Beneficial Role of HO-1-SIRT1 Axis in Attenuating Steatohepatitis
Received: 12-Aug-2024 / Manuscript No. bcp-24-144805 / Editor assigned: 14-Aug-2024 / PreQC No. bcp-24-144805 / Reviewed: 28-Aug-2024 / QC No. bcp-24-144805 / Revised: 02-Sep-2024 / Manuscript No. bcp-24-144805 / Published Date: 10-Sep-2024 DOI: 10.4172/2168-9652.1000482
Abstract
The imbalance between free radicals and antioxidant defense is defined as the critical factor in the progression of nonalcoholic fatty liver disease and obesity. Heme Oxygenase-1 (HO-1), an intrinsic antioxidant enzyme, significantly mitigates this imbalance. Sirtuin 1 (SIRT1), a protein belonging to the NAD-dependent deacetylase family linked to the cellular metabolic status of the chromatin structure, regulation of gene expression and notably influenced by redox imbalance. The hypothesis of this study suggests that fructose-induced obesity leads to an inflammatory and oxidative condition that promotes non-alcoholic steatohepatitis (NASH) development. We investigate the role of the hepatocyte HO-1-SIRT1 axis in attenuating steatohepatitis. This study analyzed the effects of fructose supplementation on hepatic lipid metabolism in murine hepatocytes and liver tissues of mice subject to a high-fructose diet. The experiments were conducted both in the presence and absence of Cobalt protoporphyrin (CoPP) (HO-1 inducer) and Tin mesoporphyrin (SnMP) (HO-1 activity inhibitor). Fructose supplementation promoted a significant increase in oxidative stress while concurrently resulting in the attenuation of HO-1 and SIRT1 levels within hepatocytes. Furthermore, fructose led to increased Fatty acid synthase (FAS) expression, as well as elevated triglyceride levels; these changes caused by fructose were significantly attenuated with CoPP intervention. Concurrently, the co-treatment of CoPP and siRNA targeting SIRT1 to hepatocytes increased FAS expression and triglyceride levels. This outcome postulates that HO-1 potentially operates upstream of SIRT1 within the signal transduction cascade and suppression of SIRT1 decreases the positive effects of HO-1. Markers of oxidative stress, blood pressure, insulin resistance, and lipogenesis significantly increased in a high-fructose diet, as well as, the HO-1 induction, led to an increase in SIRT1 expression, thus attenuating fructose-induced lipid accumulation. The positive effects of CoPP were reversed by SnMP. In summary, our study demonstrates that HO-1 induction alleviates fructose-induced NASH by activating SIRT1 gene expression. This finding highlights the potential of targeting the HO-1-SIRT 1 axis as a therapeutic strategy for treating NASH.
keywords
Heme oxygenase 1; Sirtuin 1; Steatohepatitis; Hepatocytes; Oxidative stress
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver condition in the world that has been increasing in parallel with the global prevalence of obesity. In the subset of NAFLD patients undergoing random liver biopsies, the observed prevalence of non-alcoholic steatohepatitis (NASH) was recorded at 6.67%. For NAFLD patients necessitating a liver biopsy due to clinical indications, the prevalence of NASH escalated to 59.10%. However, the average prevalence of NASH was estimated to be between 1.5% and 6.45% [1,2]. Studies have shown that NASH imparts a substantial economic burden in the United States, with a projected lifetime cost for NASH patients amounting to $222.6 billion and advanced NASH amounting to $95.4 billion [3,4]. NASH is strongly associated with the development of metabolic disorders, and is characterized by modulating the lipogenic pathways, increasing the hepatic lipid accumulation, compromising insulin sensitivity, free fatty acid (FFA) levels, and inflammatory markers [5,6]. Furthermore, approximately 30% of NAFLD cases escalate into NASH-related liver fibrosis, marked by inflammatory infiltration and subsequent fibrotic progression [7]. It has been shown that high fructose dietary has a substantial influence on metabolic perturbations and NASH development [8-10]. The deleterious effects of reactive oxygen species (ROS) play a crucial role in the transition from NAFLD to NASH [11]. High-fructose (HFr) diets modulates different signaling pathways, including the de novo lipogenesis pathway, related to lipid metabolism, culminating in intrahepatic lipid accumulation, triglyceride synthesis, insulin resistance, hyperglycemia, as well as oxidative stress production pathways; linking fructose intake to NASH pathogenesis [12-16]. Sirtuin 1 (SIRT1) is a class III protein deacetylase, located primarily in the cell nucleus that is known involved in important metabolic pathways, modulating inflammation, cellular senescence, and cell cycle regulation by deacetylating many crucial transcription factors [17,18]. SIRT1 exerts regulatory control over glucose and lipid metabolism, as well as decreasing fatty liver levels by downregulating lipogenic enzyme expression [19]. Its already described thatSIRT1 activity and expression is modulated by redox modifications and ROS; and antioxidants were able to rescue SIRT1 in different tissues under oxidative stress conditions [18,20-23]. In this context, the heme-heme oxygenase system (HO) represents a key cellular antioxidant defense mechanism that attenuates ROS by breaking down heme (a pro-oxidant) into carbon monoxide and biliverdin [24]. Studies have shown that the inducible HO isoform, hem; heme-oxygenase isoenzyme 1 (HO-1) is up-regulated during oxidative stress and plays a role in various pathological conditions, such as metabolic syndrome [25,26]. Additionally, elevated HO-1 levels has been described to decrease fatty acid synthase (FAS), leadingto improved insulin sensitivity [27]. In this study, we have hypothesized that HO-1, a crucial antioxidant, establishes a cytoprotective module in conjunction with SIRT1 to counteract diet-induced pathways in the liver, thereby attenuating NASH progression. The primary aim of this study is to demonstrate that HO-1 induction in the liver effectively attenuates diet-induced metabolic imbalances, reduces oxidative stress, improves insulin resistance, and decreases hepatic lipid deposition. These positive effects are mediated through the activation of SIRT1 gene expression. We believe that understanding the role of HO-1-SIRT1 axis in the pathogenesis of NASH could potentially lead to the development of new therapeutic strategies to fight liver disease and its associated pathologies, such as obesity and diabetes. From a clinical standpoint, these studies are timely as the prevalence of NASH is on the rise. If left unchecked, this will pose a significant financial strain on the healthcare system and the general population.
Material and Methods
Experimental design for in vitro experiment
Cryopreserved murine hepatocytes (AML12, CRL-2254) were obtained from American Type Culture Collection (ATCC). The cells were cultivated in Dulbecco Modified Eagle Medium (DMEM) and Ham's F12 medium with appropriate additives. Subsequent to cultivation, the cells were meticulously seeded into 12-well plates and 75-cm2 culture flasks, attaining a cellular density ranging between 1 to 2 X104 cells per well and treated every alternate day for a period of 5 days in the presence and absence of fructose (500 μM). Additionally, treatments were carried out in the presence of Cobalt protoporphyrin (CoPP) (5μM), siRNA targeting SIRT1 (or non-specific siRNA control), and Tin mesoporphyrin (SnMP) (5μM). Overexpression of SIRT1 was also conducted in separate experiments. For "knockdown" studies, commercially available Ambion Silencer Select siRNA specific to SIRT1, along with an appropriate scrambled RNA control, was employed. For overexpression studies, the mouse SIRT1 full-length isoform 1 variant (Gene ID93759) was synthesized and integrated into the pJ603 vector, which was contrasted with the pJ603-GFP negative control, both of which were generated by DNA 2.0 Inc. The act of cellular transfection was executed utilizing the FuGENE HD transfection reagent.
Experimental design for in vivo experiment
All animal experiments were conducted with prior approval from the Animal Care and Use Committee, following the guidelines set forth by the National Institutes of Health for the Care and Use of Laboratory Animals. Forty male C57Bl6 mice, aged eight weeks, were fed a high-fructose (HFr) diet for eight weeks to induce fatty liver. Mice were divided into four groups: 1) control diet; 2) HFr diet; 3) HFr diet with CoPP (5 mg/kg, twice weekly) during the last four weeks; and 4) HFr diet with both CoPP (5 mg/kg, twice weekly) and SnMP (20 mg/kg, twice weekly) during the last four weeks. The mice were subjected to weekly weighing and their blood pressure was assessed using the tail-cuff method every 4 weeks. The mice were acclimatized to the tail-cuff procedure before the experiments, and the blood pressure measurements were taken in a temperature-controlled environment (36–38°C) for approximately 10 minutes and were conducted at the same time each day. After the 8 weeks, mice were fasted for eight hours and anesthetized with sodium pentobarbital (65 mg/kg, i.p.) Glucose levels and insulin concentration were determined using blood samples collected from the tail vein through a glucometer (Lifescan Inc., Milpitas, CA) and (ELISA) kit (Abcam, Cambridge, MA) respectively. Blood samples collected in K3EDTA tubes at euthanasia were used to assess alanine aminotransferase (ALT) levels for liver function evaluation Hepatic tissues were snap-frozen in liquid nitrogen and stored at -80°C for later analysis.
Analysis of isoprostane and heme
Isoprostane concentrations were assessed in conditioned media (CM) and murine plasma using an ELISA kit. The quantification of heme content within murine hepatocytes was conducted according to the manufacturer’s instructions of the assay kit.
Analysis of superoxide levels for in vitro experiment
Hepatocytes were seeded in 96-well plates and cultured until 70% confluence. Subsequently, the cells were treated with fructose (500 μM) either alone or in combination with CoPP (5 μM) and SnMP (5μM) for 2 days. Oxidative stress was assessed by incubating cells with 10 μM dihydroethidium (DHE) in the dark at 37°C for 30 minutes. Fluorescence was measured using a Perkin-Elmer Luminescence Spectrometer (excitation/emission: 530/620 nm).
Analysis of triglyceride levels for in vitro experiment
Hepatocytes were cultured in 75-cm² flasks until 70% confluence. After five days of treatment with 500 μM fructose, with or without CoPP (5 μM) and SnMP (5 μM), cells were collected, washed with ice-cold phosphate-buffered saline (PBS), and intracellular triglyceride levels were measured using an assay kit from Abcam (Cambridge, MA)
Analysis of homeostasis model assessment of insulin resistance (HOMA-IR)
HOMA-IR was calculated using the formula: HOMA-IR = [fasting insulin (μU/mL)×fasting glucose (mmol/L)] / 22.5, based on glucose and insulin concentrations from blood samples after an eight-hour fast.
Analysis of triglyceride and cholesterol content in hepatic tissue
After homogenization, the lipids were extracted using a methanol/chloroform (1:2) mixture, dried, and reconstituted in 5% fat-free bovine serum albumin. Triglyceride and cholesterol levels were quantified using an assay kit from Abcam, following the manufacturer's instructions (Abcam, Cambridge, MA).
Analysis of free fatty acids levels in hepatic tissue
Approximately 10 mg of liver tissue was homogenized in 1% Triton X-100 in chloroform. After centrifugation, the organic phase was dried, reconstituted in fatty acid assay buffer, and FFA levels were measured using a kit from Sigma-Aldrich (St. Louis, MO).
RNA extraction and real-time PCR for in vitro and in vivo experiments
Total RNA from hepatocytes and liver tissue was extracted using the RNeasy Protect Mini Kit (QIAGEN, Maryland, USA) and analyzed by quantitative real-time polymerase chain reaction (PCR) using the SYBR Green PCR Master Mix on an Applied Biosystems 7500 HT Fast Real-Time PCR System. Primers targeted HO-1, FAS, SIRT1, acetyl-CoA carboxylase (ACC), sterol regulatory element-binding protein 1c (Srebp-1c), fatty acid elongase 6 (Elvol6), stearoyl-CoA desaturase-1 (SCD-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Fold amplification was determined using the comparative threshold cycle method, with GAPDH as the housekeeping gene. Appropriate controls for siRNAs were included.
Western blot analysis
Hepatocyte pellets were homogenized using an appropriate homogenization buffer, and the supernatant was isolated post-centrifugation at 27,000 x g for 10 minutes at 4°C. SIRT1 levels were determined, with β-actin serving as the loading control.
Statistical analysis
Statistical differences between groups were determined using Tukey’s post hoc test and two-factor ANOVA via GraphPad Prism version 9 (GraphPad, San Diego, CA, USA). Significance was set at p<0.05 or p<0.01, with data presented as mean ± SE
Results
HO-1 induction improves oxidative stress and increases SIRT1 expression in cultured murine hepatocytes treated with high-fructose
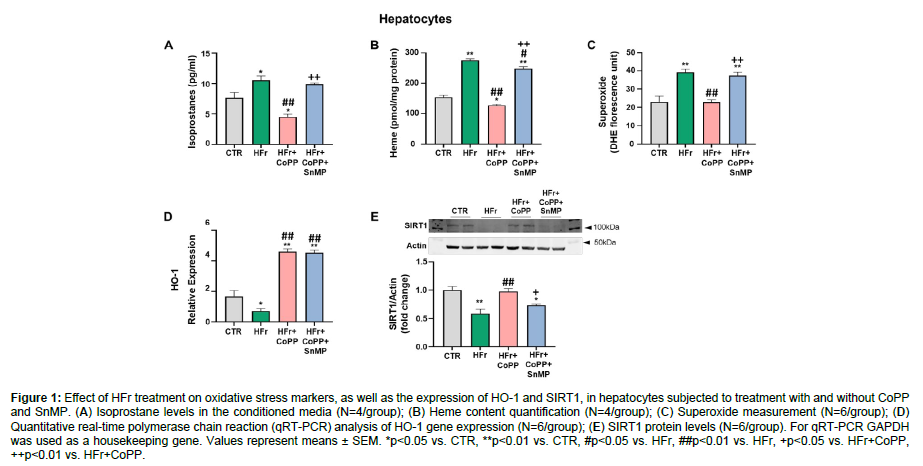
Our data showed that isoprostane, heme, and superoxide levels significantly increased after Hfr treatment in murine hepatocytes as compared to the control group. However, treatment with CoPP reduced all the oxidative marker levels (Figure 1A-C). In addition, the CoPP effects were significantly reversed by concurrent administration of SnMP, highlighting the necessity of increased HO-1 levels for reducing these oxidative markers. As shown in (Figure D), HFr-treated hepatocytes demonstrated a significant decrease in HO-1 levels compared to control. CoPP treatment increased HO-1 levels, while SnMP also upregulated HO-1 expression, consistent with previous findings that SnMP could modulate HO-1 expression despite inhibiting its activity [28,29]. In addition, our data showed that SIRT1 expression significantly decreased in hepatocytes treated with HFr. In contrast, the HO-1 induction, via CoPP, rescued SIRT1 levels and increased its expression compared to cells treated with fructose only. The concurrent treatment with SNMP had a similar effect as in our previous data, reversing the CoPP effect, in this result, leading to a decrease in SIRT1 expression (Figure 1E) (Figure 1).
Figure 1: Effect of HFr treatment on oxidative stress markers, as well as the expression of HO-1 and SIRT1, in hepatocytes subjected to treatment with and without CoPP and SnMP. (A) Isoprostane levels in the conditioned media (N=4/group); (B) Heme content quantification (N=4/group); (C) Superoxide measurement (N=6/group); (D) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of HO-1 gene expression (N=6/group); (E) SIRT1 protein levels (N=6/group). For qRT-PCR GAPDH was used as a housekeeping gene. Values represent means ± SEM. *p<0.05 vs. CTR, **p<0.01 vs. CTR, #p<0.05 vs. HFr, ##p<0.01 vs. HFr, +p<0.05 vs. HFr+CoPP, ++p<0.01 vs. HFr+CoPP.
HO-1 induction improves lipid accumulation in cultured murine hepatocytes
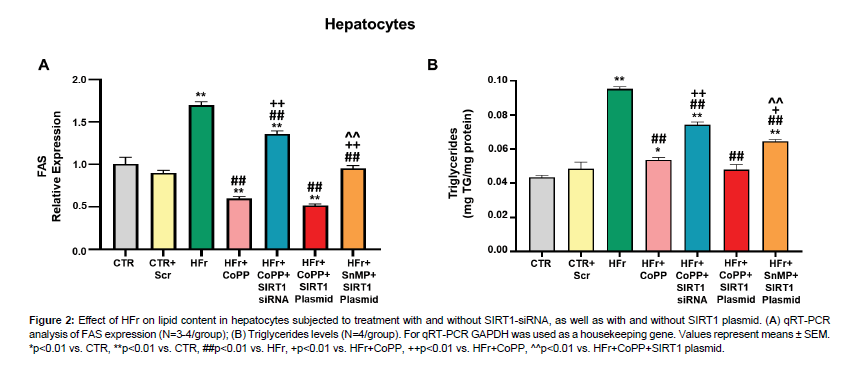
Fructose treatment led to a significant increase in FAS expression and triglyceride levels (Figure 2A, 2B). However, CoPP treatment reversed these effects. Interestingly, concurrent treatment with CoPP and SIRT1 siRNA resulted in increased FAS expression and triglyceride levels, suggesting that HO-1 functions upstream of SIRT1 and that inhibiting SIRT1 attenuates the HO-1 effects. Using the SIRT1 plasmid, we explored whether the upregulation of SIRT1 alone could counteract the adverse effects of HFr on lipid accumulation and metabolic imbalance. Fas expression and triglyceride levels significantly increased after treatment with fructose, SnMP, and SIRT1 plasmid compared to cells treated with fructose, CoPP, and SIRT1 plasmid (Figure 2A, 2B). Our data indicate that the positive effects of HO-1 are partially mediated by SIRT1 activation (Figure 2).
Figure 2: Effect of HFr on lipid content in hepatocytes subjected to treatment with and without SIRT1-siRNA, as well as with and without SIRT1 plasmid. (A) qRT-PCR analysis of FAS expression (N=3-4/group); (B) Triglycerides levels (N=4/group). For qRT-PCR GAPDH was used as a housekeeping gene. Values represent means ± SEM. *p<0.01 vs. CTR, **p<0.01 vs. CTR, ##p<0.01 vs. HFr, +p<0.01 vs. HFr+CoPP, ++p<0.01 vs. HFr+CoPP, ^^p<0.01 vs. HFr+CoPP+SIRT1 plasmid.
HO-1 induction improves the metabolic profile and liver function in mice fed a high-fructose diet
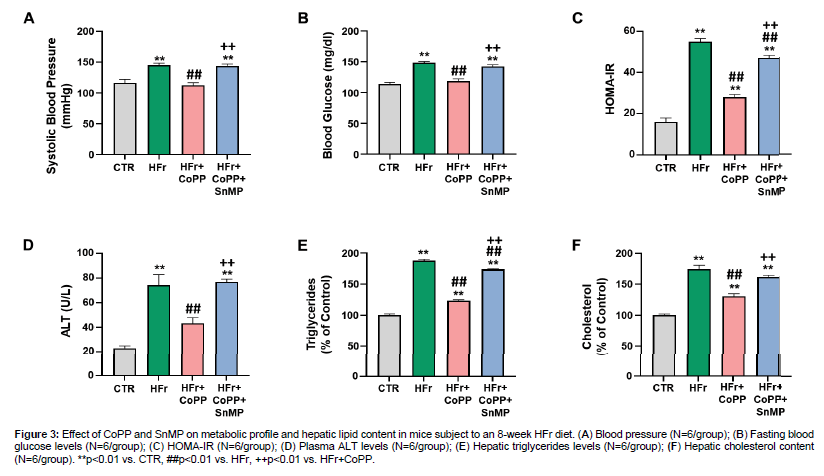
We also evaluated the role of HO-1 in the metabolic profile and liver function. Mice on an HFr diet demonstrated a significant increase in blood pressure and blood glucose levels compared to control (Figure 3A, 3B). However, the CoPP treatment was able to reverse these parameters. In addition, the SnMP co-administration was able to reverse the CoPP effect. HOMA-IR analysis was also performed and indicated increased insulin resistance in HFr-fed mice compared to controls (Figure 3C). CoPP treatment significantly decreased HOMA-IR levels compared to the HFr diet alone. Additionally, serum ALT levels, a marker of liver function, were significantly increased in HFr-fed mice (Figure 3D). CoPP treatment attenuated this increase, while SnMP reversed CoPP's effect, leading to increased ALT levels. SnMP co-administration reversed the CoPP effect. We also assessed the hepatic lipid profile. These results showed a significant increase in triglyceride and cholesterol levels in HFr-fed mice compared to control. CoPP treatment significantly reduced triglyceride and cholesterol levels, while SnMP co-administration reversed the CoPP effects (Figure 3).
Figure 3: Effect of CoPP and SnMP on metabolic profile and hepatic lipid content in mice subject to an 8-week HFr diet. (A) Blood pressure (N=6/group); (B) Fasting blood glucose levels (N=6/group); (C) HOMA-IR (N=6/group); (D) Plasma ALT levels (N=6/group); (E) Hepatic triglycerides levels (N=6/group); (F) Hepatic cholesterol content (N=6/group). **p<0.01 vs. CTR, ##p<0.01 vs. HFr, ++p<0.01 vs. HFr+CoPP.
HO-1 induction improves hepatic lipogenesis in mice fed a high-fructose diet
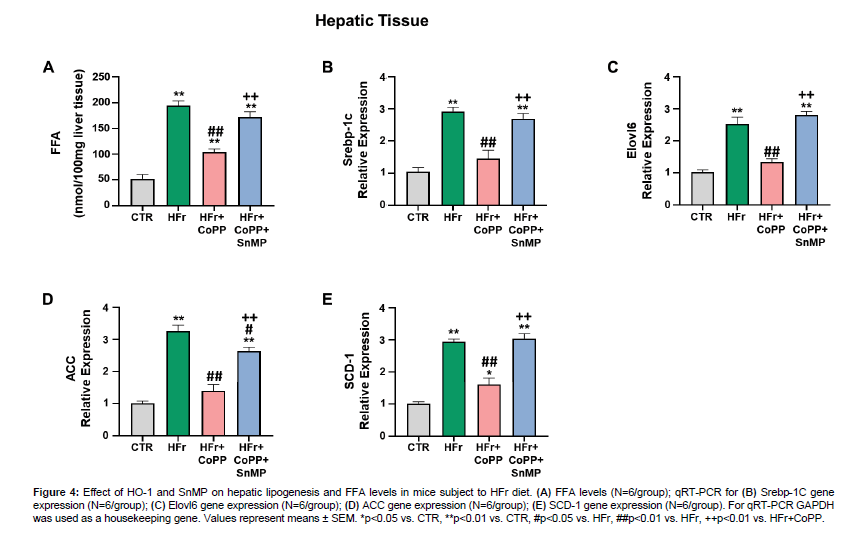
FFA levels were significantly higher in HFr-fed mice compared to control. CoPP treatment decreased hepatic FFA levels in HFr-fed mice (Figure 4A). Furthermore, mRNA expression of hepatic fatty acid synthesis-related genes, including Srebp-1c, Elovl6, ACC, and SCD-1, was significantly upregulated in HFr-fed mice compared to control (Figure 4B-E). CoPP treatment effectively attenuated these increases, while SnMP co-administration reversed the CoPP effects (Figure 4).
Figure 4: Effect of HO-1 and SnMP on hepatic lipogenesis and FFA levels in mice subject to HFr diet. (A) FFA levels (N=6/group); qRT-PCR for (B) Srebp-1C gene expression (N=6/group); (C) Elovl6 gene expression (N=6/group); (D) ACC gene expression (N=6/group); (E) SCD-1 gene expression (N=6/group). For qRT-PCR GAPDH was used as a housekeeping gene. Values represent means ± SEM. *p<0.05 vs. CTR, **p<0.01 vs. CTR, #p<0.05 vs. HFr, ##p<0.01 vs. HFr, ++p<0.01 vs. HFr+CoPP.
HO-1 induction improves hepatic oxidative stress and increases SIRT1 expression in mice fed a high-fructose diet
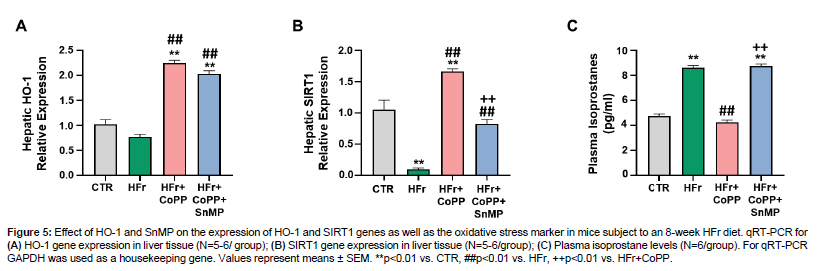
Our data showed that hepatic HO-1 expression increased compared to control in mice subjected to HFr diet as well as concurrently CoPP treatment (Figure 5A). HO-1 expression also increased after SnMP administration, despite inhibiting HO activity, as previously reported [28,29]. Furthermore, hepatic SIRT1 expression was decreased in HFr-fed mice compared to control (Figure 5B). CoPP co-administration increased SIRT1 expression, but SnMP reversed this effect. We also noticed that HFr-fed mice exhibited elevated plasma isoprostane levels compared to control (Figure 5C). CoPP treatment attenuated this increase, while SnMP reversed CoPP's effect leading to increased oxidative stress (Figure 5).
Figure 5: Effect of HO-1 and SnMP on the expression of HO-1 and SIRT1 genes as well as the oxidative stress marker in mice subject to an 8-week HFr diet. qRT-PCR for (A) HO-1 gene expression in liver tissue (N=5-6/ group); (B) SIRT1 gene expression in liver tissue (N=5-6/group); (C) Plasma isoprostane levels (N=6/group). For qRT-PCR GAPDH was used as a housekeeping gene. Values represent means ± SEM. **p<0.01 vs. CTR, ##p<0.01 vs. HFr, ++p<0.01 vs. HFr+CoPP.
Discussion
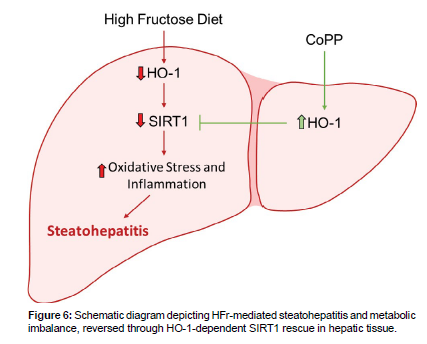
This study reports the protective effect of HO-1 system in attenuating pathologies induced by HFr diet, such as the reduction in hepatic lipid accumulation, and metabolic balance. Notably, our findings demonstrate that this protection is, in part, facilitated by the recovery of hepatic expression of SIRT1 modulated by HO-1. Consequently, our data imply the existence of a hepatic HO-1-SIRT1 axis that effectively attenuates hepatic steatosis pathways and exerts systemic effects, including the restoration of insulin sensitivity. We provide evidence that HO-1 acts via SIRT1 to establish a functional module within hepatocytes, thereby attenuating steatohepatitis and metabolic imbalance. Based on this, the main finding of this study is the relationship between altered redox status and HO-1-dependent modulation of SIRT1 in hepatocytes. It’s already been described that high-sugar diets disrupt the redox state of hepatocytes, leading to increased lipid accumulation in these cells [30, 31]. This is confirmed in our study, as we found an elevated oxidative stress in hepatocytes treated with HFr, accompanied by a suppression of hepatocytes SIRT1 levels. The modulation of SIRT1 by ROS has been demonstrated in the literature, as well as the antioxidant properties of the heme-HO-1 system [18,22,23,26]. Consequently, the protective effects of HO-1 induction on hepatocyte SIRT1 are novel but expected. However, it is crucial to emphasize that the precise molecular mechanism underlying the antioxidant-rescue of SIRT1 expression is not fully understood. Further investigations are needed to unravel the detailed mechanism involved. The use of HFr treatment has been described not only to induce oxidative stress but also to mimic fatty changes in hepatocytes involving the activation of lipogenic genes, such as FAS, a key protein in this pathological adaptation [32-34]. While previous reports have indicated the positive effects of HO-1 induction against oxidative stress [25,35,36], our data reveals that HO-1 induction can modulate the hepatic metabolism in the presence of HFr. SIRT1-dependence of this protective effect of HO-induction is another key finding of the study. Our results showed that fructose-induced changes in cellular redox and subsequent attenuation of SIRT1 mediate, at least in part, the activation of the lipogenic pathways. It has been described that SIRT1, a crucial cellular survival protein, is responsible for modulating gene expression through chromatin acetylation, reducing the access of transcription factors to the promoter region of the affected gene, as well as inhibiting pro-inflammatory pathways and modulating the energy metabolism [37-40]. Furthermore, the activation of pro-lipogenic pathways by fructose is associated with SIRT1 attenuation. Our findings using SIRT1 plasmid and siRNA in cultured hepatocytes, strongly support the idea that the positive effects of HO-1, at least in part are mediated via SIRT1. This, however, does not exclude the possibility of SIRT1-independent component of the effects of HO-1. But overall, our in vitro results indicate that at least part of the protective actions of the HO system on the metabolic pathways in hepatocytes is via SIRT1-rescue. Another key finding of the study highlights the hepato-protective effect of HO-1 in mice fed with HFr diet. For this set of experiments, mice treated with CoPP showed an improvement in hepatic steatosis progression and metabolic balance. Our findings demonstrate high redox potential in hepatic tissues of mice fed with HFr showing the possible role of ROS-related pathways in the pathophysiology of NASH [41]. One such candidate pathway is ROS-induced SIRT1 downregulation, modulating the NAD-dependent deacetylase, leading to a cellular metabolic balance, and the HO-1 system serves as the first line of defense against liver injuries. In addition, our results showed that HO-1 induction leads to a decrease in lipid accumulation and FFA, a reduction in blood glucose levels, and ROS levels in hepatocytes, which are major contributors of liver damage and subsequent insulin resistance. In summary (Figure 6), this study substantiates that the hepatic induction of HO-1 attenuates the fructose-induced changes in lipids metabolism and alleviates the metabolic imbalance linked to NASH progression. These effects are mediated through the activation of SIRT1 gene expression. Notably, the SIRT1 gene expression plays a pivotal role in the redox system. Nevertheless, further comprehensive investigations are warranted to elucidate the intricate interactions between HO-1 and SIRT1 within intact animal systems. The utilization of hepatocyte-specific SIRT1 knockout mice will undoubtedly advance our comprehension of these intricate interactions. We hold the view that a profound understanding of these interactions will pave the way for the identification of novel biomarkers and the formulation of therapeutic strategies to combat hepatic dysfunction associated with NASH (Figure 6).
Conflict of Interest
The authors have declared that no competing interests exist.
Acknowledgment
The authors wish to thank Ms. Jennifer Brown for their contribution to the preparation of this manuscript.
Author Contributions
Designed the experiments: KS and JIS. Performed the experiments: GF and AC. Analyzed the data: AC and AN. Edited the manuscript: RR. Contributed reagents/materials/analysis tools: JIS. Wrote the paper: KS.
References
- Younossi ZM (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64: 73-84.
- Hamid O (2022) The epidemiology of non-alcoholic steatohepatitis (NASH) in the United States between 2010-2020: a population-based study. Ann Hepatol 27: 100727.
- Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, et al. (2016) The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64: 1577-1586.
- Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossiet IM, et al. (2019) Burden of Illness and Economic Model for Patients With Nonalcoholic Steatohepatitis in the United States. Hepatology 69: 564-572.
- Hoek AM, Verschuren L, Worms N, Nieuwkoop A, Ruiter C, et al. (2020) A Translational Mouse Model for NASH with Advanced Fibrosis and Atherosclerosis Expressing Key Pathways of Human Pathology. Cells 9.
- Tacer FK, Rozman D (2011) Nonalcoholic Fatty liver disease: focus on lipoprotein and lipid deregulation. J Lipids 783976.
- Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK, et al. (2009) NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 104: 861-7.
- Siyu Y, Chunlin L, Guang J, Zhang L (2021) The Contribution of Dietary Fructose to Non-alcoholic Fatty Liver Disease. Front Pharmacol 12: 783393.
- Jin R, Vos MB, (2015) Fructose and liver function--is this behind nonalcoholic liver disease? Curr Opin Clin Nutr Metab Care 18: 490-5.
- Jegatheesan P, Bandt JPD, (2017) Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 9.
- Pettinelli P, Obregon AM, Videla LA, (2011) Molecular mechanisms of steatosis in nonalcoholic fatty liver disease. Nutr Hosp 26: 441-50.
- Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Lipkin EW, et al. (2012) Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 56: 952-60.
- Abdelmalek MF, Suzuki A, Guy C, Arida AU, Colvin R, et al. (2010) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51: 1961-71.
- Basaranoglu M, Basaranoglu G, Sabuncu T, Sentürk H, et al. (2013) Fructose as a key player in the development of fatty liver disease. World J Gastroenterol 19: 1166-72.
- Johnson RJ, Santos EPP, Sautin YY, Manitius J, Lozada GSL, et al. (2009) Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 30: 96-116.
- Federico A, Rosato V, Masarone M, Torre P, Dallio M, et al. (2021) The Role of Fructose in Non-Alcoholic Steatohepatitis: Old Relationship and New Insights. Nutrients 13.
- Salminen A, Kaarniranta K, Kauppinen A (2013) Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int J Mol Sci 14: 3834-59.
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, et al. (2010) Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 501: 79-90.
- Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, et al. (2009) Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am J Physiol Endocrinol Metab 297: 1179-86.
- Singh CK, Chhabra G, Ndiaye MA, Peterson LMG, Mack NJ, et al. (2018) The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal 28: 643-661.
- Ogura Y, Kitada M, Koya D (2021) Sirtuins and Renal Oxidative Stress. Antioxidants 10: 1198.
- Shi L, Zhang J, Wang Y, Hao Q, Chen H, et al. (2020) Sirt1 Regulates Oxidative Stress in Oxygen-Glucose Deprived Hippocampal Neurons. Front Pediatr 8: 455.
- Zhang T, Chi Y, Ren Y, Du C, Shi Y, et al. (2019) Resveratrol Reduces Oxidative Stress and Apoptosis in Podocytes via Sir2-Related Enzymes, Sirtuins1 (SIRT1)/Peroxisome Proliferator-Activated Receptor gamma Co-Activator 1alpha (PGC-1alpha) Axis. Med Sci Monit 25: 1220-1231.
- Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, et al. (2003) Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J17: 1724-6.
- Chiang SK, Chen SE, Chang LC, (2021) The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells 10.
- Dongdong L, Zhao D, Jinghua D, Dong S, Aldhamin Z, et al. (2020) Heme oxygenase-1 alleviated non-alcoholic fatty liver disease via suppressing ROS-dependent endoplasmic reticulum stress. Life Sci 253: 117678.
- Kuo NK, Huang SY, Yang CY, Shen HH, Lee YM, et al. (2020) Involvement of HO-1 and Autophagy in the Protective Effect of Magnolol in Hepatic Steatosis-Induced NLRP3 Inflammasome Activation In Vivo and In Vitro. Antioxidants 9.
- Abate A, Zhao H, Wong R, Stevenson D (2007) The role of Bach1 in the induction of heme oxygenase by tin mesoporphyrin. Biochem Biophys Res Commun 354: 757-63.
- Schillingmann DA, Riese SB, Vijayan V, Zimmermann ST, Schmetzeret H, et al. (2019) Inhibition of Heme Oxygenase-1 Activity Enhances Wilms Tumor-1-Specific T-Cell Responses in Cancer Immunotherapy. Int J Mol Sci20.
- Basciano H, Federico L, Adeli K, (2005) Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab 2: 5.
- Pan JH, Kim HS, Beane KE, Montalbano AM, Lee JH, et al. (2018) IDH2 Deficiency Aggravates Fructose-Induced NAFLD by Modulating Hepatic Fatty Acid Metabolism and Activating Inflammatory Signaling in Female Mice. Nutrients 10(6).
- Softic S, Meyer JG, Wang G, Gupta MK, Batista TM, et al. (2019) Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab 30: 735-753.
- Softic S, Cohen DE, Kahn CR. (2016) Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig Dis Sci 61: 1282-93.
- Vos MB, Lavine JE, (2013) Dietary fructose in nonalcoholic fatty liver disease. Hepatology 57: 2525-31.
- Zhang J, Cai W, Fan Z, Yang C, Wang W, et al. (2019) MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis 290: 9-18.
- Converso DP, Taillé C, Carreras MC, Jaitovich A, Poderoso JJ, et al. (2006) HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J 20: 1236-8.
- Zhang T, Kraus WL (2010) SIRT1-dependent regulation of chromatin and transcription: linking NAD(+) metabolism and signaling to the control of cellular functions. Biochim Biophys Acta 1804: 1666-75.
- Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, et al. (2022) Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol 13: 831-168.
- Majeed Y, Halabi N, Madani AY, Engelke R, Bhagwat AM, et al. (2021) SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci Rep 11: 8177.
- Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, et al. (2010) SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab 298: 419-28.
- McCullough AJ (2006) Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol 40 1: 17-29.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Sodhi K, et al. (2024) Beneficial Role of HO-1-SIRT1 Axis in AttenuatingSteatohepatitis. Biochem Physiol 13: 482. DOI: 10.4172/2168-9652.1000482
Copyright: © 2024 Sodhi K, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 971
- [From(publication date): 0-2024 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 645
- PDF downloads: 326