Basic Concepts of Genotype by Environment Interaction and Stability Analysis on Linseed: Review
Received: 01-Jul-2024 / Manuscript No. acst-24-142260 / Editor assigned: 04-Jul-2024 / PreQC No. acst-24-142260 / Reviewed: 18-Jul-2024 / QC No. acst-24-142260 / Revised: 22-Jul-2024 / Manuscript No. acst-24-142260 / Published Date: 29-Jul-2024
Abstract
Linseed is currently revitalized worldwide owing to its multiple values for food, feed, medicinal, and industrial uses. It has beneficial effects in preventing cancer, coronary heart disease, and sudden death from arrhythmia for its rich source of omega-3 fatty acids, plant lignans, and soluble fiber that can contribute vital to healthy life. As a result, it is now designated as one of the healthy foods for the 21st century. This paper reviewed GxE interaction effects and basic concepts of stability on Linseed to discern useful information that could be used for future research input and development endeavors. Genotype and Environment (GxE) Intuitive are characterized as a wonder in which phenotypes react to genotypes in an unexpected way concurring with diverse natural variables. The concept of genotype by environment interactions leads to measuring the agronomic stability of the genotype. Linseed grain abdicates steadiness is affected by the capacity of a genotype to respond to natural conditions, which is decided by the genotype’s hereditary composition. The stability analysis and adaptability of promising breeding materials are basic and important measuring concepts for wide and specific recommendations for known agricultural systems. The method commonly used for the analysis of G×E interaction is the Linear Regression model in which the bi-values give information about adaptability and S2d is used as a measure of the stability of performance, Additive Main Effects, and Multiplicative Interaction (AMMI) method as a measure of stability and adaptability. Linseed productivity is a direct consequence of how well adapted an individual’s genotype is to the surrounding environment and specific environment.
keywords
AMMI; G×E interaction; Linear; Omega-3; Fatty acids; Stability
Introduction
In Ethiopia, the major highland oilseeds which the oil millers currently use as raw materials are Noug (Niger), linseed, and Ethiopian mustard, especially by large oil millers. Although Noug and linseed are dominantly used for edible oil, their productivity and oil content are lower than sunflower, and their oil, especially for linseed, is sensitive to autoxidation making the oil rancid [1]. Linseed (Linum usitatissimum L.) has been a traditional crop in Ethiopia and it is the second most important oil crop in production after Niger seed (Guizotia abyssinica CASS) in the higher altitudes (Adugna & Adefris, 2000). Any living thing living on earth develops variations due to either genetic effects or environmental effects or both, as a result, a change in the genetic sequence due to genetic effects is defined as genetic variation, and the variation due to environmental effects is defined as environmental variation (Yash, 2015). GxE interactions are principal to estimate the performance stability regarding the variety yield or genotype. According to Nath & Dasgupta (2013) in plant breeding, the basic concept of stable genotype shows little or no deviation from the already-known trait status when tested over several environments [2]. The growing environment can alter the quantitative trait behavior and expression of an organism. Different scholars depicted epigenetic variation as occurring due to the mechanism to survive and express its plasticity nature for specific traits of the genotypes across mega-environments (Malosetti et al., 2013; Zhang et al., 2013).
During GxE correlation analysis, the magnitude indicates the degree of shared genetic control and the sign indicates the direction of the allelic effect over the environments. This viewpoint has provided an important basis for interpreting and handling GxE in plant breeding programs (Misra and Patnaik. 2009) [3]. According to Falconer (1966), the development of statistical tools to quantify, understand, and interpret GxE in plant breeding is crucial. Efforts made in high land oil crop breeding research were very high in studies of Genotype and Environment (GxE) Interactions on Linseed under Diverse Agro-ecologies. However, there are fragmented research works on different institutions that need to be compiled. This paper reviewed some of the research results on genotype by environment interactions studies on Linseed generated during the last decades along with its overall research methodologies of GxE and stability analysis together with basic concepts [4].
Literature Review
Genetic resources of linseed
The International Plant Genetic Resource Institute (IPGRI) can handle Suitable maintenance procedures of plant genetic resources; and would keep the composite available materials for distribution to any interested breeder; operations to make it more useful and effective (Chweya & Eyzanguire, 1999). Plant Gene Resources Canada (PGRC), the Canadian plant germplasm repository in Saskatchewan, houses, conserved more than 3300 accessions of Linum L. germplasm and is one of the largest collections of Linseed in the world. At IPGRC cultivated Linseed accessions for oil and fiber type are collected from 72 producing countries from all regions of the world (Diederichsen, A. and Fu, Y.B., 2008) [5].
Linseed center of origin and diversity
The development of linseed goes back to the first light of civilization, as appeared by ancient Swiss Lake Tenants (Vavilov, 1926). There are solid signs that Linum started in a range east of the Mediterranean locale, outstandingly adjacent to India (Lühs & Friedt, 1994), and it spread northwards and it spread northwards and westwards; most researchers believe that linseed originated in an area east of the Mediterranean Sea because of the great diversity forms found in this region; since one indication for origin (Lay and Dybing, 1989) [6]. Linseed was grown for its oil and was developed primarily in southwestern Asia, whereas flax, or the fiber type was developed in the Mediterranean region (Berglund, D.R., 2002). Linseed was used as a food during the Greek and Roman eras, a practice that has continued in both India and Ethiopia even today (Adugna, 2000; Berglund, D.R., 2002). Ethiopia was also reported as a center of diversity for linseed (Vavilov, 1926 & 1951; Harlan, 1969; Mengesha & Jembere, 1975) [7].
Current scenario of linseed area coverage, production and uses
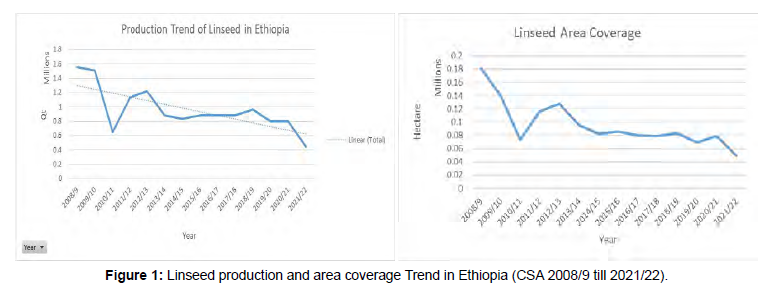
Linseed is grown for oil or fiber, isolated from the stem of the plant, flax. Canada is the world’s largest producer of Linseed, about 50% of total world production, most of which is grown in the Prairie Provinces of Canada for seed oil. It is widely grown in Ethiopia for edible oil; the country has immense potential for Linseed production and is ranked as the third-largest producer of Linseed after China (Faostat, 2009). In Small-scale production, farmers have been producing it organically, without applying any chemicals (fertilizers, herbicides, pesticides) and with minimum inputs (Adugna, 2000). According to the Central Statistical Authority of the country, Linseed production and area coverage have decreased tremendously over the last ten production years (Fig.1). Even though the area coverage of linseed is small compared to other crops, the country has immense potential and large agricultural suitable land for Linseed production (CSA, 2008/9-2021/22) (Figure 1) [8].
According to estimates of CSA, the average annual production of linseed in Ethiopia was about 0.96 million t from an area of 0.1m ha for the last 14 production years, with an average productivity of 1.16 t/ha and the main producing regions were Oromia, Amhara, Tigray, and SNNPR (CSA, 2021). It has been grown in Ethiopia primarily for food and fiber to generate cash revenues for the farmers either on local markets or by exporting abroad (Adefris et al., 1991). For food, the seeds are usually roasted, ground, and mixed with spices and some water to be served along with different local breads. It is also consumed in various forms of soups, soft drinks, porridge, cooked potatoes, etc. It is particularly consumed during fasting days. Hence, Linseed has been cultivated in Ethiopia for its seed and oil, and its use as a fiber crop is very rare in Ethiopia but nowadays some initiations have been started by the national program at Holeta Agricultural Research Center [9].
Linseed oil has many food and industrial applications, while its seed cake is also a valuable feed for livestock. The oil has 55-58% of unique alfa-linolenic fatty acid, an essential omega-3 fatty acid, which has beneficial effects on health and the auto-immune system (Moris, 2007). The soluble fiber of Linseed helps to lower blood cholesterol, while insoluble fiber promotes laxative effects. Moreover, its lignan (phytoestrogen, a plant compound with estrogen-like activity) was found useful for women's health (Payne, 2000). Unfortunately, Linseed is currently becoming the most healthy and popular food worldwide. Undoubtedly, Linseed has customarily been devoured and acknowledged for different restorative values in nations like Ethiopia, India, and China since old times (Seegler, 1983; Adugna, 2002) [10].
Linseed oil has been mostly used for industrial purposes (Lay & Dybing, 1989; Rowland et al., 1994) in making varnishes, paints, and the like due to its high linolenic fatty acid, which is known for its fast-drying quality because of its auto-oxidative three double bonds. All things considered, this character of Linolenic corrosive that promptly oxidizes and produces off-flavor, rancidity, has constrained the utilization of Linseed oil for consumable purposes, especially for cooking oil [11]. To fulfill the natural gap that linseed lacks “Linola” and “Solin” oil-type varieties were developed in Australia and Canada, respectively (Adugna et al., 2004). Their linolenic acids were reportedly reduced to about 2%, with an increase of linoleic acid, while the level of other fatty acids remained constant. These achievements are believed to expand the market opportunities and uses of Linseed globally [12].
Basic concepts, and uses of GxA interactions
Genotype refers to an individual’s genetic makeup. Concerning the comparison of plant material in a set of multi-environment yield trials, the term genotype refers to a cultivar rather than to an individual’s genetic makeup (Annicchiarico, 2002). Environment can be defined as the total surrounding scenario or situation that an organism or group of organisms faces to express its genetic potential (Kang, 1997). In an agricultural context, it is defined as a general term that covers conditions under which plants grow and may involve locations, years, management practices, or all combinations of these factors (Romagosa and Fox, 1993). To a geneticist, it is the total of physical, chemical, and biological factors that influence the development of an organism (Dabholkar, 1992). The phenotype of an individual can be determined by the effects of the genotype and the environment surrounding it (Dabholkar, 1992). phenotype refers to the physical appearance or dissemble traits of an individual, which may be observable at a physical, morphological, anatomical, or biological level (Kang, 1993) [13,14].
Genotype by Environment Interaction (GxE); according to Kang and Magari (1996) there are three types of genotypes by environment interaction: I) crossover interaction (COI) or genotypic rank changes across environments, the most crucial interaction in crop improvement and production, II) non-crossover interaction (non-COI) or scale changes among environments and III) combination of both. Major genotype by environment interaction can be expected when there is wide variation between genotypes for morphological characters conferring resistance to one or more stresses, and wide variation between environments for incidence of the same stress(es) as determined by climate, soil, biotic, and management factors (Annicchiarico, 2002) [15].
GxE is a major concern in plant breeding for two main reasons; it reduces progress from selection, and it makes cultivar recommendation difficult because it is statistically impossible to interpret the main effects (Allard and Bradshaw, 1964; Kang and Magari, 1996). Knowledge of Genotype by Environment (GxE) interaction can help plant breeders reduce the cost of extensive genotype evaluation by eliminating unnecessary testing sites (Shaffi et al., 1992; Kang and Magari, 1996; Basford and Cooper, 1998) [16]. Conversely, the presence of a large GxE interaction may necessitate the establishment of additional testing sites. Subsequently, on the off chance that cultivars are chosen for a huge gathering of situations, solidness and cruel surrender over all situations are more vital than surrender for particular situations (Piepho, 1998). Differential genotypic expressions of varieties across environments force plant breeders to examine genotypic adaptation and measure genotype by environment interactions carefully to determine an optimum breeding strategy for releasing genotypes, with adequate adaptation to target environments (Romagosa and Fox, 1993) [17].
Crop performance, the observed phenotype, is a function of genotype or variety or cultivar, environment, and GxE. To occur GxE is significant when genotypes or varieties perform or respond differently across texted locations or environments. Researchers agree that GxE is important only when it is signi?cant and causes signi?cant change in genotype ranks in different environments, i.e., different genotypes are superior in different environments (Haldane, 1946). For GxE to be recognized through factual methods, there must be at least two particular genotypes or cultivars assessed in at least two diverse situations. Thus, a basic model that includes GE Interaction is:
P = G + E + GxE.
This model can be written from a statistical standpoint as Pij = µ + Gi + Ej + (GE)ij. Here, µ is the overall mean. It follows from this model that, for a given genotype, there can be many phenotypes depending on the environment and GxE interaction (Falconer, 1966) [18]. The minimum number of genotypes and environments for such a model is two (Simmonds, 1981). Within the improvement of cultivars or assortments, that can be adjusted to a wide extent of broadened situations; soundness; is one of the major goals of plant breeders in an edit change program. Concurring to Eberhart and Russell (1966) solidness is the capacity to appear a least interaction with the environment. Subsequently, the soundness of genotype execution is straightforwardly related to the impact of GxE Interaction. The two fundamental concepts of phenotypic solidness are a natural concept; which alludes to the steady execution of a genotype over a wide run of situations and the agronomic concept; which states that a steady genotype ought to continuously grant a tall surrender anticipated at the level of efficiency of the particular situations, i.e., a assortment with genotype by environment interaction as little as conceivable (Becker, 1981). In this way, successful varieties of linseed ought to be adjusted to a wide run of natural conditions in Ethiopia to guarantee their abdicate steadiness and financial productivity, as agriculturists are more curious about assortments that create reliable yields beneath their developing conditions and breeders moreover need to fulfill these needs (Adugna and Labuschagne, 2003) [19,20].
Inferences of GxE and stability on linseed improvement
Agricultural researchers have long been cognizant of the various implications of GxE in breeding programs (Medina, 1992; Yates and Cochran, 1938). GxE hurts heritability, the lower the heritability, the higher the difficulty in improving the target trait through selection. Understanding the structure and nature of GxE is important in plant breeding programs because a significant GxE can seriously impair efforts in selecting superior genotypes relative to new variety development (Sha?i and Price, 1998; Shukla, 1972) [21]. Information on the structure and nature of GxE is particularly useful to breeders because it can help determine if they need to develop cultivars for all environments of interest and speci?c target environments (Bridges, 1989). Gauch and Zobel (1996) explained the importance of GxE as follows: “Were there no interaction, a single variety of wheat (Triticum aestivum L.) or corn (Zea mays L.) or any can feed the world and also a single variety trial needed to provide universal results [22].
Extracting and interpretation of GxE study
A significant GxE interaction increases, and the reliability and importance of the main effects harmoniously decrease. Several methods have been proposed to Analyze G×E interactions or phenotypic stability (Lin et al., 1986) and classified into univariate and multivariate stability analysis (Lin et al., 1986). According to Becker and Leon (1988), Joint regression (JR) is one of the univariate analysis methods popularly used by scholars because of its ease and understandable calculation and application procedure, whereas AMMI has become popular recently and also currently the main option for multivariate analysis to the joint regression analysis in most of the national breeding skim (Annicchiarico, 1997) [23]. Multi-environment testing (MET) is important to identify a variety that has consistent performance across locations and over years showing in-significant variability. The extensive testing, however, allows the application of stability analyses to identify genotypes that are widely adopted. Such analyses also make it possible to identify those genotypes that are speci?cally adapted to a particular environment (Yan and Kang, 2002) [24].
Estimations of the fluctuation components showed that G×E intelligence clarified a sizable extent of the phenotypic fluctuation (Ruud, 2015). Plant enhancement includes together the control of hereditary characteristics to optimize efficiency with the confinements of natural components (Nath & Dasgupta, 2013). According to Leflon et al. (2015) the difference between the value of phenotypic to expected value of an additive model that considers the overall mean as well as the genetic constitution as genotypic and the surrounding environment's main effects refers to genotype by environment interaction [25]. Determining the yield-hindering factors and integrating these factors for further analyses of the interaction for the other tested genotypes under different environments is an important task of Crop diagnosis (Leflon et al., 2015). Linear Regression model is widely used for the analysis of G×E interaction in agricultural experiments (Eberhart and Russell, 1966). The S2d and bi-values give information about the stability of performance and adaptability of tested genotypes respectively. The use of the AMMI model as a measure of stability and adaptability. AMMI show could be a way better strategy for the examination of G×E interaction in duplication varietal trials (Zobel et al., 1988). It does not allow an assessment of the full G×E interaction impact of each genotype but also segments it into interaction impacts due to situations (Abuali et al., 2014). AMMI is one of the vital components of examination and a common strategy in quantitative hereditary think about it's a combination of measurable shows joining both ANOVA and PCA for analyzing two-way (G×E) information structures (Gomez and Gomez, 1984) [26].
Brief on mega environment trials management
In plant breeding genotype evaluations for mega environments on the performance of genotypes have depended on multi-location field trials that represent potential production environments and those are used to identify and recommend varieties (de Leon et al., 2016). Over-location trials provide two-way tables of means for genotypes across different environments [27]. The data collected were subjected to analysis of variance and least significant difference to separate the means that showed significant differences at 5% or 1% probability level and the combined analysis of variance will lead to estimates of genotype, environmental, genotype by environment interaction with the addition of source of variation as; the residual experimental error. The PC and the GGE biplot computer program can alter that by translating complex information sets as effectively as several keystrokes and several minutes of seeing easy-to-understand realistic shows [28]. GGE bi-plot gives a clue of the extent of the change that suggests the most ideal genotype compared to GxE, but it is restricted in terms of giving interaction effect over the environment. In a mega environmental study consideration of fundamental concepts on elucidations of the line shows a higher mean yield, the line that passes through the origin and is perpendicular to the ATC with dual arrows represents the stability of the genotypes, either direction away from the biplot origin on this axis indicates greater GxE interaction and reduced stability and so, the ideal genotypes are the closest to the concentric circle and having a line through the origin and is perpendicular to the ATC with either direction arrows, high yielder and stable [29].
Work done on GEI and stability in linseed
According to Adugna and Labuschagne (2003), significant GxE Interactions among ten linseed genotypes were tested across eighteen environments in Ethiopia. They noted fluctuations of genotypes in their responses to different environments. They also identified a stable genotype (R12-N10D) for wider adaptation and narrowly adapted genotypes for specific environments. Mostafa and Ashmawy (1998) also evaluated eight linseed genotypes at three locations for two seasons for seventeen parameters. And reported they that both environment and genotypes affected seed yield and identified three stable genotypes across tested environments. Similarly, Mishra and Rai (1993) studied GxE Interaction and stability in ten varieties of Linseed and their 45 F1 hybrids for their seed yield and oil content across four environments in India. They reported significant GxE Interaction and identified stable genotypes as well [30].
According to Adane (2008) Significant differences between Linseed genotypes were observed at all environments for days to flowering (DF); at seven environments for days to maturity (DM), plant height cm (PH), harvest index (HI), thousand seed weight (TSW); at five environments for seed yield in kg/ha (SYH,), oil content (OC) and oil yield in kg/ha (OYH); at four environments for BMH. Also, the Author Adane indicated in his study GEI was statistically significant for DF, DM, SKH, HI, OKH, TSW, BMH, wilt, and PM. The significance of GEI indicates the differential response of genotypes across locations for these characters. Adugna and Labuschagne (2003) also reported significant GEI for DF, DM, PH and SYH. However, the GEI was not significant for oil content. Non-significant GEI for oil content in Indian mustard was also reported by Dhillon et al., 1999 [31].
Out of the total sums of squares of seed yield, the environment has taken the major share contributing 75.27%, followed by GEI, error, and genotypes contributing 8.72%, 8.47%, and 5.25%, respectively (Adane, 2008). The larger portion of the sums of squares due to the environment and the higher the GEI contributions to the total sums of squares as compared to the genotypes denotes the significant influence of the environment in the evaluation of linseed genotypes. Similar results were also reported in linseed (Adugna and Labuschagne, 2002) and in sesame (Mekonnen & Mohammed, 2010). Partitioning of the total variances of oil content also indicated that location (E) contributed 34.87%, genotypes 19.85%, GEI 13.99%, replications within locations 6.46%, and error 24.84% [32]. The relatively lower contribution of sums of squares due to environment and higher contribution of sums of squares due to genotypes on oil content as compared to seed yield indicates that environmental influence on oil content was not as large as its influence on seed yield. Similarly, Adane (2008) also indicated in his study; that stability analysis of variance revealed highly significant (P<0.01) differences between genotypes for both yields, suggesting the considerable differential performance of the genotypes. The environment and the GEI were not significant for both yields, demonstrating that there were no differences among slopes of regression lines and the regression model was inadequate in describing the stability of the linseed genotypes but the deviation from the regression (S2d) was significant, indicating the importance of only nonlinear sensitivity in the expressions of these traits [33].
Studies in Chillán, Yungay, Los Ángeles, and Osorno for different cropping years confirmed that there were significant differences among genotypes for biomass yield and the GxE interaction was not significant. And the authors depicted there were significant differences among genotypes for the harvest index, the GxE interaction was not significant. The genotypes Rahab and Carapé had the highest harvest index of 37.7%. This value is considered high for an oilseed crop whose levels generally fluctuate between 20 and 30% (Aufhammer et al., 2000) [34].
Genetic diversity studies undertaken using randomly amplified polymorphic DNA analysis show genetic variation in line with the four centers of Linseed diversity (i.e., Abyssinian, Mediterranean, Central Asian, West Asian) but not associated with any particular morpho-type (Mekbib, 1986 & Engels, 1991). A core Linseed(flax) world collection of 500 distinct Linseed accessions has been selected by Diecherichson (2006), curator of PGRC (A.A.F.C.) based on genetic diversity studies, phenology, morphological, and agronomic traits, as well as importance to Canadian agriculture. The core world collection was grown in a modified augmented design in the 2010 growing season in two locations, Kernen and AAFC Research Station, and evaluated for days to flowering and maturity as well as for morphological and agronomic traits [35]. The Linseed core collection is being screened for oil, protein, mucilage, lignans, cyclolinopeptides, carotenoids, and cyanogenic glycosides (Shim, 2015). The potential negative implications of GxE during selection and variety deployment have been understandable and work should be done to minimize its effect and, take advantage of positive interactions (Sadras and Richards, 2014).
The genotypes, which were found to be stable for all or most of the characters, were considered to be highly stable. A study done in the Shambu area showed from the pooled mean yield, it can be seen that the best-yielding genotype was CI-1528 with a mean yield of 1.576 tons ha followed by Kulumsa-1 (1.565 tons/ha), Berene (1.562tons/ha), and CI-1652 (1.51 tons/ha) (Temesgen et al., 2014) [36]. The study also depicted the analysis of variance for seed yield over six environments based on the AMMI model for Linseed was Significantly different (p≤0.01) among environments, genotypes, and genotypes by environment interaction. The significant interactions among genotypes by environment showed that the genotypes varied across the environments in seed yield. GxE interaction was further partitioned into principal component axes and only the first IPCA was significantly different and explained the largest proportion (62.25%) of the interaction, while the remaining proportions of the interaction sum of squares were not significant. As a result, the analysis is entirely dependent on the AMMI1 model. The AMMI biplot involved the values of the IPC1 axis and mean yield in which the genotypes and environments were dispersed around the center biplots, indicating a substantial amount of variability in genotypes and environments. Their findings are in coincide with the findings of many studies on common bean (Abeya et al., 2008; Lirie et al., 2013); durum wheat (Alamnie et al., 2004), barley (Alamnie et al., 2004) and maize (Wende and Labuschangne, 2005) [37].
According to Fikere et al., (2013) research study on 49 linseed accessions collected from five regions of Ethiopia together with fifteen exotic cultivars was used to investigate biochemical diversity between and within germplasms. The higher oil percentage (39.8%) was recorded from genotype PI-52335 and the lowest was from genotype Acc. 219333 (30.63%). The highest palmitic acid percentage (7.06%) was observed from Acc. 237494. The maximum and minimum stearic acid content was found from Acc. 13545 and Acc. 13756 with 6.21% and 4.74% respectively. Maximum oleic acid of 21.4% was also observed in Acc. 13545 and the crude protein, crude fat, and iodine values were analyzed (Fikere et al., 2013). Accordingly, the author depicted the first three principal components accounted for more than 73.3% of the total variation. The variation between linseed genotypes revealed that there was a highly significant variation showing remarkable genetic variability of studied genotypes [38].
A study done in India reported that, at five different locations during the main season, 30 linseed varieties were evaluated for their stability under varying environmental conditions. GxE and E + (G×E) were significant for all the characters except the number of seeds per capsule. Significant differences for G×E, linear, were observed for traits of primary branches per plant, secondary branches per plant, aerial biomass yield per plant, seed yield per plant, and 1000-seed weight. According to the author, the bi-value, and S2di-value of the genotypes Him Alsi-2, KL-241, and Nagarkot showed the genotypes are highly stable and most adaptive to overall environments and can be utilized as a donor in crossing programs for traits of interest (Sharma and Paul, 2016).
According to Sharma and Paul (2016), the stability performance of 30 linseed genotypes including cultivars and elite lines was compared by using regression on environmental means for grain yield and related traits for five locations during the main season, and Significant differences were observed among tested genotypes for traits of interest. AMMI analysis showed a highly significant (P≤ 0.001) variation due to locations and varieties for yield and oil yield, oil content, whereas GxE interaction was significant for oil content and oil yield, and not for grain yield indicating that the stability of the genotypes over the range of locations tested (Lirie et al., 2013). According to the author, five varieties showed stable oil content, and three varieties showed stable oil yield depicting they were stable and adaptable for wider recommendation in the Amhara region. The result of AMMI analysis, based on oil content and yield for specific locations included variety Kassa-2 for all tested locations; variety Jeldu for Holetta and Kokate and similar areas; variety Kulumsa-1 for Didamidore, Hossaina, and Kokate and similar areas; variety CI-1525 for Holetta and Hossaina and similar areas; variety Dibannee for Hossaina for both oil content and oil yield but for Kokate area only for oil yield. The GxE Interaction showed a significant difference for oil content and oil yield but not for grain yield and non-significant GxE Interaction for grain yield depicts the stability of the genotypes over all tested locations (Misra et al., 2009) [39].
According to Cherinet and Taddese (2014) study on the Northwest Amhara region, there were significant variations among environments and genotypes for most of the characters; indicating the existence of variability among the tested genotypes. From all tested environments debark was the most ideal and suitable production location for target traits of seed yield, oil content, and oil yield, and most importantly fatty acid profile content of linolenic acid. The combined analysis of variance for traits of seed yield, oil content and yield, and linolenic content, the variety Kulumsa-1 can be used for a wider recommendation for the Northwest Amhara area and similar agroecological regions of the country [40].
Conclusions
Linseed is currently revitalized in the world owing to its multiple values for food, feed, medicinal, and industrial uses. It has beneficial effects in the prevention of cancer, coronary heart disease, and sudden death from arrhythmia for its rich source of omega-3 fatty acids, plant lignans, and soluble fiber that can offer vital contributions to healthy life. As a result, it is now designated as one of the healthy foods for the 21st century. In this paper, efforts of research work on linseed GxE were reviewed to discern useful information that could be used in future development endeavors. A multi-location trial that was conducted to determine the adaptation potential, GxE interaction, and yield stability of linseed genotypes across environments, for a majority of works, has shown highly substantial variances among the genotypes for different traits of interest.
However, when unpredictable environments prevail due mainly to unreliable weather conditions, the strategy needs to focus on developing stable genotypes. So, Plant production and productivity are a direct consequence of how well-adapted and stable the genotype is to the target environment. This duly enhances the effectiveness of production and productivity of the crop as well. A pertinent point is to predict accurately the performance of a variety across a wide environment and an understanding of the target environment with its components gives a brief clue about the status of linseed varieties' GxE interactions and supports breeders' ability to recommend appropriate variety for the target environment or wider recommendation. Finally, the author would like to say and recommend the importance of GxE study in linseed and other oil crops is important for linseed breeding strategy development and also for the linseed extension system as a whole to penetrate the current cropping system and meet the needs of upcoming world population growth demand.
Acknowledgment: The current study has not been sponsored by any sector or organization. Even, the author would like to acknowledge the Holeta agricultural research center and the national high and mid-land oil seed research program staff who worked valuable research, and information as a whole.
Declaration of interest statement
There are no potential conflicts of interest.
References
- Abeya Temesgen AT, Chemeda Chaba CC, Girma Mangistu GM, Dagnachew Lule DL, Negash Geleta N G, et al (2008) Regression and additive main effects and multiple interactions (AMMI) in common bean (Phaseolus vulgaris L.) genotypes.
- Abuali AI, Abdelmula AA, Khalafalla MM, Hamza NB, Abdalla AH, et al. (2014) Assessment of yield stability and adaptability of parental inbred lines and F1-hybrids of grain maize (Zea mays L.) Using AMMI Analysis. British Biotechnology Journal 4: 339-349.
- Adugna W, Labuschagne MT (2002) Genotype‐environment interactions and phenotypic stability analyses of linseed in Ethiopia. Plant breeding, 121: 66-71.
- Adugna W, Labuschagne MT (2003) Parametric and nonparametric measures of phenotypic stability in linseed (Linum usitatissimum L.). Euphytica 129: 211-218.
- Annicchiarico P (1997) Joint regression vs AMMI analysis of genotype-environment interactions for cereals in Italy. Euphytica 94: 53-62.
- Annicchiarico P (2002) Genotype x environment interactions: challenges and opportunities for plant breeding and cultivar recommendations.
- Aufhammer W, Wägner W, Kaul HP, Kübler E (2000) Radiation use by oilseed crops-a comparison of winter rape, linseed and sunflower. Journal of Agronomy and Crop Science 184: 277-286.
- Basford KE, Cooper M (1998) Genotype× environment interactions and some considerations of their implications for wheat breeding in Australia This review is one of a series commissioned by the Advisory Committee of the Journal. Australian Journal of Agricultural Research 49: 153-174.
- Becker HC (1981) Correlations among some statistical measures of phenotypic stability. Euphytica, 30: 835-840.
- Becker HC, Leon JI (1988) Stability analysis in plant breeding. Plant breeding 101:1-23.
- Bridges Jr WC (1989) Analysis of a plant breeding experiment with heterogeneous variances using mixed model equations. Applications of mixed models in agriculture and related disciplines 145-151.
- de Leon N, Jannink JL, Edwards JW, Kaeppler SM (2016) Introduction to a special issue on genotype by environment interaction. Crop Science 56: 2081-2089.
- Diederichsen A, Raney JP (2006) Seed colour, seed weight and seed oil content in Linum usitatissimum accessions held by Plant Gene Resources of Canada. Plant Breeding 125: 372-377.
- Haldane JBS (1946) The interaction of nature and nurture. Annals of eugenics 13: 197-205.
- Kang MS (1993) Simultaneous selection for yield and stability in crop performance trials: Consequences for growers. Agronomy Journal 85: 754-757.
- Kang MS (1997) Using genotype-by-environment interaction for crop cultivar development. Advances in agronomy 62:199-252.
- Kang MS, Magari R (1996) New developments in selecting for phenotypic stability in crop breeding. Genotype-by-environment interaction. CRC Press, Boca Raton, FL 1-14.
- Leflon M, Lecomte C, Barbottin A, Jeuffroy MH, Robert N, et al. (2005) Characterization of environments and genotypes for analyzing genotype× environment interaction: some recent advances in winter wheat and prospects for QTL detection. Journal of Crop Improvement 14: 249-298.
- Lin CS, Binns MR, Lefkovitch LP (1986) Stability analysis: where do we stand? 1. Crop science 26: 894-900.
- Lirie E, Zeleke H, Wakjira A (2013) AMMI analysis of yields and oil content in some linseed (Linum usitatissimum L.) genotypes in south and central Ethiopia. Ethiopian Journal of Agricultural Sciences 24: 79-98.
- Malosetti M, Ribaut JM, van Eeuwijk FA (2013) The statistical analysis of multi-environment data: modeling genotype-by-environment interaction and its genetic basis. Frontiers in physiology 4: 37433.
- Medina RC (1992) Some exact conditional tests for the multiplicative model to explain genotype-environment interaction. Heredity 69: 128-132.
- Mekbib H (1986) Crop Germplasm multiplication, characterization, evaluation and utilization at PGRC/E. Plant genetic resources of Ethiopia 258-267.
- Mekonnen Z, Mohammed H (2010) Study on genotype x environment interaction of oil content in sesame (Sesamum indicum L.). World Journal of Fungal and Plant Biology 1: 15-20.
- Misra RC, Das S, Patnaik MC (2009) AMMI model analysis of stability and adaptability of late duration finger millet (Eleusine coracana) genotypes. World Applied Sciences Journal 6: 1650-1654.
- Nath D, Dasgupta T (2013) Genotype× environment interaction and stability analysis in mungbean. IOSR J Agric Vet Sci 5: 62-70.
- Piepho HP (1998) Methods for comparing the yield stability of cropping systems. Journal of Agronomy and Crop Science 180: 193-213.
- Sadras VO, Richards RA (2014) Improvement of crop yield in dry environments: benchmarks, levels of organisation and the role of nitrogen. Journal of experimental botany 65: 1981-1995.
- Seegler CJP (1983) Linum usitatissimum L. (2n= 30). Oil Plants in Ethiopia, their Taxonomy and Agricultural Significance 151-197.
- Shafii B, Price WJ (1998) Analysis of genotype-by-environment interaction using the additive main effects and multiplicative interaction model and stability estimates. Journal of Agricultural, Biological, and Environmental Statistics 335-345.
- Shafii B, Mahler KA, Price WJ, Auld DL (1992) Genotype✕ Environment interaction effects on winter rapeseed yield and oil content. Crop Science 32: 922-927.
- Sharma D, Paul S (2016) Genotype×environment interaction and phenotypic stability analysis of linseed (Linum usitatissimum L.) in mid-hills of North-West Himalayas. Journal of Applied and Natural Science 8: 2040-2048.
- Shim YY, Gui B, Wang Y, Reaney MJ (2015) Flaxseed (Linum usitatissimum L.) oil processing and selected products. Trends in Food Science & Technology 43: 162-177.
- Simmonds NW (1981) Genotype (G) environment (E) and GE components of crop yields. Experimental Agriculture, 17: 355-362.
- Temesgen A, Mammo K, Lule D (2014) Genotype by Environment Interaction (G x E) and grain yield stability analysis of Ethiopian linseed and Niger seed varieties. Journal of Applied Biosciences 80: 7093-7101.
- Wakjira A, Labuschagne MT, Hugo A (2004) Variability in oil content and fatty acid composition of Ethiopian and introduced cultivars of linseed. Journal of the Science of Food and Agriculture 84: 601-607.
- Wende A, Labuschangne MT (2005) Stability analysis of Ethiopian maize varieties using AMMI model. Ethiopian Journal of Agricultural Sciences 18: 173-180.
- Yates F, Cochran WG (1938) The analysis of groups of experiments. The Journal of Agricultural Science, 28: 556-580.
- Zhang YY, Fischer M, Colot V, Bossdorf O (2013) Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytologist 197: 314-322.
- Zobel RW, Wright MJ, Gauch Jr HG (1988) Statistical analysis of a yield trial. Agronomy journal 80: 388-393.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Tilahun M (2024) Basic Concepts of Genotype by Environment Interactionand Stability Analysis on Linseed: Review. Adv Crop Sci Tech 12: 716.
Copyright: © 2024 Tilahun M. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 671
- [From(publication date): 0-2024 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 474
- PDF downloads: 197