Research Article Open Access
Beta Endorphin and Alcohol Urge Responses in Alcoholic Patients Following an Acute Bout of Exercise
Athanasios Z Jamurtas1,2*, Nikos Zourbanos1, Kalliopi Georgakouli1,2, Panagiotis Georgoulias3, Eirini Manthou3, Ioannis G Fatouros4, Marios Goudas1, Yiannis Koutedakis1,2,5 and Yannis Theodorakis1
1Department of Physical Education and Sport Science, University of Thessaly, Karies, Trikala 42100, Greece
2Department of Kinesiology, Institute for Research and Technology - Thessaly, Greece
3Department of Medicine, University of Thessaly, Larissa 41110, Greece
4Department of Physical Education and Sport Science, University of Thrace, Komotini 69100, Greece
5School of Sports, Performing Arts and Leisure, University of Wolverhampton, UK
- Corresponding Author:
- Athanasios Z Jamurtas
Department of Physical Education & Sport Science
University of Thessaly, Karies, Trikala 42100, Greece
Phone: +30 24310 47054
Fax: +30 24310 47054
E-mail: ajamurt@pe.uth.gr
Received date: July 09, 2014; Accepted date: September 29, 2014; Published date: October 05, 2014
Citation: Jamurtas AZ, Zourbanos N, Georgakouli K, Georgoulias P, Manthou E, et al. (2014) Beta Endorphin and Alcohol Urge Responses in Alcoholic Patients Following an Acute Bout of Exercise. J Addict Res Ther 5:194. doi:10.4172/2155-6105.1000194
Copyright: © 2014 Jamurtas AZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Objective: To examine the effects of low intensity exercise on beta endorphin (β-Ε) levels and urge for alcohol in
alcoholic patients.
Methods: Nine alcoholic patients (M= 41.2 + 6.7 yrs) and 9 healthy controls (M=38.2 + 10.7 yrs) exercised for 30
minutes at a low intensity (61.1 + 4.9 % of their maximum heart rate). Blood was collected prior to and immediately
following exercise and was analyzed for a complete blood count (CBC), β-E and lactic acid. Furthermore, an alcohol
urge questionnaire was filled by the subjects prior to and immediately following exercise.
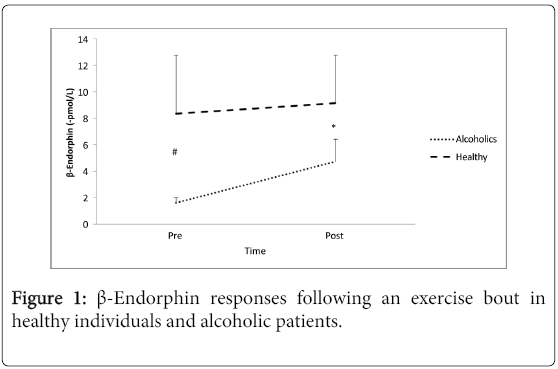
Results: Data analysis revealedthat β-E levels were significantly lower in alcoholic patients whereas exercise
resulted in significant (p< .001) increases in β-Ε (pre: 1.57 + 0.39 pmol/L, post: 4.8 + 1.6 pmol/L) only in alcoholic
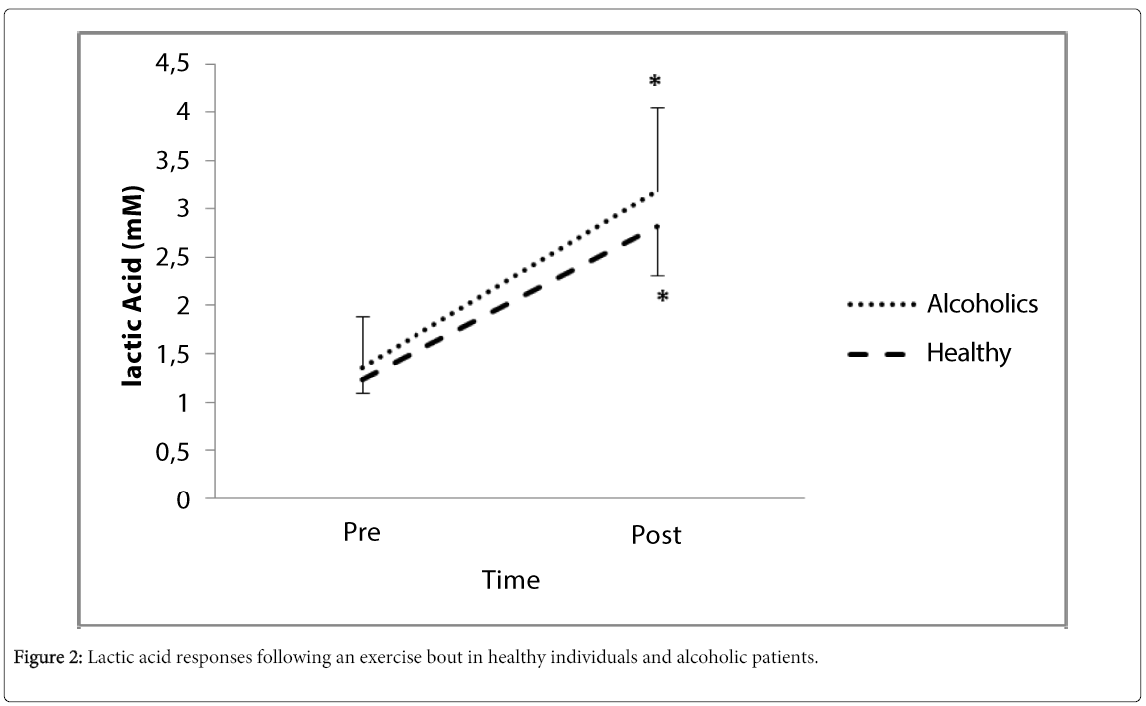
patients. Lactic acid increased significantly in both groups. There was a 17% decrease in alcohol urge in alcoholic
patients without however this difference being significant. No differences in the parameters assessed through the
CBC were seen between the two groups whereas exercise resulted in significant increases in red blood cells,
hemoglobin and hematocrit in both groups.
Conclusion: These results indicate that a bout of low intensity exercise affects the endogenous opioids in
alcoholic patients.
Keywords
Physical activity; Alcoholism; Opioids; Beta endorphin; Addiction; Ethanol
Introduction
Moderate alcohol consumption is associated with improved health [1,2]. However, uncontrolled and excessive alcohol drinking can have negative effects in mental and physical aspects of humans [3]. Alcoholism and alcohol related disorders are a major health concern for all countries and are responsible for approximately 4.5% of all diseases and injuries worldwide, making alcohol abuse a significant cause of death [4].
Alcohol consumption has been reported to influence the activity of the endogenous opioid system [5]. Reports indicate that acute exposure to ethanol leads to an enhanced release of brain β-endorphin (β-E) which through its interaction with μ and δ receptors mediates, at least in part, neurobehavioral effects such as reinforcement and acquisition of ethanol drinking behavior [5]. Specifically, ethanol intake has been shown to increase β-E release by the pituitary and hypothalamus [6], an action that is mediated by the increase of corticotropin releasing hormone [7] in a dose dependent manner [8]. Furthermore, some reports indicate a biphasic effect of ethanol on hypothalamic β-E release [8]. However, the ethanol-induced increase of β-E release is fast and transient, lasting about 15-20 minutes before normalizing again [9]. Besides its effects on pituitary and hypothalamic β-E, ethanol administration enhances β-E release in the nucleus accumbens [10-12], a brain region important for the processes of reward and reinforcement. Therefore, the activation of μ and δ receptors by the increase in β-E levels due to ethanol intake may be pivotal in reinforcing properties of alcohol intake. On the other hand, chronic exposure to ethanol may cause adaptive responses of neuronal systems linked to negative reinforcement. Decreased β-E production following chronic ethanol exposure may be responsible for some of the feelings of discomfort and the presence of negative reinforcement [5]. Reports indicate that chronic ethanol abuse results in lower concentration of β-E in the cerebrospinal fluid and plasma of male and female alcoholics [5,13]. Therefore, chronic ethanol abuse might result in a central opioid deficiency. That deficiency might be related to decreased synthesis and release of β-E in the hypothalamus and pituitary as well as lower density and activity of the opioid receptors.
Relapse remains one of the most significant problems in alcoholic patients and relapse rates range between 60 and 90% [14]. Different treatments have been used in order to combat this significant problem. Marlatt [15] provided a social learning model of the relapse process in addictive disorders and suggested treatment approaches to prevent relapse some of which include lifestyle modifications. One factor of the positive lifestyle modifications that could reduce relapse rates that has not been sufficiently investigated is physical exercise. In fact, exercise provides several advantages as a relapse prevention strategy [16-18]. These advantages include improved health and wellness, the potential to be cost-effective, flexible, and accessible and with minimal side effects compared to pharmacological treatment [19]. Recent reports indicate that pleasure ratings after exercise are higher compared to drinking alcohol in alcohol dependent patients [1]. Furthermore, Zourbanos et al. [20] suggested a theoretical framework of possible relationships between exercise, alcohol urge and β-Elevels indicating the possible use of exercise as an adjunctive strategy. To our knowledge, there is lack of information regarding the effects of acute aerobic exercise on alcohol urge of alcoholic patients [21]. Therefore, the purpose of this study was to examine the effects of an acute aerobic exercise session of moderate intensity on the urge for alcohol and plasma β-Elevels in chronic alcoholics participating in a rehabilitation program at the time of testing.
Methods
Participants
Nine chronic alcoholic patients (8 males and 1 female) undergoing alcohol detoxification were recruited from a psychiatric hospital in Greece and 9 healthy controls volunteered to participate. Patients were diagnosed as being alcohol dependent according to the DSM-IV and the Alcohol Use Disorders Identification Test (AUDIT; [22]). AUDIT consists of 10 questions scored individually from 0 = never to 4 = 4 or more times per week. A total score of > 8 is an indication of alcohol abuse, a score of > 15 indicates serious abuse/addiction whilst a score between 8 and 10 is an indication of being at risk. Cronbach’s alpha coefficient was .73. Alcoholic patients were young and the medical exam revealed no presence of cardiovascular or metabolic disease. However, five patients were receiving antidepressant medicine, five were receiving anticonvulsant medicine (two subject were receiving both) and seven of them were receiving Thiamine, Pyridoxine, Cyanocobalamine (3 times a day) and folic acid (5 mg per day).
Research Design
After gaining institutional ethics approval and obtaining assent and written informed consent, volunteers participated in a single exercise session on a cycle ergometer (Monark Vansbro, Sweden). Exercise in alcoholic patients was administered 10-14 days after hospitalization. The exercise session was performed at 9:00 a.m. Exercise was of low intensity (55-60% of maximum heart rate, MHR= 220-age) and moderate duration (30 minutes). Heart rate was monitored continuously during exercise by short-range telemetry (Sports Tester PE 3000, Polar Electro, Kempele, Finland). Blood samples were collected and the alcohol urge questionnaire was administered prior to and immediately following exercise. Blood sampling and fitness evaluation (battery of fitness assessment tests, Table 1) was performed before and after exercise.
| Variable | Healthy | Alcoholics |
|---|---|---|
| Age (yrs) | 38.2 + 10.7 | 41.2 + 6.7 |
| Weight (kg) | 73.2 + 11.9 | 75.0 + 12.9 |
| Height (cm) | 170.3 + 8.7 | 171.0 + 6.7 |
| % Body Fat | 21.3 + 9.8 | 20.3 + 12.0 |
| Flexibility (cm) | 18.8 + 8.1 | 17.8 + 10.1 |
| Push-ups | 16.1 + 8.0 | 14.3 + 6.0 |
| Sit-ups | 15.3+ 4.3 | 17.3+ 3.3 |
| Exercise Heart rate (bpm) | 114.1 + 21.4 | 110.5 + 18.4 |
| AUDIT score | 0.0 + 0.0 | 28.6 + 6.4 |
Table 1: Anthropometric and physiologic characteristics (mean+SD) of the subjects.
Alcohol Urge Questionnaire
The Alcohol Urge Questionnaire [23]; 1 = strongly disagree, 7 = strongly agree) was assessed twice to examine alcohol urges. In completing the questionnaire it was emphasized to respondents that they should describe their current feelings based on the instructions that were provided to them, not as they wished to feel in the future. Participants were informed that there were no right or wrong answers and that their individual responses would remain anonymous and confidential. Cronbach’s alpha coefficient before exercise was 0.68 and immediately after exercise was 0.70.
β-E Analysis
Venous blood samples were collected in tubes containing EDTA and Trasylol® (5000 KIU Trasylol in a 10 ml vacutainer tube). The samples were cooled in an ice-bath immediately. Plasma was separated by centrifugation at 4°C. Plasma samples were stored at -20° C or lower until assayed. Plasma levels of β-E were measured using a human RIA (Radioimunoassay) diagnostic kit (KIPERB301), produced by DIASource Europe SA (Belgium) for human plasma β-E with negligible cross reactivity against other human polypeptides (ß-lipotropin, leu-enkephalin, etc.). The inter-assay and intra-assay variation was 7.2% and 7.1%, respectively. RIA (KIPERB301) kits were calibrated against valid international standards. The radiotracer used in all kits was 125Iodine (I-125, half-life T1/2 60 days, 35.5 keV gamma rays, 27-32 keV x-rays, no beta radiation). All sample assays were performed in duplicate. If a difference between duplicate results of a sample was more than 7%, the measurement was repeated. The in-run coefficient of variation for β-E was 5.7%. An automatic gamma counter (type: Cobra II/5010, company: Packard, USA) was used to count the radioactivity and calculate the results.
Lactic Acid and Complete Blood Count
Whole blood lactic acid concentration was determined spectrophotometrically (Dr Lange LP 20, Berlin, Germany). An automated hematological analyzer (Mythic 18, Orphee, Switzerland) was used to analyze for the complete blood count (CBC).
Statistical analysis
A 2 x 2 (group x time) repeated measures analysis of variance was used to analyse the data. If a significant interaction was obtained, pairwise comparisons were conducted using the Bonferroni test method. A Pearson correlation was performed to assess a possible correlation between changes in β-E and urge for alcohol. The significance level was set at p<0.05. The statistical program used for all analyses was SPSS version 15.0 (SPSS Inc., USA).
Results
Anthropometric and physiologic characteristics of the subjects appear in Table 1. All patients had a history of addiction of 10 years or more.All subjects were able to complete a 30-minute workout and the mean relative exercise heart rate was 61.1 + 4.9 % and 62.2 + 3.5% of their maximum heart rate for the alcoholic patients and healthy controls, respectively. β-E levels were significantly lower (p<0.001) in alcoholic patients whereas exercise resulted in significant increases (p<0.001, Cohen’s D: 3.31) only in the alcoholic group (Figure 1). Lactic acid at baseline was not significantly different between groups and increased significantly (p<0.001) after exercise in both groups (Figure 2). Analysis for CBC parameters revealed a significant time effect for red blood cells, hemoglobin and hematocrit. None of the remaining parameters was significantly different between groups nor was changed due to exercise (Table 2).
| Healthy | Alcoholics | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| WBC (X103/μL) | 6.8 ± 1.1 | 7.7 ± 1.4 | 8.7 ± 1.9 | 8.8 ± 1.9 |
| LYM (%) | 32.2 ± 5.3 | 35.1 ± 7.3 | 27.93 ± 5.45 | 26.4 ± 4.9 |
| MON (%) | 11.2 ± 3.6 | 11.1 ± 4.0 | 9.04 ± 2.46 | 9.6 ±2.8 |
| GRA (%) | 56.6 ± 6.7 | 53.7 ± 8.5 | 63.01 ± 5.34 | 64.0 ±5.4 |
| RBC (x106/μL) | 4.6 ± 0.4 | 4.8 ± 0.3* | 4.32 ± 0.43 | 4.4 ± 0.4* |
| HGB (g/dL) | 13.6 ± 0.9 | 14.2 ±0.9* | 14.46 ± 1.11 | 14.8 ± 1.2* |
| HCT (%) | 41.5 ± 2.3 | 43.6 ± 2.3* | 42.2 ± 3.42 | 43.0 ± 3.4* |
| MCV (fl) | 90.5 ± 3.0 | 90.5 ± 3.2 | 97.7 ± 2.8 | 97.6 ± 29.1 |
| MCH (pg) | 29.7 ± 1.4 | 29.5 ± 1.2 | 33.5 ± 1.1 | 33.3 ± 35.3 |
| MCHC (%) | 32.9 ± 1.3 | 32.7 ± 1.5 | 34.2 ± 0.6 | 34.1 ± 0.6 |
| RDW (%) | 11.8 ± 0.6 | 11.7 ± 0.7 | 13.8 ± 1.3 | 13.8 ± 11.5 |
| PLT (X103/μL) | 263.9 ± 68.3 | 247.4 ± 110.0 | 240.89 ± 54.89 | 251. 6 ± 54.0 |
| MPV (fl) | 8.1 ± 0.8 | 8.2 ± 0.7 | 9.1 ± 1.2 | 9.3 ± 1.6 |
*Sig vs. pre at the same group. WBC: white blood cells, LYM: lymphocytes, MON: monocytes, GRA: granulocytes, RBC: red blood cells, HGB: hemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, RDW: red blood cell distribution width, PLT: platelets, MPV: mean platelet volume.
Table 2: Anthropometric and physiologic characteristics (mean+SD) of the subjects.
Pearson correlation analysis revealed non-significant relationships between β-E and urge for alcohol (r = 0.23, p = 0.58). Finally, results revealed no significant changes in scores for alcohol urge in alcoholic patients (pre: 2.3 + 1.17; post: 1.87 + 1.17).
Discussion
This is the first study, to our knowledge, that assessed concurrently changes in β-E and alcohol urge following an acute bout of exercise in alcoholic patients under treatment. Even though a significant increase in this opioid peptide was found, no significant change in alcohol urge was evident.
Exercise has been suggested as an alternative approach in the treatment of alcoholism [e.g., 16,17]. However, there has been limited research on exercise as an adjunctive strategy in alcohol abuse treatment programs. We were able to find only one study that examined the acute effects of exercise on alcohol urge. The results from that study indicated that an acute bout of short duration (10 minutes) and moderate intensity exercise results in significant decrease in alcohol urge compared to an equal duration but of light intensity exercise when the alcohol urge was assessed immediately following exercise [21]. However, no difference was observed between the two conditions when alcohol urge was assessed 5 and 10 minutes post-exercise. These results are in contrast with results from this study where no significant changes in alcohol urge, assessed immediately post-exercise, were observed. However, taking a closer look at the results from the two studies it can be seen that the percent reduction in alcohol urge in Ussher et al. [21] study was approximately 17% and in the current study was close to 19%. The low number of subjects in this study could be a reason for the lack of statistical significance in the decline of alcohol urge. Furthermore, it has to be pointed out that our subjects were hospitalized patients and the initial alcohol urge ratings were low. Higher initial alcohol urge ratings probably would lead to a greater alcohol urge decline.
On the other hand a few attempts have been made to elucidate the effects of a chronic exercise on alcohol urge, alcohol consumption and abstinence from alcohol [e.g. 24-26]. Mixed results have been reported even though all of them indicate an improvement in fitness status. In this study, 30 minutes of aerobic exercise resulted in a 3-fold increase in β-E levels. To our opinion there are two possible explanations for this response. The first one relates to the increased levels of lactic acid during exercise and the second one to the low initial β-E levels. Previous research suggests that increased intensity of exercise and its associated acidity is related to changes in plasma β-E levels [27-29]. Lactic acid levels increased by two-fold towards the end of exercise in this study implicating elevated exercise-induced acidity. In a previous report, β-E was highly correlated with markers of acidosis (pH, PCO2, HCO3, base excess, lactate) during an incremental exercise protocol whereas treatment with alkaline buffer that maintained pH above 7.40 resulted in a significant suppression of β-E release [29]. These results support the notion that acidosis may mediate the exercise-induced increase of β-E release. Therefore, we may assume that in this study exercise resulted in increased acidity which in turn caused an elevation in β-E levels. The second explanation for the 3-fold increase in β-E levels relates to its initial low levels. It has been shown that β-E levels are lower in the plasma [13] and cerebrospinal fluid [30] of alcoholic patients. There is experimental evidence suggesting that untreated chronic alcoholics may exhibit attenuated synthesis of β-E in hypothalamus and pituitary gland, leading to a central opioid deficiency [5]. Therefore, it may be proposed that exercise, acting as a strong stimulant of β-E synthesis, may play a significant role by partially correcting this central opioid deficiency thereby leading to attenuation of ethanol intake by exercising alcoholics. This proposal merits further investigation.
Although previous research suggests that CBC parameters may differ in alcoholic patients as compared to healthy controls [31], this was not evident in the present study. This finding may be attributed to a good general health status of our patients at the point of assessment. This is further supported by a lack of differences in the fitness parameters that were used to assess participants' fitness level. We were unable to find another study that assessed CBC responses of alcoholic patients following an exercise bout. Fluid shifts might have accounted for the significant increases in hematocrit and hemoglobin following exercise. Fluid intake should be recommended when an alcoholic patient is exercising whereas dehydration levels could be assessed through refractometry. One of the possible limitations of this study could be the sample size. Even though the sample size appears small, it is efficient to detect, and statistically support the desirable effect. Power analysis (GPower 3.0 calculation) suggested that with 8 participants per group and adopting an alpha of .05, the study would be powered at .80 to detect a large, based on Cohen’s recommendation, effect size that was expected.
Physical activity is unique in the sense that is available to people who may not have access to other forms of treatment such as psychological intervention or medication. However, treatment with the implementation of physical activity, as opposed to medication, requires active participation of the individual and not a passive acceptance of treatment. It is of great importance to emphasize that efforts need to be made to identify ways through which an appropriate treatment will be developed to reduce alcoholism relapse rates. It seems that a single approach does not produce significant results and a combination treatment of pharmacotherapy, behavioral, psychological therapy and perhaps exercise training could produce better results. Future research should also look into the delayed effects of exercise on alcohol urge. Finally, the response of other molecules known to be involved in the reward system (i.e. dopamine) may be also altered by exercise. Their assessment following an exercise bout or a training period could delineate pathways involved in exercise-induced attenuation of the relapse rates to alcoholism.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
Appreciation is extended to Professor Christina Gianoulakis for her thoughtful and constructive comments on the paper. This research has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program "Education and Lifelong Learning" of the National Strategic Reference Framework (NSRF) - Research Funding Program: THALES. Investing in knowledge society through the European Social Fund.
References
- O'Brien CP, Gastfriend DR, Forman RF, Schweizer E, Pettinati HM (2011) Long-term opioid blockade and hedonic response: preliminary data from two open-label extension studies with extended-release naltrexone. Am J Addictions 20: 106-112.
- Huang PH, Chen YH, Tsai HY, Chen JS, Wu TC, et al. (2010) Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscl Throm Vas 30: 869-877.
- Caan W, de Belleroche J (2002) Drink, Drugs and Dependence: From Science to Clinical Practice. (1rst edn), Routledge, London.
- World Health Organization (2011) Global status report on alcohol and health. WHO, Italy.
- Gianoulakis C (2004) Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 4: 39-50.
- Thiagarajan AB, Mefford IN, Eskay RL (1989) Single-dose ethanol administration activates the hypothalamic-pituitaryadrenal axis; exploration of the mechanism of action. Neuroendocrinology 50: 427-432
- de Waele JP, Gianoulakis C (1993) Effects of single and repeated exposures to ethanol on hypothalamic beta-endorphin and CRH release by the C57BL/6 and DBA/2 strains of mice. Neuroendocrinology 57: 700-709.
- Gianoulakis C (1990) Characterization of the effect of acute ethanol administration on the release of ß-endorphin peptides by the rat hypothalamus.
- Keith LD, Crabbe JC, Robertson LM, Kendall JW (1986) Ethanol stimulated endorphin and corticotropin secretion in vitro. Brain Res 367: 222-229.
- Jarjour SJ, Bai L, Gianoulakis C (2009) Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alc Clin Exp Res 33: 1033-1043.
- Marinelli PW, Quirion R, Gianoulakis C (2004) An in vivo profile of b-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience 127: 777-784.
- Lam MP, Nurmi H, Rouvinen N, Kiianmaa K, Gianoulakis C (2010) Effects of acute ethanol on b-endorphin release in the nucleus accumbens of selectively bred lines of alcohol-preferring AA and alcohol-avoiding ANA rats. Psychopharmacology 208: 121-130.
- Vescovi PP, Coiro V, Volpi R, Giannini A, Passeri M (1992) Plasma beta-endorphin, but not met-enkephalin levels are abnormal in chronic alcoholics. Alcohol Alcoholism 5: 471-475.
- Miller WR, Walters ST, Bennett ME (2001) How effective is alcoholism treatment in the United States? J Stud Alcohol 62: 211-220.
- Marlatt GA (1985) Lifestyle modification. In: Marlatt GA & Gordon JR (ed) Relapse prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. (1rst edn), Guilford, New York, pp 280-344.
- Donaghy ME, Mutrie N (1999) Is exercise beneficial in the treatment and rehabilitation of the problem drinker? A critical review. Phys Ther Rev 4: 153-166.
- Read JP, Brown RA (2003) The role of physical exercise in alcoholism treatment and recovery. Prof Psychol Res Pr 34: 49-56.
- Donaghy ME, Ussher M (2005) Exercise interventions in drug and alcohol rehabilitation. In: Faulkner G and Taylor A (ed) Exercise as therapy: Emerging relationships. (1rst edn), Routledge, London, pp 48-69.
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, et al. (1998) Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. Am J Psychiat 155: 603-609
- Zourbanos N, Jamourtas AZ, Staveri E, Hatzigeorgiadis A, Theodorakis Y (2011) Physical exercise as strategy in alcohol abuse treatment. Hell J Psychol 8: 123-145.
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC (2004) Acute effect of a brief bout of exercise on alcohol urges. Addiction 99: 1542-1547.
- Moussas G, Dadouti G, Douzenis A, Poulis E, Tzelembis A, et al. (2009) The Alcohol Use Disorders Identification Test (AUDIT): reliability and validity of the Greek version. Ann Gen Psychiat 8: 11. 23.
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19: 600-606.
- Sinyor D, Brown T, Rostant L, Seraganian P (1982) The role of physical fitness program in the treatment of alcoholism. J Stud Alcohol 43: 380-386.
- Murphy TJ, Pagano RR, Marlatt GA (1986) Lifestyle modification with heavy alcohol drinkers: Effects of aerobic exercise and meditation. Addict Behav 11: 175-186.
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, et al. (2009) Aerobic exercise for alcohol recovery: Rationale, program description, and preliminary findings. Behav Modif 33: 220-249.
- Goldfarb AH, Hatfield BD, Armstrong D, Potts J (1990) Plasma beta-endorphin concentration: response to intensity and duration of exercise. Med Sci Sport Exer 22: 241-244.
- Goldfarb AH, Jamurtas AZ (1997) Beta-endorphin response to exercise. An update. Sports Med 24: 8-16.
- Taylor DV, Boyajian JG, James N, Woods D, Chicz-Demet A, et al. (1994) Acidosis stimulates beta-endorphin release during exercise. J Appl Physiol 77: 1913-1918.
- Genazzani AR, Nappi G, Facchinetti F, Mazzella GL, Parrini D, et al. (1982) Central deficiency of beta-endorphin in alcohol addicts. J Clin Endocr Metab 55: 583-586.
- Cylwik B, Naklicki M, Gruszewska E, Szmitkowski M, Chrostek L (2013) The distribution of serum folate concentration and red blood cell indices in alcoholics. J Nutr Sci Vitaminol (Tokyo) 59: 1-8.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 16677
- [From(publication date):
October-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12028
- PDF downloads : 4649