Benzimidazoles - A Promising Lead for Antipyschotic Drug Design
Received: 03-Jun-2022 / Manuscript No. jabt-22-67302 / Editor assigned: 05-Jun-2022 / PreQC No. jabt-22-67302(PQ) / Reviewed: 19-Jun-2022 / QC No. jabt-22-67302 / Revised: 23-Jun-2022 / Manuscript No. jabt-22-67302(R) / Published Date: 30-Jun-2022 DOI: 10.4172/2155-9872.1000466
Abstract
Benzimidazole is outstandingly effective compounds and there are a number of reviews available for biochemical and pharmacological studies which confirmed that these molecules are useful. This review presents a comprehensive overview on selected synthetic routes towards antipsychotic drug containing benzimidazole as published in both journal and patent literature. Owing to the vast number of potential structures, we have concentrated only on antipsychotic drugs containing benzimidazole and focused principally on the assembly of the heterocyclic core. In order to target on benzimidazole this is most representative chemical entities. Researchers around the world are paying greater attention to and showing increasing interests in this field. However, challenges such as drug resistance, relatively little knowledge of structure of receptors and rare convenient methods for synthesis of benzimidazoles still exist. We hope this review will form a comprehensive foundation and reference source that will open up new opportunities for researchers interested in benzimidazole containing antipsychotic agents and drug designing.
Introduction
Benzimidazole is widely known as a biologically active scaffold which possesses a diverse nature of activities. The combination of different pharmacophores in a benzimidazole ring system has led to the formation of more active compounds. It is a benzannulated ring system in which benzene ring is fused with a five member ring system having hetero atom at 1 and 3 positions. The properties of benzimidazole and its analogs have been studied since over hundred years. Benzimidazole analogs are of great significant because of their various clinical applications and biological activity. In recent years, attention has increasingly been given to the synthesis of benzimidazole derivatives. The synthesis of novel benzimidazole derivatives remains a main focus of medicinal research. Benzimidazoles are considered as an optimistic class of bioactive heterocyclic compound that possesses a range of psychotic activity. Recent advances in understanding the pathophysiology of underlying psychotic disorder and subsequent development of new antipsychotic drugs to treat these diseases have altered clinical pharmacological approach. It has also helped researcher to produce a new generation antipsychotic agents which could show better clinical results. Attention has been gradually more given to the synthesis of benzimidazole derivatives as a source of new antipsychotic agents.
Literature review on benzimidazole derivatives as antipsychotic drugs as follows:
Sladjana Dukic et al. reported Synthesis of several substituted phenylpiperazines behaving as mixed D2/5HT1A Ligands and 22 different compounds have been synthesized with the aim of creating new, mixed D2/5HT1A ligands. For this purpose 1 substituted phenylpiperazines attached by the N4 nitrogen to dopaminergic pharmacophores of the 2(5-benzimidazole)ethyl, 2(5-benztriazole)ethyl,2[5-(benzimidazole- 2-thione)]ethyl and 2-[6(1,4dihydroquinoxaline-2,3-dione)]ethyl type were selected according to known structure affinity requirements of 1-arylpiperazines.All the new compounds were evaluated for in vitro binding affinity at the dopamine (D1 and D2) and 5-HT1A receptors [3H]SCH 23390 (D1 selective), [3H]spiperone (D2 selective) and 8-OH-[3H]DPAT (5-HT1A selective) were employed In the 8-OH- [3H]DPAT-displacement assay compounds behaved as moderate competitors and as rather strong competitors; 4-[2-(5-benztriazole) ethyl]-1-(2-methoxyphenyl)piperazine, 2a had the highest binding affinity at the 5-HT1A receptors (Ki = 2.6 nm).Because many antipsychotic and anxiolytic agents behave as mixed dopaminergic and serotonergic ligands, the high affinity of several of these new ligands for binding at both D2 and 5-HT1A receptors make them promising candidates deserving further pharmacological evaluation as antipsychotic or anxiolytic pharmaceuticals [1].
Nisar Ullah reported A series of new 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones have been synthesized and evaluated for dual D2 and 5-HT1Areceptor binding affinities. The synthesized ligands are structurally related to bifeprunox, a potential atypical antipsychotic, having potent D2 receptor antagonist and 5-HT1Areceptor agonist properties. The Suzuki–Miyaura reaction of cyclic vinyl boronate with appropriate aryl halide yielded arylpiperidine, which was eventually transformed to piperidinyl-1Hbenzo[ d]imidazol-2(3H)-one. The reductive aminationof the latter with appropriate biarylaldehdyes rendered the synthesis of 5-piperidinyl- 1H-benzo[d]imidazol-2(3H)-ones. The structure-activity relationship studies showed that cyclopentenylpyridine and cyclopentenylbenzyl groups contribute significantly to the dual D2 and 5-HT1A receptor binding affinities of these compounds [2]. (Figure-2)
Deana Andric et al. synthesized a series of 8 new compounds with halogen atom introduced into the benzimidazole-2-thione dopaminergic pharmacophore of 5-[2-(4-arylpiperazin-1-yl)ethyl]- 1,3-dihydro-2H-benzimidazole-2-thiones with the arylpiperazine part of the molecule being selected according to known structure affinity requirements, have been synthesized. All the new compounds were evaluated for the in vitro binding affinity at the dopamine (DA) D1 and D2 and serotonin 5-HT1A receptors by the competitive radioassays, performed on synaptosomal membranes prepared from fresh bovine caudate nuclei and hippocampi. All the new compounds were strong competitors for the binding of the radioligands to the D2 and 5-HT1A receptors, with the most active of them having 34 and 170 time higher affinity than non-halogenated congeners in the D2DA receptor radioassays Divergently, these compounds were without significant affinities for the D1 DA receptors [3]. (Figure-3)
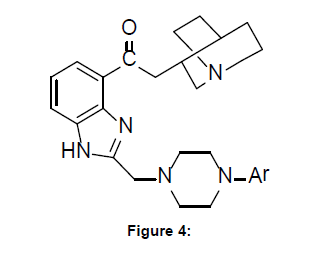
Lopez-Rodriguez et al designed a series of new mixed benzimidazole–arylpiperazine derivatives by incorporating in general structure shown below the pharmacophoric elements of 5-HT1A and 5-HT3 receptors. Compounds thus synthesized were evaluated for binding affinity at both serotoninergic receptors, all of them exhibiting high 5-HT3R affinity, and derivatives with an o-alkoxygroup in the arylpiperazine ring showed nanomolar affinity for the 5-HT1AR. Additionally, all the synthesized compounds were selective over 1-adrenergic and dopamine D2 receptors. These novel mixed 5-HT1A/5-HT3 ligands were also effective in preventing the cognitive deficits induced by muscarinic receptor blockade in a passive avoidance learning test, suggesting a potential interest in the treatment of cognitive dysfunction [4]. (Figure-4)
Mewshaw et al described synthesis several bioisosteric analogs based on the 3-OH-Nl-phenylpiperazine dopamine D2 agonist template. The indolone and 2-CF3-benzimidazole were observed to have excellent affinity for the D2 receptor. Several D4 selective compounds were also identified. A conformational analysis was performed on some compounds in order to rationalize affinity, intrinsic activity, and selectivity. Also based on their recently discovered DA D2 template, they have identified several potent heterocyclic piperazinyl derivatives which were found to have low intrinsic activity [6]. (Figure-5)
Stewart et al described the first synthesis of 1-(1, 2-dihydro-2- acenaphthylenyl) piperazine - a new arylpiperazine. Preliminary binding studies on this new arylpiperazine revealed the affinity for the 5-HT1A and 5-HT2A receptor subtypes. Incorporation of a dopamine pharmacophore onto this arylpiperazine provided a compound which is a potential antipsychotic with an atypical profile. The quest to identify and synthesize a novel arylpiperazine has led to the synthesis of 1,2-dihydro-2-acenaphthylenyl piperazine, synthesized by oxidation of 1,2-dihydroacenaphthylene with red lead in acetic acid to yield the 2-acetoxy compound, which was converted to the crystalline hydroxy compound by saponification. The hydroxy compound underwent a comfortable regio-selective S2 displacement reaction to give 2-bromo- 1,2-dihydro acenaphthylene. Nucleophilic substitution of which using anhydrous piperazine gave the new arylpiperazine. All known and reported arylpiperazines have been established to have serotonergic potential, at least at one of the 5-HT receptor subtypes. Since the new arylpiperazine had a high lipophilicity, their focus was to determine the binding affinities for the compound at the central 5-HT receptors (5-HT1A and 5-HT2A) targeting various CNS disorders, where the dopamine (D2) receptor has also been widely implicated. Introduction of D2 receptor blockade onto this arylpiperazine improved affinity for the 5-HT1A and the 5-HT2A receptor indicating that the strategy of mimicking a phenolic group present in both serotonin and dopamine has succeeded at more than one receptor simultaneously [6]. (Figure-6)
Srinivas et al discovered a series of subtype selective dopamine D4 receptor ligands from the hetroarylmethylphenylpiperazine class have that exhibits a remarkable structure-activity relationship (SAR), revealing a substituent effect in which region substitution on the terminal arylpiperazine ring can modulate functional or intrinsic activity. Other structure-dependent efficacy studies in the dopamine D4 field have suggested a critical interaction of the heteroarylmethyl moiety with specific protein microdomains in controlling intrinsic activity. These studies indicate that for some binding orientations, the phenylpiperazine moiety also plays a key role in determining efficacy. Structural similarity and consistent binding affinities within a set of benzimidazoylmethylarylpiperazine ligands, which display a range of intrinsic activity, indicate these ligands have poor selectivity for high- and low-affinity states of the dopamine D4 receptor. These data support a sequential binding and conformational stabilization model under kinetic control within the cell. Agonists are a class of ligands for small-molecule drug development that possess complex combinations of dissociation constants and kinetic parameters as part of their biochemical profile, both of which has been manipulated, somewhat independently, through SAR strategies [7]. (Figure-7)
Sukalovic et al synthesized 5-[3-(4-Arylpiperazin-1-yl)propyl]- 1H-benzimidazoles and 5-[2-(4-arylpiperazin-1-yl)ethoxy]-1Hbenzimidazoles and their affinity for the D1, D2 and 5-HT1A receptors examined. They expressed a rather high affinity for the D2 dopamine receptor. The main features of ligand–D2 receptor interactions revealed by docking analyses were: salt bridge between piperazine ring protonated N1 and Asp 86, hydrogen bonds of ligand benzimidazole part with Ser 141, Ser 122 and His 189, edge-to-face interactions of arylpiperazine aromatic ring with Phe 178, Tyr 216 and Trp 182 and hydrogen bond between ethereal oxygen in ethylenoxy ligands and hydrogen of Phe 185 or Trp 115. The most active 5-{2-[4-(2-methoxyphenyl)- piperazin-1-yl]ethoxy}-1,3-dihydro-2H-benzimidazole-2-thione has a maximal number of attractive interactions. A satisfactory correlation between docking of the compounds into the D2 receptor and competition binding results was observed. Within the scope of the program aimed at the discovery of new DA-ergic ligands, they synthesized a series of benzimidazoles that can be considered as noncatechol bioisosteres of catecholamines. The most active compounds were obtained by connecting benzimidazole ring through a flexible spacer with N-arylpiperazines. It was observed that the affinity of the obtained ligands for the binding to the D2 DAR depends on both the structure of benzimidazole and arylpiperazine part of the molecule and the spacer itself. In order to obtain a better structure/DA-ergic activity relationship and to evaluate the influence of the spacer in this type of ligands on their binding affinity; synthesis, evaluation and docking analyses were performed of a series of new compounds, derivatives of 5-[3-(4-arylpiperazin-1-yl)propyl]-1H-benzimidazole and 5-[2-(4-arylpiperazin-1-yl)ethoxy]-1H-benzimidazole [8]. (Figure-8)
Tomic et al synthesized two new series of substituted arylpiperazines with heterocyclic 3-propoxy-benzimidazole or 3-propoxybenzimidazole- 2-thione groups were synthesized and their in vitro binding affinities for the D2, 5-HT1A, 5-HT2A, and α1-adrenergic receptors were determined. Among them, only two compounds with phenyl aryl-constituent showed 5-HT2A/D2 pKi binding ratios proposed for atypical neuroleptics. As to their behavioral screening on rodents, both compounds exhibited a non-cataleptic action in rats and antagonized D-amphetamine-induced hyperlocomotion in mice, suggesting their possible atypical antipsychotic potency. Here, some selected newly synthesized ligands containing benzimidazole or benzimidazole-2-thione linked by Propyloxy Bridge to four different arylpiperazines, already characterized by improved interaction with the D2 receptors have been presented. Aryl parts of the ligands were chosen in accordance to their best pharmacological profiles and their intention was also to further evaluate the influence of the propyloxy linker of the new ligands (instead of ethyloxy link found in our previously synthesized compounds) on the receptor binding profiles [9]. (Figure-9)
Tomic et al had produced over 100 new compounds that were screened for their affinities at bovine brain D1, D2 and 5-HT1A receptors by in vitro radioligand binding assays that were obtained by variations of heterocyclic and aryl groups. One of the synthetic approaches was design and synthesis of heterocyclic arylpiperazines, with a specific structure of heteroaryl group, that mimics catechol moiety of the dopamine (benzimidazole, substituted benzimidazoles, benztriazoles and 1,4-dihydroquinoxaline- 2,3-diones). Keeping this in mind, two new series of substituted arylpiperazines with heterocyclic 3-propoxy-benzimidazole or 3-propoxy-benzimidazole-2- thione groups were synthesized and their in vitro binding affinities for the D2, 5-HT1A, 5-HT2A, and α1-adrenergic receptors were determined. Among them, only two compounds with phenyl aryl-constituent showed 5-HT2A/D2 pKi binding ratios proposed for atypical neuroleptics. As to their behavioral screening on rodents, both compounds exhibited a non-cataleptic action in rats and antagonized D-amphetamine-induced hyper locomotion in mice, suggesting their possible atypical antipsychotic potency [10]. (Figure-10)
Wright et al discovered a novel series of 2-[4-[3-(4-aryl-1- piperazinyl)propoxy]phenyl] benzimidazole dopamine D3 receptor partial agonists. The aryl group was crucial for activity and Topliss analysis confirmed that phenyl was optimal for DA D3 receptor binding and selectivity. The phenyl analogue was a partial agonist in a second messenger assay. It increased DA synthesis in rat brain and inhibited exploratory locomotors activity in rodents. Despite significant agonist effects in an in vitro second messenger assay, the phenyl analogue increased DA synthesis in vivo, a profile usually associated with DA antagonists. Phenyl analogue also inhibited exploratory locomotors activity in rodents. These results appear to support the suggestion that activation of DA D3 receptors in rodent brain causes an increase in DA synthesis and inhibition of locomotors activity, opposite to the effects seen when DA D2 receptors are activated [11]. (Figure-11)
The optimization of an HTS hit series leading to the identification of structurally novel, selective, orally bioavailable mGluR2 positive modulators GSK1331258 and GSK1331268 is described. Structure– activity relationships, attenuation of dopaminergic activity, and potentiation of mGluR2 responses in rat hippocampal MPP-DG synapses are also reported.
Investigation of a novel amino-aza-benzimidazolone structural class of positive allosteric modulators (PAMs) of metabotropic glutamate receptor 2 (mGluR2) identified [2.2.2]-bicyclic amine 12 as an intriguing lead structure due to its promising physicochemical properties and lipophilic ligand efficiency (LLE). Further optimization led to chiral amide 18, which exhibited strong in vitro activity and attractive pharmacokinetic (PK) properties. Hypothesis-driven target design identified compound 21 as a potent, highly selective, orally bioavailable mGluR2 PAM, which addressed a CYP time-dependent inhibition (TDI) liability of 18, while maintaining excellent drug-like properties with robust in vivo activity in a clinically validated model of antipsychotic potential.
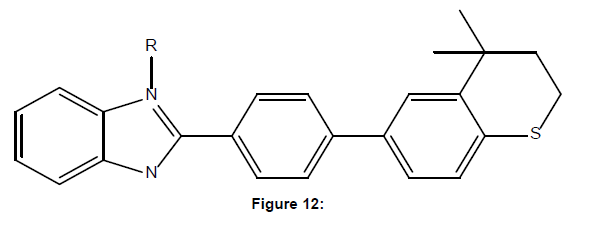
Orjales et al. reported synthesis of novel benzimidazole-2- carboxylic acid amides and esters were 18 with a quinolidine or a tropane moiety. It was evaluated for in vitro affinity for the 5-HT3 receptor. Synthesized compounds having 5-HT3 receptor antagonist activity (12.7, 18.4, 24.4) with ED50 values of (10.6-19.1) mg/kg i.v. among these compound 16a having higher affinity for 5-HT3 receptor [12]. (Figure-12)
Serotonin 5-HT6 receptor has been proposed as a promising therapeutic target for cognition enhancement though the development of new antagonists is still needed to validate these molecules as a drug class for the treatment of Alzheimer’s disease and other pathologies associated with memory deficiency. As part of our efforts to target the 5-HT6 receptor, new benzimidazole-based compounds have been designed and synthesized. Site-directed mutagenesis and homology models show the importance of a halogen bond interaction between a chlorine atom of the new class of 5-HT6 receptor antagonists identified herein and a backbone carbonyl group in transmembrane domain. In vitro pharmacological characterization of 5-HT6 receptor antagonist indicates high affinity and selectivity over a panel of receptors including 5-HT2B subtype and hERG channel, which suggests no major cardiac issues.
On the basis of previously described pharmacophore model for serotonin 5-HT(6) receptor (5-HT(6)R) antagonists, they have designed, synthesized, and pharmacologically characterized a series of benzimidazole derivatives that represent a new family of potent antagonists at the human 5-HT(6)R. Site-directed mutagenesis and a beta(2)-adrenoceptor-based homology model of the 5-HT(6)R were used to predict the mode of binding of antagonist SB-258585 and the new synthesized ligands. Substitution of W6.48, F6.52, or N6.55 by Ala fully impedes compound 4 to block 5-HT-induced activation. Thus, we propose that D3.32 in TM 3 anchors the protonated piperazine ring, the benzimidazole ring expands parallel to EL 2 to hydrogen bond N6.55 in TM 6, and the aromatic ring is placed between TMs 3 and 5 in CH(2)- containing compounds and between TMs 3 and 6 in CO-containing compounds. This combined experimental and computational study has permitted to propose the molecular mechanisms by which the new benzimidazole derivatives act as 5-HT (6) R antagonists.
Spasov et al. Studied in vitro the 5HT2A antagonistic activity of 16 imidazo[1,2a] benzimidazole derivatives. Using the radioligand method we showed the binding of 9 (2diethylaminoethyl) 2 (4methoxyphenyl) imidazo [1,2a] benzimidazol dinitrate to the 2A subtype serotonin receptor [13].
Takeshi Kamato et al. assessed the 5-HT3-receptor antagonist effects of 4,5,6,7-1H-benzimidazole compounds which are derivatives of YM060, a potent and selective 5-HT3-receptor antagonist, in isolated guinea pig colon. YM114 (KAE-393), YM-26103-2, YM-26308-2 (3 x 10-9 to 3 x 10-8 M) produced concentration-dependent shifts to the right of the dose-response curves for both 5-HT and 2-methyl-5-HT (2-Me-5-HT). YM114 (pA2=9.08 against 5-HT, pA2=8.88 against 2-Me-5-HT), YM-26103-2 (pA2=8.27 against 5-HT, pA2 = 8.19 against 2-Me-5-HT), and YM-26308-2 (pA2 = 8.58 against 5-HT, pA2 = 8.4 against 2-Me-5-HT) showed similar pA2 values irrespective of the agonist used, suggesting that they have 5-HT3-receptor blocking activity irrespective of the N-position at the aromatic ring. Since these compounds have an asymmetric center, their enantiomers exist. The S-isomers were one to three orders of magnitude less potent than the respective R-isomer compounds, indicating that the stereochemical configuration of 4, 5, 6, 7-tetrahydro-lHbenzimidazoles is an important determinant of their affinity for 5-HT3 receptors. These results suggest that the highly potent 5-HT3 receptor antagonism and high selectivity for 5-HT3 receptors of 4, 5, 6, 7-tetrahydroIHBenzimidazole derivatives are conserved irrespective of the position of the nitrogen atom in the aromatic ring and that 5-HT, recentors favor the R-isometric conformation of these compounds [14]. (Figure-13)
A series of benzimidazole and imidazopyridine derivatives are claimed in this patent to have utility in the treatment of neuropsychiatric conditions, especially schizophrenia and related psychoses. This claim is based on nanomolar affinities of these compounds for the dopamine D3 receptor, and the relative selectivity of these agents for the D3 vs.the D2 receptor
Murray et al synthesized a novel series of arylpiperazines which showed high affinity for dopamine D3 receptors. Several of these compounds exhibited ca.100 fold selectivity for the dopamine D3 receptor over D1, D2 and D4 receptors. In vivo studies suggest that many of them may have an atypical antipsychotic profile. As part of a programme to identify selective D3 receptor antagonists, evaluation of a series of arylpiperazines from their database had previously shown to possess D2 affinity. One of these compounds had high affinity for the D3 receptor and, strikingly, displayed ca. 100 fold selectivity over the D2 receptor. Furthermore, it showed 100 fold selectivity over D4 receptors and 1000 fold selectivity over D1 receptors. This was in marked contrast with typical antipsychotics, such as haloperidol, which show little D3/D2 receptor selectivity. However, given the propensity for the arylpiperazines to display affinity for several classes of seven Trans membrane receptor, these compounds also showed appreciable affinity for 5-HT1A and α1 receptors. (Figure-14)
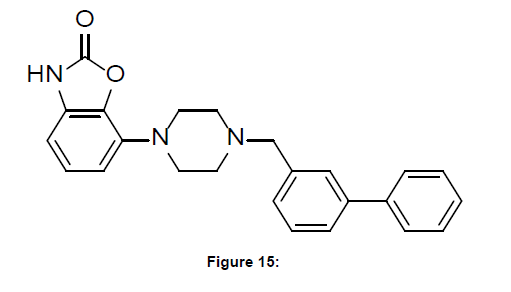
Wadenberg discussed here the complete profile of the drug Bifeprunox and its effect on treatment of psychosis (schizophrenia). He discussed that most second generation, atypical, dopamine (DA)D2/5- HT2 blocking antipsychotics still induce extrapyramidal side effect (EPS) in higher doses. Also, weight gain and metabolic disturbances are also a problem, and negative and cognitive symptoms have not been sufficiently addressed and the current brain DA mesolimbic hyperactive/mesocortical hypoactive hypothesis of schizophrenia suggests that DA D2/5-HT1A receptor partial agonist properties may be more efficacious with less side effects and may stabilize a hyperactive/hypoactive DA condition. Additional 5-HT1A stimulation may enhance therapeutic efficacy and also improve EPS liability profile. In clinical trials in schizophrenic patients the novel DA D2/5-HT1A partial agonist bifeprunox indeed demonstrates therapeutic efficacy, a safe EPS profile and appears beneficial weight gain, prolactin, blood lipid and glucose levels and cardiac rhythm. The data on bifeprunox are promising and suggest that combined DA D2/5-HT1A partial agonism may well be important properties for future generation antipsychotics. (Figure-15)
As a G-protein coupled receptor, the 5-hydroxytryptamine 2A (5- HT2A) receptor is known for its critical role in the cognitive, behavioral and physiological functions, and thus is a primary molecular target to treat psychiatric diseases, including especially depression. With purpose to explore the structural traits affecting the inhibitory activity, currently a dataset of 109 arylpiperazine derivatives as promising 5-HT2A antagonists was built, based on which the ligand-based threedimensional quantitative structure-activity relationship (3D-QSAR) study by using both comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) approaches was carried out. The resultant optimal CoMSIA model displays proper validity and predictability with cross-validated correlation coefficient Q2 = 0.587, non-cross-validated correlation coefficient R2ncv = 0.900 and predicted correlation coefficient for the test set of compounds R2pre = 0.897, respectively. Besides, molecular docking was also conducted to investigate the binding mode between these ligands and the active site of the 5-HT2A receptor. Meanwhile, as a docking supplementary tool to study the antagonists’ conformation in the binding cavity, molecular dynamics (MD) simulation was also performed, providing further elucidation about the changes in the ligand-receptor complex. Lastly, some new molecules were also newlydesigned based on the above results that are potential arylpiperazine antagonists of 5-HT2A receptor. We hope that the present models and derived information may be of help for facilitating the optimization and design of novel potent antagonists as antidepressant drugs as well as exploring the interaction mechanism of 5-HT2A antagonists.
Benzimidazole and indole derivatives that act as Selective modulators of CRF 1 receptors are provided. The present invention relates to novel benzimidazole and indole compounds that bind with high Selectivity and/ or high affinity to CRF receptors (Corticotropin Releasing Factor Receptors). This invention also relates to pharmaceutical compositions comprising such compounds and to the use of Such compounds in treatment of psychiatric disorders and neurological diseases, including major depression, anxiety-related disorders, post-traumatic Stress disorder. (Figure-16)
Serotonin 5-HT6 receptor has been proposed as a promising therapeutic target for cognition enhancement though the development of new antagonists is still needed to validate these molecules as a drug class for the treatment of Alzheimer’s disease and other pathologies associated with memory deficiency. As part of our efforts to target the 5-HT6 receptor, new benzimidazole-based compounds have been designed and synthesized. Site-directed mutagenesis and homology models show the importance of a halogen bond interaction between a chlorine atom of the new class of 5-HT6 receptor antagonists identified herein and a backbone carbonyl group in transmembrane domain .In vitro pharmacological characterization of 5-HT6 receptor antagonist indicates high affinity and selectivity over a panel of receptors including 5-HT2B subtype and hERG channel, which suggests no major cardiac issues. Compound exhibited in vivo procognitive activity (1 mg/kg, ip) in the novel object recognition task as a model of memory deficit. (Figure-17)
Marco Turconi et al synthesized a series of 2,3- dihydro-2-oxo- 1H- benzimidazole-1- carboxylic acid esters and amides, a basic azacyclo or azabicycloalkyl moiety and their modeling study showed that compound, was a recently proposed pharamacophoric model for 5-HT3 antagonistic activity.
Rivara et al developed a novel series of non-imidazole H3-receptor antagonists. The greatest H3-receptor affinity was obtained for the piperidine substituted compounds. It was possible to get good H3- antagonist potencies with 2-aminobenzimidazoles having a tertiary amino group at appropriate distance.
Marco Mor et al reported the design, synthesis, QSPR and QSAR of a new class of H3- antagonists, having a 2- aminobenzimidazole moiety connected to the 4(5) position of an imidazole ring through di or tri methylene chains (60).Compound lipophilicity (log P), basicity (pKa) and H3-receptor affinity and antagonist potency were determined and submitted to QSPR and QSAR investigations. When a three-methylene spacer was inserted between the imidazole ring and the 2-aminobenzimidazole nucleus, very potent compounds were obtained.
Synthesis of 2-arylbenzimidazole derivatives were reported by Dutra et al and found to bind with high affinity to the human histamine H4 receptor. Compounds shown their antihistaminic activity, among three of them one showed moderate affinity for H4 receptor.(Ki = 124 nM) and others (Ki = 65, 95). (Figure-18)
Conclusion
It is concluded that benzimidazole derivatives possess a broad spectrum of antipsychotic activity. The benzimidazole ring is an important pharmacophore in modern drug discovery. The benzimidazole derivatives are a resource for further medicinal research. Thus, the benzimidazole nucleus can be optimized to generate new, safer, and more effective antipsychotic drugs that satisfy the increasing need of patients afflicted with psychiatric disorders. Therefore, it is worthwhile to get insight into the discovery and development of benzimidazole containing antipsychotic drugs along with their mechanism of action for future endeavors. Therefore, in our opinion novel drugs can be discovered by combining benzimidazole– piperazine-biphenyl based heterocycles. This concept can represent the next major step forward in the development of broad-spectrum, more potent, and less toxic antipsychotic drugs. We are hopeful that these perspectives could provide the impetus to investigate novel and more efficacious drugs in future.
Acknowledgements
Authors are grateful to Dr Rani S Kankate, Department of Pharmaceutical Chemistry, for exemplary technical assistance and critical proof reading of the manuscript, Dr. Sanjay J. Kshirsagar, Principal MET’s Institute of Pharmacy, Bhujbal Knowledge City,Nashik, Trustee,Bhujbal Knowledge City, Nashik for providing the necessary facilities for institutional research platform.
Conflicts of interest
There are no conflicts of interest
References
- Dukic S, Rajacic SK, Dragovic D, Soskic V, Joksimovic J (1997) Synthesis of several substituted phenylpiperazines behaving as mixed D2/5-HT1a ligands. J Pharm Pharmacol 49: 1036-1041.
- Ullah N (2014) Synthesis and dual D2 and 5-HT1A receptor binding affinities of 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones. J Enzyme Inhib Med Chem 29: 281-291.
- Deana A, Gordana T, Goran R, Vukic S, Mirko T, et al. (2007) 6-[2-(4-Arylpiperazin-1-yl)ethyl]-4-halo-1,3-dihydro-2H-benzimidazole-2-thiones: synthesis and pharmacological evaluation. J Serb Chem Soc 72: 747-755.
- Lopez-Rodriguez ML, Benhamu B, Morcillo MJ, Tejada I, Avila-Brande D,et al. (2004) Benzimidazole derivatives, Part 5: design and synthesis of new benzimidazole-arylpiperazine derivatives acting as mixed 5-HT1A/5-HT3 ligands. Bioorganic Med Chem Lett 12: 5181-5191
- Mewshaw RE, Verwijs A, Shi X, McGaughey GB, Nelson JA, et al. (1998) New generation dopaminergic agents, 5. Heterocyclic bioisosteres that exploit the 3-OH-N1-phenylpiperazine dopaminergic template. Bioorganic Med Chem Lett 8: 2675-2680.
- Stewart AO, Cowart MD, Moreland RB, Latshaw SP, Matulenko MA, et al. (2004) Dopamine D4 ligands and models of receptor activation : 2-(4-Pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole and related heteroarylmethylarylpiperazines Exhibit a substituent effect responsible for additional efficacy tuning. J Med Chem 47: 2348-2355.
- Srinivas P, Brust P, Subramanian AR, Raghavan SAV, Parimoo P, et al. (1999) Synthesis and preliminary binding affinities of of 1’(1 ,2-dihydro-2-acenaphthylenyl / piperazine - a new arylpiperazine. Pharm Acta Helv 74: 73-76.
- Sukalovic V, Andric D, Roglic G, Kostic-Rajacic S, Schrattenholzc A, et al. (2005) Synthesis, dopamine D2 receptor binding studies and docking analysis of 5-[3-(4-Arylpiperazin-1-yl)propyl]-1H-benzimidazole, 5-[2-(4-arylpiperazin-1-yl)ethoxy]-1H-benzimidazole and their analogs. Eur J Med Chem 40: 481-493.
- Tomic M, Ignjatovic D, Tovilovic G, Andric D, Roglic G, et al. (2007) Two new phenylpiperazines with atypical antipsychotic potential. Bioorg Med Chem Lett 17: 5749-5753.
- Tomic M, Kundakovic M , Butorovic B, Janac B, Andric D, et al. (2004) Pharmacological evaluation of selected arylpiperazines with atypical antipsychotic potential. Bioorg Med Chem Lett 14: 4263-4266.
- Wright J, Heffner T, Pugsley T, Mackenzie R, Wise L (1995) Discovery of selective dopamine D3 ligands : II. 2-[4-[3-(4-Aryl-1-piperazinyl)propoxy]phenyl]benzimidazole partial agonists. Bioorg Med Chem Lett 5: 2547-2550.
- OrjalesA, Cires LA, Tudanca PL, Tapia I, Mosquera R, et al. (1999) Benzimidazole-2-carboxylic acid amides and esters: a new class of 5HT3 ligands. Eur J Med Chem 34: 415-422.
- Spasov AA, Yakovlev DS, Maltsev DV, Zhukovskaya ON, Anisimova VA, et al. (2016) The derivatives of imidazo[1,2-a]benzimidazole as 5-HT2A receptor antagonists. Russ. J. Bioorganic Chem 42: 397-403
- Kamato T, Ito H, Suzuki T, Miyata T, Honda K (1995) Studies on Serotonin (5-HT)3-Receptor Antagonist Effects of Enantiomers of 4,5,6,7-Tetrahydro-1H-Benzimidazole Derivatives. Jpn J Pharmacol 67: 185-194.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Kankate RS, Wagh DD, Kshirsagar SJ (2022) Benzimidazoles - A Promising Lead for Antipyschotic Drug Design. J Anal Bioanal Tech 10: 465. DOI: 10.4172/2155-9872.1000466
Copyright: © 2022 Kankate RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2626
- [From(publication date): 0-2022 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 1723
- PDF downloads: 903