Benthos Composition and Abundance in Lentic Ecosystems
Received: 06-Jun-2017 / Accepted Date: 01-Jul-2017 / Published Date: 05-Jul-2017 DOI: 10.4172/2332-2608.1000240
Abstract
The Benthic invertebrates such as nymphs of stonefly, mayfly, caddisfly larvae, snails, mussels, crustaceans, rat-tailed maggot, etc., convert and transport nutrients from one part of the water body to another, influencing nutrient cycling. In the present study, phytobenthos comprised of three major groups namely Bacillariophyceae, Chlorophyceae, Myxophyceae, whereas zoobenthos comprised of eleven major groups namely Protozoa, Rotifera Cladocera, Ostracoda, Coleoptera, Diptera, Ephemeroptera, Hemiptera, Trichoptera, Gastropoda and Odonata. The study revealed that zoobenthos were more dominant than phytobenthos. Among zoobenthos, Dipterans were found to be abundant followed by Cladocerans and least were Trichopterans, whereas among phytobenthos Bacillariophyceae was found to be most dominant followed by Chlorophyceae and Myxophyceae. The negative but significant correlation between zoobenthos and phytobenthos in all selected water bodies during study indicated grazing of former on latter proving top down control in these lentic ecosystem.
Keywords: Benthos; Phytobenthos; Zoobenthos; Lentic waterbodies
Introduction
Freshwater ecosystems are considered as one of the most essential natural resources for the survivability and success of all the living organisms including man. The habitat is generally divided into Lentic and Lotic ecosystems. The term lentic refers to standing bodies of water such as lakes, reservoirs, and ponds. These ecosystems generally have three zones – Littoral, Limnetic and Benthic zone. The term Benthos is derived from two Greek words “Ben” meaning ‘the collection of organisms living in or on the sea or lakes’ and “Thos” ‘the bottom of sea or lakes’. Benthos can be classified on a number of basis i.e., on the basis of size; Macrobenthos, Meiobenthos and Micro benthos; On the Basis of Location; Endobenthos, Epibenthos and Hyperbenthos; On the basis of Type; Zoobenthos includes animals and Phytobenthos which comprises of plants. The Benthic invertebrates such as nymphs of stonefly, mayfly, caddisfly larvae, snails, mussels, crustaceans, rattailed maggot, etc., convert and transport nutrients from one part of the water body to another, influencing nutrient cycling. They ingest organic matter such as leaf litter and detritus and in turn serve food for higher aquatic organisms such as fish, forming a basic link between organic matter and higher aquatic animals in food web. They are sensitive to changes in habitat and pollution, especially to organic pollution [1].
Materials and Methods
Sites (Plate 1-4)
The present study was carried out on four fresh water bodies of Aligarh (latitude 27° 30' N and longitude 79° 40' E), namely Shekha Jheel, Nai Basti pond, Laldiggi pond and Chautal pond. Laldiggi, Chautal and Nai Basti ponds having 1 ha area, located in the vicinity of the Aligarh Muslim University campus receive water from domestic discharge and rain water which accumulates during rainy season. These are used by washer men extensively for washing clothes, thus adding detergents and certain chemicals that bring changes in its chemical composition. The Shekha Jheel is a 25 ha lake near the village of Shekha, 17 km east of Aligarh. It is a fresh water perennial water body that came into existence after the formation of the Upper Ganges Canal which flows adjacent to the lake. It is maintained by the Forest Department. Sampling was done fortnightly from 9th March, 2016 to 23rd April, 2016. Samples were collected from selected water bodies between 8 am and 9 am and were analysed for following physicochemical parameters were analysed: Air and water temperatures, dissolved oxygen (DO) and free carbon dioxide (CO2).
Benthos collection, separation and identification
The bottom mud scrapper with low towline designed and described by Miclhae [2] was used to collect the samples from the waterbodies. For benthos analysis, samples were diluted with tap water to prepare slurry in a bucket and sticks, leaves, debris were removed. Then slurry was divided into ten subsamples. Each subsample was first sieved by B.S. no. 30 (0.5 mm) mesh sieve kept above the sieve B.S. no. 72 (0.2 mm) in order to retain smaller organisms (meio) on the latter. Organisms were kept in separate vials and fixed in 10% formalin solution (4% formaldehyde) and labelled. For qualitative and quantitative analysis 1 mL of fixed sample was taken on glass slide and studied under dissecting microscope. Individuals were identified up to genus level with the help of keys given by Edmondson et al. [3] and Needham and Needham [4] and frequency of each taxon was noted and expressed as individual/m² [2-4].
Results And Discussion
Physico-chemical parameters
In all selected water bodies air temperature ranged from a minimum of 26.4°C to a maximum of 38.8°C from 8th March to 23rd April, 2016 whereas water temperature ranged from a minimum of 23.1°C to a maximum of 33.2°C from 8th March to 23rd April, 2016. The surface water temperature of all selected water bodies followed closely the trend of air temperature during study period. Reduction in solar radiation due to shorter day length may explain lower temperature during the month of March. Increase in both air and water temperature during the month of April is attributed to the increase in solar radiation comparatively due to longer day length. pH values of all selected water bodies ranged from a minimum of 7.0 to a maximum of 8.0 during month of March, 2016 whereas during the month of April, it ranged from a minimum of 7.5 to a maximum of 8.5. Increased values of pH in all selected waterbodies during the study period could be related to increased level of photosynthesis carried out by phytoplankton and macrophytes, wherein CO2 is consumed, and hence pH is raised. The decrease in dissolved oxygen and the increase in Carbon dioxide in all the selected water bodies from 8th March to 23rd April, 2016 clearly justify the fact that as temperature increases oxygen holding capacity of water decreases while carbon dioxide increases due to high rate of decomposition. Lower values of carbon dioxide were observed in Nai Basti The decrease in dissolved oxygen and the increase in Carbon dioxide in all the selected water bodies from 8th March to 23rd April,2016 clearly justify the fact that as temperature increases oxygen holding capacity of water decreases while carbon dioxide increases due to high rate of decomposition. Lower values of carbon dioxide were observed in Nai Basti The decrease in dissolved oxygen and the increase in Carbon dioxide in all the selected water bodies from 8th March to 23rd April, 2016 clearly justify the fact that as temperature increases oxygen holding capacity of water decreases while carbon dioxide increases due to high rate of decomposition. Lower values of carbon dioxide were observed in Nai Basti The decrease in dissolved oxygen and the increase in Carbon dioxide in all the selected water bodies from 8th March to 23rd April, 2016 clearly justify the fact that as temperature increases oxygen holding capacity of water decreases while carbon dioxide increases due to high rate of decomposition (Tables 1-4).
| Parameters | Temperature (°C) | pH | D.O. (mg/L) | Co2 (mg/L) | |
|---|---|---|---|---|---|
| Dates | Air | Water | |||
| 9/3/2016 | 26.4°C | 23.1°C | 7.5 | 4.6 mg/L | 21.0 mg/L |
| 23-03-2016 | 29.0°C | 27.1°C | 8 | 4.0 mg/L | 30.0 mg/L |
| 8/4/2016 | 33.0°C | 30.8°C | 8 | 1.8 mg/L | 35.0 mg/L |
| 23-04-2016 | 34.3°C | 31.1°C | 8.5 | 2.0 mg/L | 39.0 mg/L |
Table 1: Fortnight variations in physicochemical parameters in Lal Diggi pond.
| Parameters | Temperature (°C) | pH | D.o. (mg/L) | Co2 (mg/L) | |
|---|---|---|---|---|---|
| Dates | Air | Water | |||
| 9/3/2016 | 28.3°C | 26.0°C | 8 | 3.0 mg/L | 18.0 mg/L |
| 23-03-2016 | 31.2°C | 29.4°C | 7.5 | 2.5 mg/L | 16.0 mg/L |
| 8/4/2016 | 34 .0°C | 31.0°C | 8.5 | 1.8 mg/L | 20.0 mg/L |
| 23-04-2016 | 35.9°C | 33.2°C | 8.5 | 1.6 mg/L | 25.0 mg/L |
Table 2: Fortnight variations in physicochemical parameters in Nai Basti pond.
| Parameters | Temperature (°C) | pH | D.O. (mg/L) | Co2 (mg/L) | |
|---|---|---|---|---|---|
| Dates | Air | Water | |||
| 9/3/2016 | 27 .8°C | 23.6°C | 7 | 3.1 mg/L | 26 mg/L |
| 23-03-2016 | 30.8°C | 26.9°C | 7.5 | 2.4 mg/L | 24 mg/L |
| 8/4/2016 | 33.0°C | 30.2°C | 7.5 | 2.1 mg/L | 28 mg/L |
| 23-04-2016 | 35.0°C | 33.1°C | 7.5 | 1.9 mg/L | 36 mg/L |
Table 3: Fortnight variations in physicochemical parameters in Chautal pond.
| Parameters | Temperature (°C) | pH | D.O. (mg/L) | Co2 (mg/L) | |
|---|---|---|---|---|---|
| Dates | Air | Water | |||
| 8/3/2016 | 27.0°C | 24.0°C | 7.5 | 4.9 mg/L | 17.0 mg/L |
| 22-03-2016 | 28.9°C | 25.2°C | 7.5 | 4.0 mg/L | 21.0 mg/L |
| 7/4/2016 | 34.2°C | 30.0°C | 8.5 | 3.2 mg/L | 19.0 mg/L |
| 20-04-2016 | 38.8°C | 33.0°C | 8 | 2.2 mg/ | 24.0 mg/L |
Table 4: Fortnight variations in physicochemical parameters in Shekha Jheel.
Lower values of carbon dioxide were observed in Nai Basti pond during study period (Table 2), might be due to high photosynthesis of phytobenthos and macrophytes.
Benthos
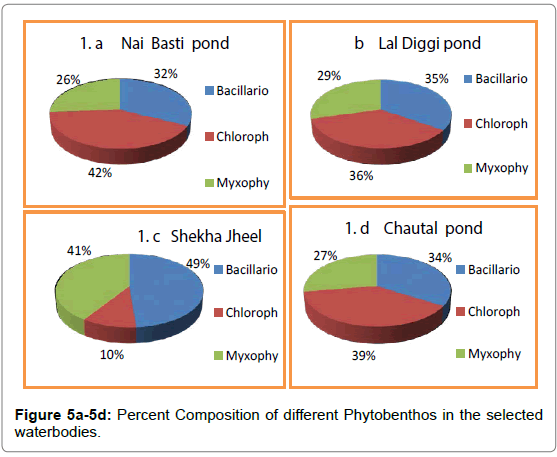
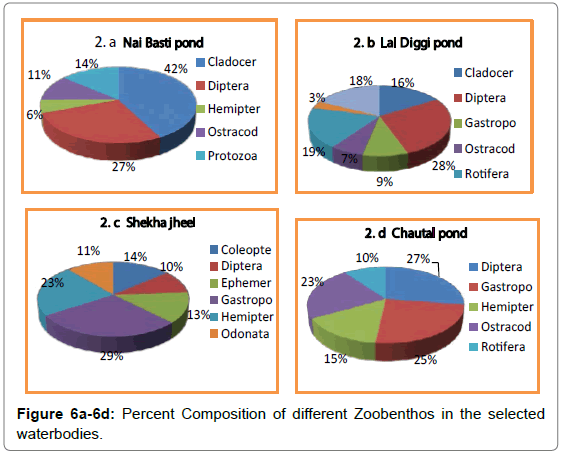
In distribution of benthic flora, light plays a very important role when the water is sufficiently shallow. The studied waterbodies, being shallow light reaches the bottom sediments in plenty and as a result of it, phytobenthos grow in greater abundance. The phytobenthos comprised of three major groups namely Bacillariophyceae, Chlorophyceae and Myxophyceae. The variations in Phytobenthos density in the selected water bodies were recorded from a minimum of 38 No/m² to a maximum of 151 No/m² (Tables 5-7; Figures 1-4) in Nai Basti pond ranged from a minimum of 85 No/m² to a maximum of 151 No/m²;. in Shekha jheel, from a minimum of 39 No/m² to a maximum of 74 No/m². Phyto benthos of Chautal pond; from a minimum of 51 No/m² to a maximum of 117 No/m² and in Lal diggi pond, phyto benthos ranged from a minimum of 38 No/m² to a maximum of 73 No/m² (Tables 5-7). Chlorophyceae formed the most abundant group followed by Baillariophyceae and Myxophyceae in Lal Diggi, Chautal and Nai Basti pond whereas Bacillariophyceae formed most abundant group in the Shekha jheel followed by Myxophyceae and Chlorophyceae (Figure 5a-5d). Chlorophyceae showed a direct relation with the temperature. Kumar et al. [5] reported that higher water temperature and low dissolved oxygen support the growth of Chlorophyceae. Statistically, phytobenthos showed positive significant correlation with Water temperature, Carbon dioxide and pH whereas as negative but significant correlation with zoobenthos, in all the four studied waterbodies. With dissolved oxygen significant positive correlation in shekha jheel only whereas significant negative in rest of the waterbodies (Tables 8-11). Benthic fauna are widespread in their distribution and can live on all bottom types and thus found even in the soil beneath puddles. The zoobenthos comprised of eleven major groups namely Protozoa, Rotifera, Cladocera, Ostracoda, Coleoptera, Diptera, Ephemeroptera, Hemiptera, Trichoptera, Gastropoda and Odonata (Tables 12-15). Among zoobenthos, Dipterans were found to be abundant followed by Cladocerans and least were Trichopterans. In the present investigation, zoobenthos of Nai Basti pond ranged from a minimum of 59 No/m² to a maximum of 117 No/m²: in Lal Diggi pond, it was ranged from a minimum of 121 No/m² to a maximum of 179 No/m²; in Chautal pond, it was ranged from a minimum of 75 No/m² to a maximum of 128 No/ m²: in Shekha jheel, it ranged from a minimum of 92 No/m² to a maximum of 120 No/m² (Tables 8-11) (Figure 6a-6d). During study period it was observed that Nai Basti pond is the most productive in terms of phyto benthos whereas Lal Diggi pond in terms of zoobenthos. During the present investigation Cladocerans were found to be abundant in Nai Basti pond while Dipterans in Chautal pond and Gastropods in Shekha jheel. The abundance of dipterans was represented by Chironomus and Culex. Chironomus can survive in low oxygen condition as well as polluted water body. Therefore, its high number in Chautal pond indicated polluted nature [6]. The availability of maximum number of Gastropods could be correlated to the cumulative effect of alkaline nature of water, high calcium contents and macrophytic vegetation [7]. Trichopterans were found to be the least abundant in all ponds. Kabir et al. [6] reported that these insects are sensitive to pollution. The zoobenthos showed negative but significant correlation with Water temperature and pH in Chautal pond, Shekha jheel and in Lal Diggi; with CO2 in Chautal pond and Shekha jheel, whereas in Nai Basti pond zoobenthos showed positive significant correlation with water temperature, pH and CO2. However, with dissolved oxygen these animals showed positive significant correlation in all water bodies (Tables 10-13). The result of present investigation revealed that zoobenthos were more dominant than phytobenthos (Table 16). The negative but significant correlation between zoobenthos and phytobenthos in all selected water bodies during study indicated grazing of former on latter proving top down control in these lentic ecosystems [8-10].
| Date/Genera | 9/3/2016 | 23-03-2016 | 8/4/2016 | 23-04-2016 |
|---|---|---|---|---|
| Bacillariophyceae | ||||
| Navicula spp. | 13 | 17 | 25 | 31 |
| Diatoma spp. | 12 | 17 | 26 | 21 |
| Cocconeis spp. | - | - | - | - |
| Total | 25 | 34 | 51 | 52 |
| Chlorophyceae | ||||
| Chlorella spp. | 16 | 22 | 19 | 26 |
| Ulothrix spp. | - | - | - | - |
| Clorococcus spp. | - | - | - | - |
| Oedogonium spp. | - | - | - | - |
| Tetrapedia spp. | - | - | - | - |
| Crucigenia spp. | 15 | 20 | 24 | 29 |
| Hydrodictyon spp. | 5 | 9 | 13 | 11 |
| Total | 36 | 51 | 56 | 66 |
| Myxophyceae | ||||
| Gomphosphaeria spp. | 14 | 18 | 23 | 21 |
| Oscillatoria spp. | 10 | 14 | 19 | 12 |
| Nostoc spp. | - | - | - | - |
| Total | 24 | 32 | 42 | 33 |
| Grand total | 85 | 117 | 149 | 151 |
Table 5: Fortnight distribution of phytobenthos (no/m²) in nai basti pond.
| Date /Genera | 9/3/2016 | 23-03-2016 | 8/4/2016 | 23-04-2016 |
|---|---|---|---|---|
| Bacillariophyceae | ||||
| Navicula spp. | 5 | 8 | 13 | 7 |
| Diatoma spp. | - | - | - | - |
| Cocconeis spp. | 11 | 16 | 23 | 29 |
| Total | 16 | 24 | 36 | 36 |
| Chlorophyceae | ||||
| Chlorella spp. | - | - | - | - |
| Ulothrix spp. | - | - | - | - |
| Clorococcus spp. | 13 | 19 | 11 | 26 |
| Oedogonium spp. | - | - | - | - |
| Tetrapedia spp. | 10 | 12 | 16 | 21 |
| Crucigenia spp. | - | - | - | - |
| Hydrodictyon spp. | - | - | - | - |
| Total | 23 | 31 | 27 | 47 |
| Myxophyceae | ||||
| Gomphosphaeria spp. | - | - | - | - |
| Oscillatoria spp. | 7 | 12 | 9 | 14 |
| Nostoc spp. | 5 | 8 | 16 | 20 |
| Total | 12 | 20 | 25 | 34 |
| Grand total | 51 | 75 | 88 | 117 |
Table 6: Fortnight distribution of phytobenthos (no/m²) in chautal pond.
| Date /Genera | 9/3/2016 | 23-03-2016 | 8/4/2016 | 23-04-2016 |
|---|---|---|---|---|
| Bacillariophyceae | ||||
| Navicula spp. | 8 | 13 | 18 | 15 |
| Diatoma spp. | - | - | - | - |
| Cocconeis spp. | 11 | 16 | 12 | 17 |
| Total | 19 | 29 | 30 | 32 |
| Chlorophyceae | ||||
| Chlorella spp. | - | - | - | - |
| Ulothrix spp. | 5 | 7 | 3 | 9 |
| Clorococcus spp. | - | - | - | - |
| Oedogonium spp. | - | - | - | - |
| Tetrapedia spp. | - | - | - | - |
| Crucigenia spp. | - | - | - | - |
| Hydrodictyon spp. | - | - | - | - |
| Total | 5 | 7 | 3 | 9 |
| Myxophyceae | ||||
| Gomphosphaeria spp. | - | - | - | - |
| Oscillatoria spp. | 6 | 5 | 8 | 11 |
| Nostoc spp. | 9 | 12 | 19 | 22 |
| Total | 15 | 17 | 27 | 33 |
| Grand total | 39 | 53 | 60 | 74 |
Table 7: Fortnight distribution of phytobenthos (no/m²) in shekha jheel
| Date /Genera | 9/3/2016 | 23-03-2016 | 8/4/2016 | 23-03-2016 |
|---|---|---|---|---|
| Bacillariophyceae | ||||
| Navicula spp. | 12 | 17 | 27 | 21 |
| Diatoma spp. | - | - | - | - |
| Cocconeis spp. | - | - | - | - |
| Total | 12 | 17 | 27 | 21 |
| Chlorophyceae | ||||
| Chlorella spp. | - | - | - | - |
| Ulothrix spp. | - | - | - | - |
| Clorococcus spp. | - | - | - | |
| Oedogonium spp. | 3 | 2 | 1 | 5 |
| Tetrapedia spp. | 11 | 18 | 15 | 25 |
| Crucigenia spp. | - | - | - | - |
| Hydrodictyon spp. | - | - | - | - |
| Total | 14 | 20 | 16 | 30 |
| Myxophyceae | ||||
| Gomphosphaeria spp. | - | - | - | - |
| Oscillatoria spp. | 4 | 6 | 5 | 7 |
| Nostoc spp. | 8 | 9 | 11 | 15 |
| Total | 12 | 15 | 16 | 22 |
| Grand total | 38 | 52 | 59 | 73 |
Table 8: Fortnight distribution of phytobenthos (no/m²) in lal diggi.
| Date /Genera | 9-03-2016 | 23-03-2016 | 8-04-2016 | 23-04-2016 |
|---|---|---|---|---|
| Coleoptera | ||||
| Berosus sp. | - | - | - | - |
| Total | - | - | - | - |
| DIPTERA | ||||
| Chironomus sp. | ||||
| Culex sp. | 27 | 25 | 21 | 28 |
| Total | 27 | 25 | 21 | 28 |
| Ephemeroptera | ||||
| Cynigmula sp. | ||||
| Total | - | - | - | - |
| Hemiptera | ||||
| Belostoma sp. | - | - | - | - |
| Notonecta sp. | 5 | 4 | 8 | 3 |
| Ptilostomis sp. | - | - | - | - |
| Total | 5 | 4 | 8 | 3 |
| Odonata | ||||
| Libellula sp. | - | - | - | - |
| Total | - | - | - | - |
| Trichoptera | ||||
| Phryganaea larvae | - | - | - | - |
| Total | - | - | - | - |
| Cladocera | ||||
| Bosmina sp. | - | - | - | - |
| Moina sp. | 15 | 23 | 13 | 6 |
| Chydorus sp. | 14 | 17 | 7 | 4 |
| Daphnia sp. | 21 | 18 | 12 | 6 |
| Total | 50 | 58 | 32 | 16 |
| Ostracoda | ||||
| Cypris sp. | 13 | 9 | 12 | 7 |
| Cypridopsis sp. | - | - | - | - |
| Total | 13 | 9 | 12 | 7 |
| Gastropoda | ||||
| Amnicola sp. | - | - | - | - |
| Gyraulus sp. | - | - | - | - |
| Campeloma sp. | - | - | - | - |
| Rotifera | ||||
| Asplanchna sp. | - | - | - | - |
| Keratella sp. | - | - | - | - |
| Protozoa | ||||
| Euglena sp. | 19 | 21 | 7 | 5 |
| Total | 19 | 21 | 7 | 5 |
| Grand total | 114 | 117 | 80 | 59 |
Table 9: Fortnight distribution of zoobenthos (no/m²) in nai basti pond.
| Date/Genera | 9/3/2016 | 23-03-2016 | 8/4/2016 | 23-04-2016 |
|---|---|---|---|---|
| Coleoptera | ||||
| Berosus spp. | 28 | 16 | 11 | - |
| Total | 28 | 16 | 11 | - |
| Diptera | ||||
| Chironomus spp. | 3 | 8 | 18 | 11 |
| Culex spp. | - | - | - | - |
| Total | 3 | 8 | 18 | 11 |
| Ephemeroptera | ||||
| Cynigmula spp. | 25 | 18 | 9 | - |
| Total | 25 | 18 | 9 | - |
| Hemiptera | ||||
| Belostoma spp. | 21 | 15 | 8 | 13 |
| Notonecta spp. | 19 | 12 | 7 | 4 |
| Ptilostomis spp. | - | - | - | - |
| Total | 40 | 27 | 15 | 17 |
| Odonata | ||||
| Libellula spp. | 11 | 3 | 8 | 23 |
| Total | 11 | 3 | 8 | 23 |
| Trichoptera | - | |||
| Phyrganaea larvae | - | - | - | - |
| Total | ||||
| Cladocera | ||||
| Bosmina spp. | - | - | - | - |
| Moina spp. | - | - | - | - |
| Chydorus spp. | - | - | - | - |
| Daphnia spp. | - | - | - | - |
| Total | ||||
| Gastropoda | ||||
| Amnicola spp. | 9 | 9 | 15 | 18 |
| Gyraulus spp. | 4 | 11 | 17 | 22 |
| Campeloma spp. | - | - | 2 | 10 |
| Total | 13 | 20 | 34 | 50 |
| Ostracoda | ||||
| Cypris spp. | - | - | - | - |
| Cypridopsis spp. | - | - | - | - |
| Total | ||||
| Rotifera | ||||
| Asplanchna spp. | - | - | - | - |
| Keratella spp. | - | - | - | - |
| Total | ||||
| Protozoa | ||||
| Euglena spp. | - | - | - | - |
| Total | ||||
| Grand total | 120 | 92 | 95 | 101 |
Table 10: Fortnight distribution of zoobenthos (no/m²) in shekha jheel.
| Date /Genera | 9/3/2016 | 23-03-2016 | 8/4/2016 | 23-04-2016 |
|---|---|---|---|---|
| Coleoptera | ||||
| Berosus spp. | - | - | - | - |
| Total | ||||
| Diptera | ||||
| Chironomus spp. | 28 | 8 | 22 | 17 |
| Culex spp. | 30 | 21 | 19 | 13 |
| Total | 58 | 29 | 41 | 30 |
| Ephemeroptera | ||||
| Cynigmula spp. | - | - | - | - |
| Total | ||||
| Hemiptera | ||||
| Belostoma spp. | - | - | - | - |
| Notonecta spp. | - | - | - | - |
| Ptilostomis spp. | - | - | - | - |
| Total | ||||
| Odonata | ||||
| Libellula spp. | - | - | - | - |
| Total | ||||
| Trichoptera | ||||
| Phryganaea larvae | 10 | 7 | 2 | - |
| Total | 10 | 7 | 2 | - |
| Cladocera | ||||
| Bosmina spp. | 26 | 18 | 7 | 3 |
| Moina spp. | 20 | 13 | 5 | 1 |
| Chydorus spp. | - | - | - | - |
| Daphnia spp. | - | - | - | - |
| Total | 46 | 31 | 12 | 4 |
| Gastropoda | ||||
| Amnicola spp. | - | - | - | - |
| Gyraulus spp. | 8 | 15 | 11 | 17 |
| Campeloma spp. | - | - | - | - |
| Total | 8 | 15 | 11 | 17 |
| Ostracoda | ||||
| Cypris spp. | - | - | - | - |
| Cypridopsis spp. | 18 | 11 | 7 | 2 |
| Total | 18 | 11 | 7 | 2 |
| Rotifera | ||||
| Asplanchna spp. | 6 | 8 | 11 | 15 |
| Keratella spp. | 10 | 14 | 19 | 23 |
| Total | 16 | 22 | 30 | 38 |
| Protozoa | ||||
| Euglena spp. | 23 | 20 | 27 | 30 |
| Total | 23 | 20 | 27 | 30 |
| Grand total | 179 | 135 | 130 | 121 |
Table 11: Fortnight distribution of zoobenthos (no/m²) in lal diggi pond.
| Date /Genera | 9/3/2016 | 23-03-2016 | 8/4/2016 | 23-04-2016 |
|---|---|---|---|---|
| Coleoptera | ||||
| Berosus spp. | - | - | - | - |
| Total | ||||
| Diptera | ||||
| Chironomous spp. | 9 | 17 | 11 | 17 |
| Culex spp. | 8 | 13 | 9 | 16 |
| Total | 17 | 30 | 20 | 33 |
| Ephemeroptera | ||||
| Cynigmula spp. | - | - | - | - |
| Total | ||||
| Hemiptera | ||||
| Belostoma spp. | - | - | - | - |
| Notonecta spp. | 26 | 23 | 10 | 5 |
| Ptilostomis spp. | - | - | - | - |
| Total | 26 | 23 | 10 | 5 |
| Trichoptera | ||||
| Phryganaea larvae | - | - | - | - |
| Total | ||||
| Odonata | ||||
| Libellula spp. | - | - | - | - |
| Total | - | - | - | - |
| Cladocera | ||||
| Bosmina spp. | - | - | - | - |
| Moina spp. | - | - | - | - |
| Chydorus spp. | - | - | - | - |
| Daphnia spp. | - | - | - | - |
| Total | ||||
| Ostracoda | ||||
| Cypris spp. | 43 | 32 | 15 | 6 |
| Cypridopsis spp. | - | - | - | - |
| Total | 43 | 32 | 15 | 6 |
| Rotifera | ||||
| Asplanchna spp. | 20 | 15 | 8 | - |
| Keratella spp. | - | - | - | - |
| Total | 20 | 15 | 8 | - |
| Gastropoda | ||||
| Amnicola spp. | - | - | - | - |
| Gyraulus spp. | 22 | 27 | 23 | 31 |
| Campeloma spp. | - | - | - | - |
| Total | 22 | 27 | 23 | 31 |
| Protozoa | ||||
| Euglena spp. | - | - | - | - |
| Total | ||||
| Grand total | 128 | 127 | 76 | 75 |
Table 12: Fortnight distribution of zoobenthos (no/m²) in chautal pond.
| Parameters | Parameters | Correlation | Significant at p=0.05 |
|---|---|---|---|
| (r value) | |||
| Air temperature | Water temperature | 0.997 | ? |
| Water temperature | Carbon dioxide | 0.819 | ? |
| Dissolved oxygen | ?0.965 | ? | |
| pH | 0.787 | ? | |
| Zoo benthos | ?0.882 | ? | |
| Phyto benthos | 0.987 | ? | |
| Carbon dioxide | Phyto benthos | 0.849 | ? |
| Zoo benthos | ?0.821 | ? | |
| Dissolved oxygen | ?0.646 | ? | |
| pH | 0.317 | ? | |
| Dissolved oxygen | Zoo benthos | 0.92 | ? |
| Phyto benthos | -0.945 | ? | |
| pH | 0.962 | ? | |
| pH | Phyto benthos | 0.77 | ? |
| Zoo benthos | ?0.780 | ? | |
| Zoo benthos | Phyto benthos | ?0.984 | ? |
Table 13: Statistical brief of water quality parameters in chautal pond.
| Parameters | Parameters | Correlation | Significant at p=0.05 |
|---|---|---|---|
| (r value) | |||
| Air temperature | Water temperature | 0.982 | ? |
| Water temperature | Carbon dioxide | 0.982 | ? |
| Dissolved oxygen | ?0.958 | ? | |
| pH | 0.87 | ? | |
| Zoo benthos | ?0.998 | ? | |
| Phyto benthos | 0.932 | ? | |
| Carbon dioxide | Phyto benthos | 0.98 | ? |
| Zoo benthos | 0.988 | ? | |
| Dissolved oxygen | ?0.911 | ? | |
| pH | 0.947 | ? | |
| Dissolved oxygen | Zoo benthos | 0.941 | ? |
| Phyto benthos | -0.863 | ? | |
| pH | ?0.753 | ? | |
| pH | Phyto benthos | 0.981 | ? |
| Zoo benthos | ?0.888 | ? | |
| Zoo benthos | Phyto benthos | ?0.940 | ? |
Table 14: Statistical brief of water quality parameters in laldiggi pond.
| Parameters | Parameters | Correlation | Significant at p=0.05 |
|---|---|---|---|
| (r value) | |||
| Air temperature | Water temperature | 0.997 | ? |
| Water temperature | Carbon dioxide | 0.722 | ? |
| Dissolved oxygen | ?0.975 | ? | |
| pH | 0.734 | ? | |
| Zoo benthos | ?0.879 | ? | |
| Phyto benthos | 0.95 | ? | |
| Carbon dioxide | Phyto benthos | 0.885 | ? |
| Zoo benthos | ?0.802 | ? | |
| Dissolved oxygen | ?0.842 | ? | |
| pH | 0.146 | ? | |
| Dissolved oxygen | Zoo benthos | 0.946 | ? |
| Phyto benthos | 0.996 | ? | |
| pH | ?0.643 | ? | |
| pH | Phyto benthos | 0.585 | ? |
| Zoo benthos | ?0.671 | ? | |
| Zoo benthos | Phyto benthos | ?0.955 | ? |
Table 15: Statistical brief of water quality parameters in shekha jheel.
| Parameters | Parameters | Correlation | Significant at p= 0.05 |
|---|---|---|---|
| (r value) | |||
| Air temperature | Water temperature | 0.991 | ? |
| Water temperature | Carbon dioxide | 0.749 | ? |
| Dissolved oxygen | ?0.970 | ? | |
| pH | 0.563 | ? | |
| Zoo benthos | 0.869 | ? | |
| Phyto benthos | 0.96 | ? | |
| Carbon dioxide | Phyto benthos | 0.671 | ? |
| Zoo benthos | ?0.978 | ? | |
| Dissolved oxygen | ?0.773 | ? | |
| pH | 0.834 | ? | |
| Dissolved oxygen | Zoo benthos | 0.886 | ? |
| Phyto benthos | -0.989 | ? | |
| pH | ?0.715 | ? | |
| pH | Phyto benthos | 0.642 | ? |
| Zoo benthos | ?0.816 | ? | |
| Zoo benthos | Phyto benthos | ?0.808 | ? |
Table 16: Statistical brief of water quality parameters in nai basti pond.
Conclusion
Present investigation revealed that zoobenthos were more dominant than phytobenthos. Among zoo benthos, Diptera was found to be the abundant group followed by Cladocerans and least was Trichopterans. Chironomus which is a representative of Dipterans is the pollution indicator. Trichopterans are sensitive to the pollution, so they are least abundant. Chlorophyceae formed the most abundant group in Lal Diggi, Chautal and Nai Basti pond. Zoobenthos are inversely related to phyto benthos in all the ponds indicating former grazing on latter. The presence of zoo benthos along with phytobenthos in all samples indicated nutrient rich and productive pond bottom thereby proving favourable environment for benthic animals especially fish.
Acknowledgements
The first author is indebted to Chairman Professor Iqbal Pervez, Department of Zoology Aligarh Muslim University, Aligarh for providing necessary facilities to complete this project.
References
- Ramachandra TV, Ahalya N, Murthy R (2005) Aquatic ecosystem Conservation restoration and management. Capital Publishing Company New Delhi.
- Michael P (1984) Ecological methods for field and laboratory investigations. McGraw Hill Publishing Company Limited: 400.
- Edmondson WT ,Ward HB, Whipple GC(1978)Freshwater Biology (2nd edn.), John Wiley and Sons Inc New York: 1248.
- Needham JG, Needham PR (1962) A Guide to the Study of the Freshwater Biology. Holden-Dey Inc Francisco, p. 108.
- Kumar NJI, Sharma G, Kumar RN, Joseph S (2005) An assessment of eutrophication and weed growth of certain wetlands of Gujrat. Trivedy RK editor. In Recent advances in water pollution research book Enclave Jain Bhavan Jaipur pp: 129-150.
- Kabir H, Parveen S, Ahmad U and Ganai AH (2013) Benthic insect diversity in sewage fed pond of Aligarh region. Int J of Biodiversity and Conservation 5: 209-214.
- Garg RK, Rao RJ, Saksena DN (2011) correlation of molluscan diversity with physicochemical characteristics of Ramsagar reservoir Ind. International Journal of Biodiversity and Conservation 1: 202-207.
- APHA (1992) Standard methods for Examination of Water and Wastewater. American Public Health Association AWWA WPCF Washington DC USA: 1193.
- Ahmad U, Parveen S, Hassan T, Bhat BN (2013)A study on Limnology and Biodiversity of Crustaceans and Macrophyte infested water bodies of Aligarh region. International journal of plant animal and environmental sciences.
- Trivedy RK, Goel PK (1984) Chemical and biological methods for water pollution studies. Environmental Publications Karad India, p. 250.
Citation: Fatima M, Ahmad U, Bhat BN, Hassan T, Parveen S (2017) Benthos Composition and Abundance in Lentic Ecosystems. J Fisheries Livest Prod 5: 240. DOI: 10.4172/2332-2608.1000240
Copyright: © 2017 Fatima M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7218
- [From(publication date): 0-2017 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 6165
- PDF downloads: 1053