Bilateral Internuclear Ophthalmoplegia, Deficiency and High Dose Administration of Vitamin D in Pediatric-Age Multiple Sclerosis: A Pediatric Case Report and Review of the Literature
Received: 05-Apr-2018 / Accepted Date: 19-Apr-2018 / Published Date: 26-Apr-2018
Abstract
Oculomotor Disorders (OMDs) can be seen as the initial symptoms or during the course of multiple sclerosis (MS). Bilateral internuclear ophthalmoplegia (BINO) is a rare observation as clinical MS onset. BINO and its prognostic value in pediatric-age MS patients are not absolutely known. Vitamin D deficiency is thought to be associated with an increased disease activity in MS and vitamin D is supposed to be a potent immunomodulatory agent. Vitamin D deficiency, its probable effects on the disease pathogenesis, and high dose administration to a pediatric age patient presented with BINO are discussed in this report.
Keywords: Oculomotor disorders; Multiple sclerosis; Bilateral internuclear ophthalmoplegia Vitamin D; Immunomodulatory agent
Introduction
BINO in MS is thought to be related to the demyelination of the medial longitudinal fasciculi (MLF) [1]. BINO, its prognostic value and appropriate time of immunomodulation in this group of pediatric age MS patients are not well known.
Vitamin D deficiency and insufficiency are also thought to be related with MS [2]. Vitamin D treatment, regardless of vitamin D levels may have improving effects on pathophysiological mechanisms of MS. High dose administration of vitamin D for immunomodulation in MS in a pediatric-age patient is discussed in this report.
Case Report
A 15-year-old girl was admitted to the pediatric neurology department with the complaint of blurring of vision, visual confusion and a feeling of unsteadiness for almost two weeks. She had an adduction deficit in the right eye and nystagmus in the left eye on left gaze and an adduction deficit in the left eye and nystagmus in the right eye on right gaze (Video 1). Upward, downward, and primary gaze positions revealed nothing. Her tandem walking was seldom impaired. Her body mass index was: 20.5. Family and medical history revealed nothing.
Infectious parameters, vasculitis markers, and toxicology results revealed nothing. The lumbar puncture revealed that oligoclonal band was positive. The Ig G index was 1.64 (>0.6). The serum 25(OH) vitamin D level was 13 ng/mL (<20 ng/mL) in the deficiency range.
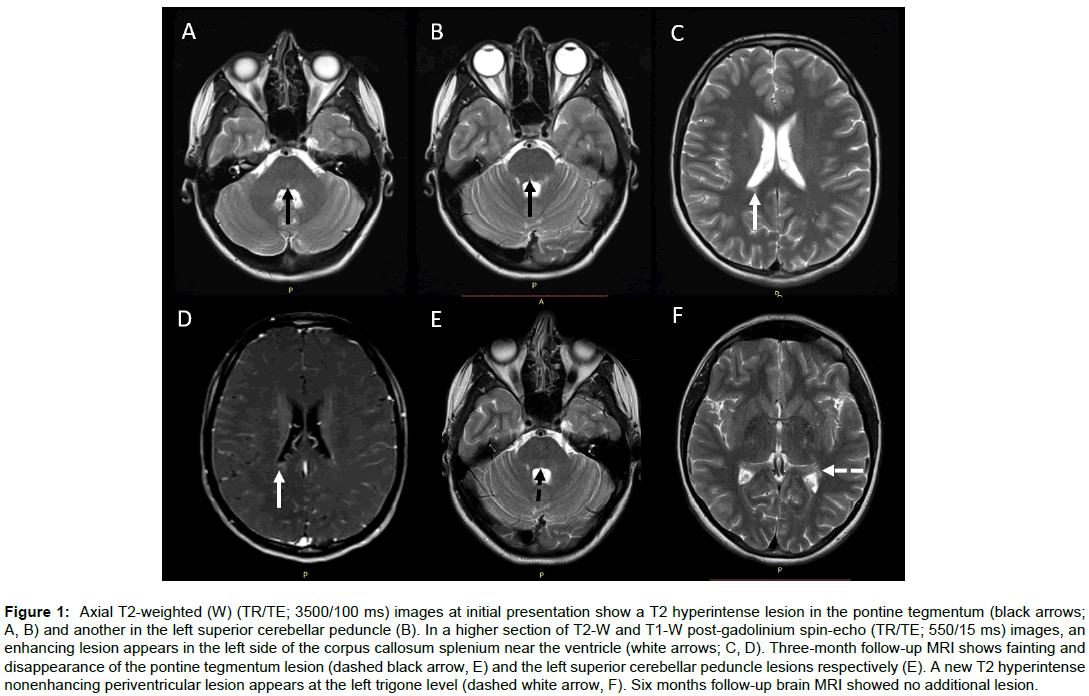
Magnetic resonance imaging (MRI) of the brain revealed T2 hyperintense nonenhancing lesions in the central pontine tegmentum, left superior cerebellar peduncle (Figures 1A and 1B); three deep white matter lesions and one additional enhancing lesion at the right side of the callosum splenium near the lateral ventricle (Figures 1C and D). A cervical and thoracic spine MRI revealed no additional intramedullary lesion. The lesions fulfilled Mc Donald revision criteria for MS regarding dissemination in time and space [3].
Figure 1: Axial T2-weighted (W) (TR/TE; 3500/100 ms) images at initial presentation show a T2 hyperintense lesion in the pontine tegmentum (black arrows; A, B) and another in the left superior cerebellar peduncle (B). In a higher section of T2-W and T1-W post-gadolinium spin-echo (TR/TE; 550/15 ms) images, an enhancing lesion appears in the left side of the corpus callosum splenium near the ventricle (white arrows; C, D). Three-month follow-up MRI shows fainting and disappearance of the pontine tegmentum lesion (dashed black arrow, E) and the left superior cerebellar peduncle lesions respectively (E). A new T2 hyperintense nonenhancing periventricular lesion appears at the left trigone level (dashed white arrow, F). Six months follow-up brain MRI showed no additional lesion.
She received pulse steroid treatment for five days. At the end of the treatment her eye movements were normal. As our patient had vitamin D levels in the deficiency range, she was treated with the dosage of 800 IU/day D3 initially for two months. The checked level of 25(OH) vitamin D level was 12.8 ng/mL. The daily routine usage was strictly advised, and the checked level was 18 ng/mL in the control visit at the third month. This time she was admitted to the pediatric neurology department, with the complaint of feeling of unsteadiness, vomiting and vertigo for 5 days. A follow-up brain MRI in this second attack revealed disappearance of the left superior cerebellar peduncle lesion and fainting of the pontine tegmentum lesion (Figure 1E) with a new T2 hyperintense, but nonenhancing periventricular lesion near the trigone of the lateral ventricle (Figure 1F). She was advised a disease modifying drug (DMD) after this second attack and informed about the adverse effects of DMDs. She and her parents did not accept to use a DMD. A high dose D3 (2000 IU/day) was discussed and started with the consultation of pediatric endocrinology after an informed consent was taken before the treatment.
She has been using high-dose vitamin D for the last 18 months and she had no attacks since then. No adverse effects have been noted. The last measured level of vitamin D was 32 ng/ml. A follow-up at six month of the first and third month of the second episode on high dose vitamin D revealed no new T2 hyperintense or enhancing lesion as no clinical episode. A written informed consent was obtained from the parents of the patient for publication of this case report and accompanying images.
Discussion
MS patients can present with OMDs during the course of the disease or sometimes as an initial symptom [1]. In a study of 150 patients with definitive MS and 13 patients with clinically isolated syndrome, unilateral INO was found at a rate of 14.7% and BINO 4.9%, respectively [4].
Infratentorial MRI lesions were found in 76.8% of patients with OMDs [4], as seen in our patient. In a two year follow-up a group of patients with abnormal eye movements had a progression of EDSS score, supporting that the presence of OMDs may predict disability in MS [5]. A summary of literature about reasons of BINO is shown in Table 1.
| Author | Year of publication | Etiology |
|---|---|---|
| Rismonchi N et al. | 2013 | 3 Boys (11,12,15 years of age) diagnosed with primary CNS tumours as pilomyxoid variant astrocytoma, anaplastic oligoastrocytoma, gliomatosis cerebri |
| Razavi ME et al. | 2009 | CO poisoning in a 8 year old girl |
| Akdal G et al. | 2007 | Vitamin B12 deficiency |
| Ozer F et al. | 2005 | 15 Years old girl in persistant vegetative state due to diffuse axonal injury involving supratentorial and infratentorial structures and the splenium of CC |
| Tsuda H et al. | 2004 | Multiple sclerosis. 49 patients with ocular syptoms. Patients’ age of onset varied from 17 to 51 years |
| Mimenzo-Alvarado AJ et al. | 2002 | SLE vasculitis. Case 2: 19 years old |
| Frohman EM et al. | 2002 | Multiple sclerosis 51/58 patients (88%) of patients had BINO |
| Muthukumar N et al. | 2001 | Trivial head injury 7 years old child presented with BINO following head injury |
| Shin M et al. | 2000 | 3 Years old boy presented with aquaductal cyst causing intracystic high CSF pressure |
| Lagreze W D et al. | 1996 | Mesencephalic midline clefts |
| Kuroiwa T et al. | 1993 | Traffic accident trauma 18 years old boy presented with traumatic bilateral MLF syndrome |
| Mueller C et al. | 1993 | Minor occipital head trauma 6 year old boy presented with minor head trauma |
| Igarashi Y et al. | 1992 | Fisher’s syndrome. 16 years old boy exhibited with BINO |
| Müri RM et al. | 1985 | Multiple sclerosis |
| Zak TA | 1983 | Beningn episodic bilateral juvenile internal ophthalmoplegia. 9 years old girl. |
| Pryszmont M et al. | 1982 | Multiple sclerosis |
| Freeman JW et al. | 1980 | Craniopharyngioma. 15 years old girl |
| Lesser RL et al. | 1979 | Cryptococcal meningitis 17 years old girl with SLE had received prednisone and azathroprine, then devolopped cryptococcal meningitis |
Table 1: Literature reports of pediatric patients with BINO.
Hypovitaminosis D is one of the most popular environmental risk factor for MS [6]. Vitamin D had improving roles on experimental autoimmune encephalomyelitis (EAE) [7]. Epidemiologically latitude, past exposure to sun, infections, smoking and the vitamin D serum level impact the risk of MS [8]. Our patient was a middle school student and her clothing style was available for sun exposure and she was not a smoker. She had no history of documented Epstein-Barr virus infection or parasites. She had only vitamin D deficiency as a triggering risk factor. The internationally accepted norms of vitamin D deficiency is defined as a 25(OH) vitamin D below 20 ng/ml and vitamin D insufficiency as a 25(OH) D of 21-29 ng/ml [9].
There are different vitamin D receptors in many parts of the body [10]; and probably in the MLF. We hypothesize that, hypovitaminosis D in the pathophysiological pathways of BINO in MS may be explained with that there could be vitamin D receptors in the MLF tractus.
Because TEM cells have been suggested to play a pathogenetic role in MS [11,12]; the effect of 1.25(OH)2D3 on T cell memory by culturing peripheral blood mononuclear cells from MS patients regardless of the serum values and healthy controls with vitamin D or vehicles was studied. There was a reduction of the TEM cells in the vitamin D-treated cultures versus the vehicle-treated cultures [13].
The necessity of the routine usage of vitamin D regardless of calculated serum values is not absolutely known.
The appropriate vitamin D doses in MS patients are still confusing [14-16]. We administered 2000 IU/day dosage of vitamin D, firstly advised for a pediatric age MS patient in our center. She had no attack for the last 18 months and no adverse effect was seen. We believe that, if our patient did not use vitamin D for immunomodulation, the frequency and severity of the attacks might be more severe than expected. A review of the literature with high dose administration of vitamin D in multiple sclerosis is shown in Table 2.
| Author | Year of publication | Dosage and duration |
|---|---|---|
| Ashtari F et al. | 2016 | 50.000 IU D3/every 5 days/3 months |
| Sotirchos ES et al. | 2016 | 10.400 IU D3/daily/6 months |
| Rosjo E et al. | 2015 | 20.000IU D3/week/96 weeks |
| Toghionifor N et al. | 2015 | 50.000 IU D3/every 5 days/12 weeks |
| Bhargava P et al. | 2014 | 50.000 IU D3/daily/96 weeks |
| Nystad AE et al. | 2014 | Cuprizone model Intraperitoneal 0.2 µg intraperitoneal calcitriol for 3 weeks |
| Golan D et al. | 2013 | 4370 IU D3/daily/1 year |
| Steffensen LH et al. | 2013 | 20.000 IU D3/weekly/96 weeks |
| Van Amerongen BM et al. | 2012 | 6000 IU D3/daily/10 year |
| Dörr J et al. | 2012 | 10.200 IU D3/daily/18 months |
| Knippenberg S et al. | 2011 | 20.000 IU D3/daily/12 weeks |
| Kimball SM et al. | 2011 | 4000-40.000 IU D3/daily/over 28 weeks followed by 10.000 IU/daily/12 weeks |
| Burton JM et al. | 2010 | 4000-40.000 IU D3/daily/over 28 weeks followed by 10.000 IU/daily/12 weeks |
| Hiremath GS et al. | 2009 | 50.000 IU/daily/7-10 days followed by 50.000 IU/weekly or biweekly for three months |
Table 2: Literature reports of high dose administration of vitamin D in multiple sclerosis.
Conclusion
BINO is a rare and can be the only clinical sign of MS onset. As in general, BINO is believed to be a high prognostic risk factor in the course of MS; high dose administration of vitamin D3 with the dosage of 2000 IU/daily in pediatric age is safe and may have improving roles on decreasing the frequency of relapses and severity of the disease.
Acknowledgement
The authors are thankful to the patient and her family.
Conflict of Interest
None.
Informed Consent
All procedures followed were in accordance with local ethic committee in our institutions.
Animal Rights
This study does not contain any studies with animal subjects performed by the any of the authors.
Author Contributions
Dr. Genç Sel worked in the clinic, evaluated the patient, gathered the data, wrote and coordinated the manuscript. Dr. Altıaylık Özer contributed effort to ophthalmological evaluation of the patient. Dr. Aksoy and and Dr. Dedeoğlu worked in the clinic. Dr. Savaş Erdeve contributed effort to endocrinological evaluation of the patient. Dr. Oğuz evaluated the serial neuroimaging findings of the patient.
References
- Frohman FM, Frohman TC, Zee DS, McColl R, Galetta S(2005) The neuro-ophthalmology of multiple sclerosis. Lancet Neurol 4: 111-121.
- Pierrot-Deseilligny C (2009) Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol 256: 1468-1479.
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen J A, et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292-302.
- Servillo G, Renard D, Taieb G, Labauge P, Bastide S, et al (2014) Bedside tested ocular motor disorders in multiple sclerosis patients. Mult Scler Int 2014: 732329.
- Derwenskus J, Rucker JC, Serra A, Stahl JS, Downey DL, et al. (2005) Abnormal eye movements predict disability in MS: Two-year follow up. Ann N Y Acad Sci 1039: 521-523.
- Ascherio A, Munger K (2008) Epidemiology of multiple sclerosis: From risk factors to prevention. Semin Neurol 28: 17-28.
- Zhen C, Feng X, Li Z, Wang Y, Li B, et al. (2015) Suppression of murine experimental autoimmune encephalomyelitis development by 1, 25-dihydroxyvitamin D3 with autophagy modulation. J Neuroimmunol 280: 1-7
- Hayes CE, Donald Acheson E (2008) A unifying multiple sclerosis etiology linking virus infection, sunlight and vitamin D, through viral interleukin-10. Med Hypotheses 71: 85-90.
- Holick M, Binkley N, Bischoff- Ferrari H, Hanley DA, Heaney R P, et al. (2011) Evolution, treatment, and prevention of vitamin D deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96: 1911-1930.
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F, et al. (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14: 164-174.
- Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, et al. (2004) Expression of CCR7 in multiple sclerosis: Implications for CNS immunity. Ann Neurol 55: 627–638.
- Bhargava P, Gocke A, Calabresi P (2015) 1, 25-Dihydroxyvitamin D3 impairs the differentiation of effector memory T cells in vitro in multiple sclerosis in patients and healthy controls. JNeuroimmunol 279: 20-24.
- Goldberg P, Fleming MC, Picard EH (1986) Multiple sclerosis: Decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses 21: 193-200.
- Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT (2003) Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol 134: 128-134.
- Kimball SM, Ursell MR, O’Connor P, Vieth R (2007) Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr 86: 645-651.
Citation: Genç Sel C, Altıaylık Ozer P, Savaş Erdeve S, Aksoy E, Dedeoğlu O, et al. (2018) Bilateral Internuclear Ophthalmoplegia, Deficiency and High Dose Administration of Vitamin D in Pediatric-Age Multiple Sclerosis: A Pediatric Case Report and Review of the Literature. Neurol Clin Therapeut J 2: 108.
Copyright: © 2018 Genç Sel C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 3151
- [From(publication date): 0-2018 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2369
- PDF downloads: 782