However, this number is skewed due to an outlier of 13 in the dataset. Discounting the outlier, the average Charlson Comorbidity Index is 1. Postoperatively, 3 of 3 (100%) of patients received an abdominal drain, experienced leukocytosis, and ALP elevations. Patients C and E both underwent biliary ductal dilatation to clear fibrotic areas around preoperatively placed biliary stents. Patient E went on to experience total bilirubin/ ALP/AST/ALT elevation and bacteremia consistent with cholangitis resolving on a course of antibiotics. No long-term complications were noted in any member of this group.
PJS group
Five patients (A, B, D, F, G), 4 Caucasian individuals and one African American individual compose the HJS group (Tables 1 & 3). Of the five total patients, there were two females and three with an average age of 50.4 ± 11.6 years old. 2 in 5 (40%) patients were smokers (Table 3). Average time to stricture was 1373 ± 1321 years. Initial pathology was chronic pancreatitis in 4 patients. Patient G suffered chronic pancreatitis secondary to a papillary mucinous adenoma in the head of the pancreas. Patient F suffered solely from choledochal cyst. 3 of 5 (60%) of patients had prior endoscopic intervention. Only 1 of 5 (20%) patients (B) was preoperatively stented, since all of these patients underwent Roux-en-Y reconstruction during Whipple procedures. To a degree, this was unintentional as the stent was not retrievable prior or intra-operatively. The average Charlson Comorbidity Index of 1.83 was lower than the cohort average. Postoperatively, 4 of 5 (80%) of patients had intraabdominal blake drain left in place (Table 2). We believe to true number is 5 of 5, however, we were unable to confirm patient A’s record of drain placement. All patients experienced leukocytosis (Table 3). Patients B, F, G all demonstrate isolated hepatic and/or pancreatic enzyme elevations reflective of their interventions as described below: Patient B underwent intraoperative liver biopsy and subsequently developed postoperative AST, ALT, and ALP elevations. Patient F experienced intraoperative pancreatic manipulation secondary to a large collection of debris in the pancreatic head and subsequently demonstrated an isolated rise in amylase and lipase. Patient G demonstrated an isolated rise in ALP, coinciding with intraoperative manipulation of the biliary tree due to retrieval of foreign body. Long term, only 2 of 7 (28%) patients (A & B) experienced pancreatitis. Both patients, patients A and B, had a documented history of chronic pancreatitis (Table 1). Patient A suffered drug abuse, smoking, and multiple psychiatric comorbidities. Patient A had bouts of pancreatitis confirmed by elevated lipase and amylase levels as well as bouts occurring in the setting of unremarkable amylase and lipase levels. Patient B experienced bouts of chronic pancreatitis solely in the setting of unremarkable lipase and amylase levels and isolated AST/ALT elevations. This risk directly corresponds to patient B’s known liver pathology diagnosed before operation. Multiple imaging modalities (MRI, CT, ERCP, MRCP) ranging from one to seven years after the operation failed to reveal stricture for both patients A and B. In addition to the surgeon involved in their care, the scans were also ordered by independent internal medicine providers.
References
- Zhu JQ, Li XL, Kou JT, Dong HM, Liu HY, et al. (2017) Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment. Hepatobiliary Pancreat Dis Int: HBPD INT 16: 412-417.
- Davids PH, Tanka AK, Rauws EA, van Gulik TM, van Leeuwen DJ, et al. Â (1993) Benign biliary strictures. Surgery or endoscopy? Ann Surg217: 237-243.
- Dimou FM, Adhikari D, Mehta HB, Olino K, Riall TS, et al. (2016) Incidence of Hepaticojejunostomy Stricture Following Hepaticojejunostomy. Surgery 160: 691-698.
- Lillemoe KD, Melton GB, Cameron JL, Pitt HA, Campbell KA, et al. (2000) Postoperative bile duct strictures: management and outcome in the 1990s. Annals of Surgery 232: 430-441.
- Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, et al. (1996) The long-term outcome of hepaticojejunostomy in the treatment of benign bile duct strictures. Annals of Surgery 224: 162-167.
- Kapoor BS, Mauri G, Lorenz JM (2018) Management of Biliary Strictures: State-of-the-Art Review. Radiology 289: 590-603.
- Chok KS, Lo CM (2014) Prevention and management of biliary anastomotic stricture in right-lobe living-donor liver transplantation. J Gastroenterol Hepatol 29: 1756-1763.
- Ghazanfar MA, Soonawalla Z, Silva MA, Reddy S (2018) Management of pancreaticojejunal strictures after pancreaticoduodenectomy: clinical experience and review of literature. ANZ J Surg 88: 626-629.
- Csendes A, Burdiles P, Diaz JC, Maluenda F (1993) Results of Heineke-Mikulicz type choledochoplasty in benign biliary strictures. The American surgeon 59:629-631.
- Okabayashi T, Shima Y, Sumiyoshi T, Sui K, Iwata J, Â et al. (2018) Incidence and Risk Factors of Cholangitis after Hepaticojejunostomy. J Gastrointest Surg 22: 676-683.
- Vos PM, van Beek EJ, Smits NJ, Rauws EA, Gouma DJ, et al. (2000) Percutaneous balloon dilatation for benign hepaticojejunostomy strictures. Abdominal imaging 25: 134-138.
- Lee AY, Gregorius J, Kerlan RK Jr, Gordon RL, Fidelman N (2012) Percutaneous transhepatic balloon dilation of biliary-enteric anastomotic strictures after surgical repair of iatrogenic bile duct injuries. PLoS One 7: e46478.
- Bonnel DH, Fingerhut AL (2012) Percutaneous transhepatic balloon dilatation of benign bilioenteric strictures: long-term results in 110 patients. Am J Surg 203: 675-683.
- Kocher M, Cerna M, Havlik R, Kral V, Gryga A, et al. (2007) Percutaneous treatment of benign bile duct strictures. European journal of radiology 62: 170-174.
- Singh DP, Arora S (2014) Evaluation of biliary enteric anastomosis in benign biliary disorders. Indian J Surg 76: 199-203.
- Elbir OH, Karaman K, Surmelioglu A, Bostanci EB, Akoglu M (2012) The heineke-mikulicz principle for hepaticojejunostomy stricture. Case Rep Surg 2012: 454975.
- Pitt HA, Kaufman SL, Coleman J, White RI, Cameron JL (1989) Benign postoperative biliary strictures. Operate or dilate? Ann Surg 210: 417-427.
- Moris D, Papalampros A, Vailas M, Petrou A, Kontos M, et al. (2016) The Hepaticojejunostomy Technique with Intra-Anastomotic Stent in Biliary Diseases and Its Evolution throughout the Years: A Technical Analysis. Gastroenterology research and practice 2016: 3692096.
- Kikuyama M, Itoi T, Ota Y, Matsumura K, Tsuchiya T, et al. (2011) Therapeutic endoscopy for stenotic pancreatodigestive tract anastomosis after pancreatoduodenectomy (with videos). Gastrointest Endosc 73: 376-382.
- Cioffi JL, McDuffie LA, Roch AM, Zyromski NJ, Ceppa EP, et al. (2016) Pancreaticojejunostomy Stricture After Pancreatoduodenectomy: Outcomes After Operative Revision. J Gastrointest Surg 20: 293-299.
- Tsalis K, Antoniou N, Koukouritaki Z, Patridas D, Sakkas L, et al. (2014) Successful treatment of recurrent cholangitis by constructing a hepaticojejunostomy with long Roux-en-Y limb in a long-term surviving patient after a Whipple procedure for pancreatic adenocarcinoma Am J Case Rep 15: 348-351.
- Varabei A, Arlouski Y, Lagodich N, Arehay V (2018) Minimally invasive treatment of intrahepatic cholangiolithiasis after stricture of hepaticojejunal anastomosis. Wideochirurgia i inne techniki maloinwazyjne = Videosurgery and other miniinvasive techniques 13: 111-115.
- Nakeeb A (2013) Sphincter of Oddi dysfunction: how is it diagnosed? How is it classified? How do we treat it medically, endoscopically, and surgically? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 17: 1557-1558.
- Morgan KA, Fontenot BB, Harvey NR, Adams DB (2010) Revision of anastomotic stenosis after pancreatic head resection for chronic pancreatitis: is it futile? HPB (Oxford) 12: 211-216.
- Dimou FM, Adhikari D, Mehta HB, Olino K, Riall TS, et al. (2016) Incidence of hepaticojejunostomy stricture after hepaticojejunostomy. Surgery 160: 691-698.
- Strasberg SM, Drebin JA, Mokadam NA, Green DW, Jones KL, et al. (2002) Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. JACS 194: 746-760.
- Madura JA, Madura JA, Sherman S, Lehman GA (2005) Surgical sphincteroplasty in 446 patients. Arch Surg (Chicago, Ill : 1960) 140: 504-512.
- Deirmengian GK, Zmistowski B, Jacovides C, O'Neil J, Parvizi J (2011) Leukocytosis is common after total hip and knee arthroplasty. Clin Orthop Relat Res 469: 3031-3036.
- Siddique A, Kowdley KV (2013) Approach to a patient with elevated serum alkaline phosphatase. Clin Liver Dis 16: 199-229.
- Pisters PW, Hudec WA, Hess KR, Lee JE, Vauthey JN, et al. (2001) Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 234: 47-55.
Citation: Muste J, Lu S, Nigam A (2020) Biliary and Pancreatic Stricturoplasty for Recurrent Stricture after Hepaticojejunostomy and Pancreaticojejunostomy. J Gastrointest Dig Syst 10: 632. DOI: 10.4172/2161-069X.1000632
Copyright: © 2020 Muste J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.