Basal Cell Adenocarcinoma of the Parotid Gland in an Elderly Iranian Woman and Review of the Literature
Published Date: 05-Dec-2014 DOI: 10.4172/2161-119X.1000180
Abstract
Basal Cell Adenocarcinoma (BCAC) is a rare subtype of carcinoma of salivary glands. It accounts for 1.6% of all salivary gland neoplasms and 2.9% of malignant salivary gland neoplasms. BCAC is especially rare in parotid glands. The biological behavior of BCAC is invasive and destructive, with perineural and vascular invasion. This paper presents a BCAC with multiple recurrences and wide local extension in a 71-year-old Iranian woman with a 30-year history of a large mass on the right side of her neck. The importance aspects of this case were its long duration, multiple recurrences, facial nerve involvement and the use of clinicopathological criteria for diagnosis.
Background
Basal Cell Adenocarcinoma (BCAC) is one of the rare subtypes of carcinoma of salivary glands. It is a salivary gland malignancy that was first recognized as a distinct neoplastic entity in World Health Organization’s (WHO’s) classification of salivary gland tumors in 1991. BCAC of the salivary gland is a rare neoplasm, especially in the parotid gland. It composes 1.6% of all salivary gland neoplasms and 2.9% of malignant salivary gland neoplasms. The most important differential diagnosis of BCAC is Basal Cell Adenoma (BCA), but the behavior of BCAC is invasive and destructive with perineural and vascular invasion [1,2]. The histopathology of BCAC shows two cell types, i.e., small basaloid epithelial cells at the periphery and larger epithelial cells in the center of the tumour clusters . Most cases of BCAC are de novo, but 25% of BCACs originate from pre-existing basal cell adenomas. In general, BCAC has a good prognosis. Metastasis is rare but may recur locally [3,4]. BCACs are categorized into four types on the basis of their growth patterns, i.e., solid, trabecular, tubular, and membranous; the solid type is the most common [2,5]. The BCAC in our 71-year-old patient had some interesting features that were different from those in previous case reports.
Case Presentation
A 71-year-old Iranian woman with a 30-year history of a large mass on the right side of her neck was referred to the Otolaryngology Department at Shahid Sadoughi Hospital in Yazd, Iran in 2012. She presented with a firm, fixed, non-tender mass on the right side of her neck that measured 10×15 cm with normal skin with no apparent invasion. The clinical examination provided no evidence of cervical lymphadenopathy. The patient complained about mild dysphagia, and the oropharyngeal exam revealed a bulging mass in the right side of the oral cavity. Facial movements were partially asymmetrical on the right side of the lower lip (other branches of facial nerve were intact). Also, a bulge was found on the anterior wall of the right external auditory canal. A superficial parotidectomy was performed 10 years ago; subsequently, the patient experienced five recurrences of masses on her neck, and the last recurrence occurred four years ago. Unfortunately, we don’t have any documented pathology from her previous surgeries. An investigation of the patients’ family history showed no occurrence of cancer.
Investigation
Imaging
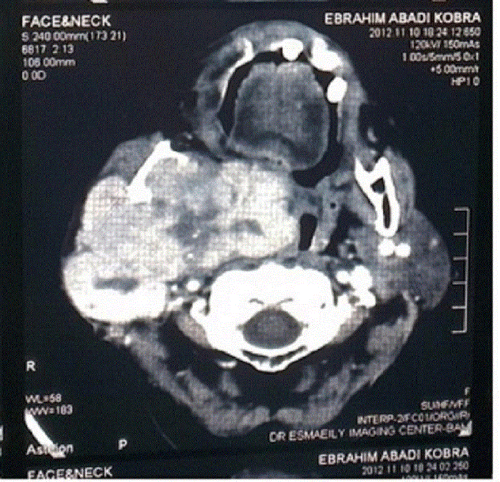
Abdominal ultrasonography revealed no abnormality, and the chest X-ray indicated no signs of metastasis. The CT scan showed a large, irregular mass in the right parotid gland, bulging into the parapharyngeal space. It extended up to the base of the skull (Figure 1).
Histopathology and other laboratory findings
In the gross pathological examination, the tumour had creamy-colored nodular tissue that measured 16×14×5 cm with firm consistency and lobular appearance in the cut section. Some of nodules had thin capsules with calcified foci. Microscopic examination revealed a tumoural lesion with a multinodular pattern composed of basaloid cells that had round-to-oval nuclei with fine nucleoli and scant-to-moderate cytoplasm. Cells arranged in lobules, sheets, aggregates cords, trabeculae and membranous pattern. Hyaline Periodic Acid Schiff (PAS) positive materials with palisading and jigsaw puzzle patterns were observed. There were no more than six mitoses in the 10 High Power Field (HPF).
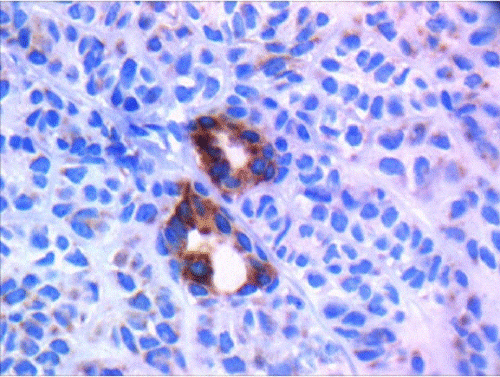
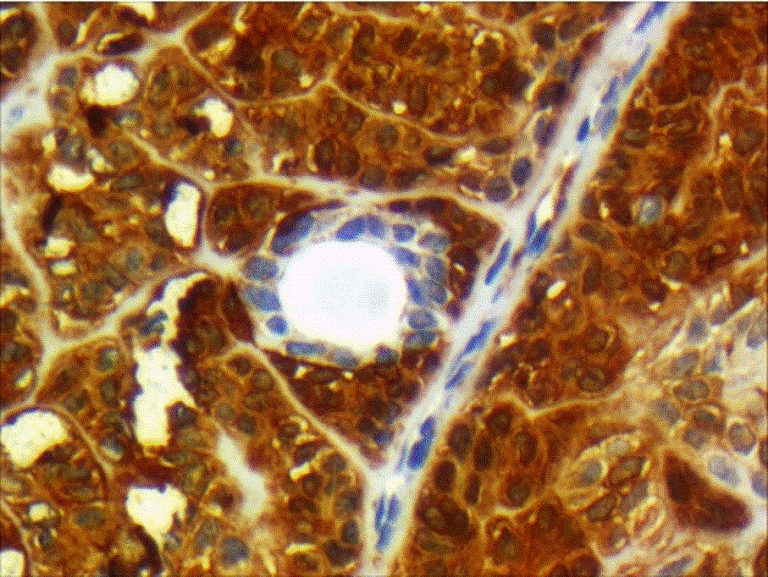
Cellular pleomorphism, necrosis, vascular and perineural invasion, and lymph node metastasis were absent. The primary diagnosis was low-grade basal cell adenocarcinoma. For ruling out other differential diagnoses, Immunohistochemistry (IHC) was performed. The tumoral cells were positive for CK AE1/3, SMA, S100 (moderately positive), EMA and CD117 (positive in luminal cells in areas of ductal differentiation), Vimentin (focally positive), and Ki67 (in 5 to 10% of the tumoral cells). CEA and GFAP were negative (Figures 2 and 3) (Table 1).
| Antibodies | clone | class | code | Antigen unmasking methods: Microwave retrieval | Dilution |
|---|---|---|---|---|---|
| Monoclonal Mouse Anti-Human Muscle Actin (SMA) | 1A4 | IG2a, kappa | M0851 | 15 minutes heating-induced epitope retrieval (750 W) in 10 mmol/Lcitrate buffer, pH 6 | 1:75 in 0.02 M PBS, pH 7.2-7.6 |
| Monoclonal Mouse Anti-Human Cytokeratin AE1/AE2 | AE1 and AE3 | AE1: IgG1, kappa; AE3: IgG1, kappa | M3515 | 15 minutes heating – induced epitope retrieval (750 W) in 10 mmol/Lcitrate buffer, pH 6 | 1:50 in 0.02 M PBS, pH 7.2-7.6 |
| Polyclonal rabbit Anti-S 100 | - | - | Z0311 Ig fraction | 15 minutes heating – induced epitope retrieval (750 W) in 10 mmol/Lcitrate buffer, pH 6 | 1: 300 in 0.02 M PBS, pH 7.2–7.6 |
| Polyclonal Rabbit Anti-Human Carcinoembryonic Antigen (CEA) | - | - | A0115 Ig fraction | 15 minutes heating – induced epitope retrieval (750 W) in 10 mmol/Lcitrate buffer, pH 6 | 1:100 in 0.02 M PBS, pH 7.2-7.6 |
| Monoclonal Mouse Anti-Human Vimentin (VIM) | V9 | IgG1, kappa | M0725 Culture supernatant | 15 minutes heating – induced epitope retrieval (750 W) in 10 mmol/Lcitrate buffer, pH 6 | 1:30 in 0.02 M PBS, pH 7.2-7.6 |
| Polyclonal Rabbit Anti-Glial Fibrillary Acidic protein (GFAP) | - | - | IS524 | 15 minutes heating – induced epitope retrieval (750 W) in 10 mmol/Lcitrate buffer, pH 6 | 1:30 in 0.02 M PBS, pH 7.2-7.6 |
Table 1: Antibodies used in the immunohistochemical investigation of BCAC
Treatment
The tumor at the base of the skull and the deep portion of the parotid gland were resected, and suprahyoid dissection of the lymph nodes was performed, including the submandibular, submental, and upper third of the jugulodigastric nodes. Except marginal mandibular branch the facial nerve was preserved and post-operative irradiation were conducted.
Outcome and follow-up
At the one-year follow up, she had no recurrence of any of the features associated with bcac. Also, the metastasis workup revealed no sign of metastasis.
Discussion
Bcac is slow-growing, locally-destructive tumor. It was first defined as a malignant tumor of the salivary gland in 1991. The most important differential diagnosis for bcac is basal cell adenoma [1]. Both of them have similar histological appearances (basaloid pattern), but infiltrative growth is the distinguishing feature of bcac. Pathological criteria for the diagnosis of bcac include infiltrative growth with vascular or perineural invasion. Other features may include nuclear pleomorphism, necrosis, and mitotic activity [3,4]. In our patient, there were no more than six mitoses inthe 10 hpf. Cellular pleomorphism, necrosis, vascular and perineural invasion, and lymph-node metastasis were absent, but, clinically, it was a huge mass (10×15 cm) with wide extention and a history of multiple recurrences. Its invasion of the adjacent soft tissues was obvious during surgery. Ihc suggested bcac and ruled out a differential diagnosis. We believe that the diagnosis of bcac is clinicopathological, and sometimes it is very difficult to distinguish it from basal cell adenoma using only pathological criteria.
The rate of metastasis is low, but the rate of local recurrence is high. Metastasis occurs in less than 10% of cases, and only one case involving the lung has been reported [3,4]. Because of their biological behavior and their prognosis, bcacs should be classified as low-grade carcinomas. In our patient, a complete metastasis workup was performed, and there were no features of metastasis. There have been a few descriptions of facial paralysis or additional destruction of the salivary gland, but interesting findings in our patient’s case were that the buccal branch of the facial nerve was weak and that the bcac extended up to the base of the skull, bulging into the parapharyngeal space. Many patients with bcac recognized after 7-10 years, but our patient had it for the long duration of about 30 years with five recurrences (may be denovo or transformation of basal cell adenoma). Most patients present with a solitary firm nodule between 1 and 3 cm that is slowly enlarging. The largest tumor described by Ellis et al. was 4 cm, but our patient’s tumor was about10x15 cm. Local recurrence occurs in about one-third of the cases, and our patient had five recurrences over a 30-year period. Our case was unique with respect to its duration, the number of recurrences, and the size of the mass [5].
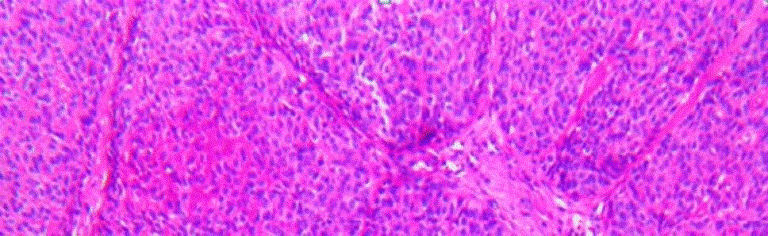
Bcac has four subtypes, i.e., solid, membranous, trabecular, and tubular. The solid areas of bcacs are composed of nonluminal cells, some of which contain tonofilaments and well-formed desmosomes; tubulo-trabecular differentiated into both luminal and nonluminal cells. Both growth patterns were associated with the formation of excess basal lamina, marginally and between nonluminal cells. The majority of these tumors are solid. They are characterized by islands and masses within the fibrous connective tissue stroma, the histological type of our patients bcac was membranous. The membranous type is actually the second most frequently-occurring type of bcac, accounting for about 20% of all occurrences. This type has thick, eosinophilic, periodic acid schiff positive hyaline laminae that surround and separate one tumor nest from another and a jigsaw puzzle formation (Figure 4). The trabecular type of bcac has anastomosing cords of basaloid epithelial cells that look like chinese characters [2,5].
Muller and Barnes conducted an extensive literature search in 1996, and they concluded that parotid is the most common salivary gland that is involved with BCAC (89% in the parotid, 10% in the submandibular gland, and 1% among the minor salivary glands). In our case, the parotid gland was involved. Most studies recommend wide loc al excision. Treatment for regional metastasis (neck) is done for significant lymphadenopathy on clinical or radiologic examination, and postoperative radiation is recommended for close surgical margins or following surgical excision of recurrent tumor [6-9].
The surgical procedure we performed was resection of the tumor at the base of the skull with deep portion resection of the parotid gland with preservation of the facial nerve. At last we summarise at least 46 cases of parotid gland BCAC from 1990-2014 in two tables and compare them with our case (Tables 2 and 3).
| Studies | Ellis et al.[5] | Muller et al. [10] | Kwang II [11] | Ikeda K [12] | Golz et al. [13] |
|---|---|---|---|---|---|
| Cases and Sex | 29 | 7(6 female and 1 male) | 1(female) | 1(female) | 1(male) |
| Age | Adults with Peak incidence 6th decade | 46-74 | 33 | 73 | 24 |
| Location | Major salivary glands | Parotid gland(Except one case) | Left Parotid | Right Parotid | Left parotid |
| Size(mm) | Variable | Variable | 20 | 50 | 30 |
| common Histologic type | Solid | - | tubulotrabecular type | Solid and Tubular | |
| Manifestation | - | Parotid mass | Infraauricular mass | Parotid mass | Retroauricular abscess |
| Facial nerve status | - | Intact | Intact | Intact | Intact |
| Cervical lymphadenopathy | 2 | none | Negative | Negative | Negative |
| Local recurrences | 7 | 2 | Negative | Negative | Negative |
| Distant metastasis | 1 | 1 | Negative | Negative | Negative |
| Site of metastasis | Lung | - | - | - | - |
| Treatment | Parotidectomy | Local excision | Parotidectomy | Parotidectomy | Total |
| Follow up duration | Few months | 30 month | 24 months | 24 months | 30 months |
| Status after follow up | One case died | Tumor free | Tumor free | Tumor free | Tumor free |
Table 2: Review of the literature
| Studies | Keiichi Jingu[14] | Markanen et al. | Michael H Elvey[15] | Hamamoto[16] | Sarafraz Z et al. |
|---|---|---|---|---|---|
| Cases and Sex | 4(2 female and 2male) | 1(male) | 1(male) | 1(female) | 1(female) |
| Age | 37-81 | 60 | 67 | 58 | 71 |
| Location | 3 cases right and 1 case in left Parotid gland | Bilateral Parotid gland | Parotid | Right parotid | Right parotid |
| Size(mm) | 19-54mm | Right:10mm Left:6mm | - | - | 150mm |
| Dominant Histologic type | - | Tubular | Solid | - | Membranous |
| Manifestation | Buccal swelling | Incidental in ultrasonography | Parotid mass Pain and swelling in his hand | Parotid mass | Parotid mass |
| Facial nerve status | Intact | Intact | Intact | Intact | Weakness in buccal branch |
| Cervical lymphadenopathy | 1 | Negative | Negative | Negative | Negative |
| Local recurrences | 1 | Negative | Negative | Negative | Negative |
| Distant metastasis | none | Negative | + | Negative | Negative |
| Site of metastasis | - | - | Second to 5th metacarpal | - | - |
| Treatment | Parotidectomy +radiotherapy | Parotidectomy | Parotidectomy +radiotherapy Distal forearm amputation | Superficial Parotidectomy +radiotherapy | Parotidectomy +radiotherapy |
| Follow up duration | 3 Months | - | 24 months | 8 months | 48 months |
| Status after follow up | No recurrence | - | No evidence of metastasis | Tumor free | No recurrence |
Table 3: Review of the literature
References
- Quddus MR, Henley JD, Affify AM, Dardick I, Gnepp DR (1999) Basal cell adenocarcinoma of the salivary gland: an ultrastructural and immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 87: 485-492.
- Sharma R, Saxena S, Bansal R (2007) Basal cell adenocarcinoma: Report of a case affecting the submandibular gland 11: 56-9.
- Markkanen-Leppanen M, Makitie AA, Passador-Santos F, Leivo I, Hagström J (2010) Bilateral Basal cell adenocarcinoma of the parotid gland: in a recipient of kidney transplant. Clin Med Insights Pathol 3: 1-5.
- Ward BK, Seethala RR, Barnes EL, Lai SY (2009) Basal cell adenocarcinoma of a hard palate minor salivary gland: case report and review of the literature. Head Neck Oncol 1: 41.
- Ellis GL, Wiscovitch JG (1990) Basal cell adenocarcinomas of the major salivary glands. Oral Surg Oral Med Oral Pathol 69: 461-469.
- Hirsch DL, Miles C, Dierks E (2007) Basal cell adenocarcinoma of the parotid gland: report of a case and review of the literature. J Oral MaxillofacSurg 65: 2385-2388.
- Jayakrishnan A, Elmalah I, Hussain K, Odell EW (2003) Basal cell adenocarcinoma in minor salivary glands. Histopathology 42: 610-614.
- Yu GY, Ubmuller J, Donath K (1998) Membranous basal cell adenoma of the salivary gland: a clinicopathologic study of 12 cases. ActaOtolaryngol 118: 588-593.
- Parashar P, Baron E, Papadimitriou JC, Ord RA, Nikitakis NG (2007) Basal cell adenocarcinoma of the oral minor salivary glands: review of the literature and presentation of two cases. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 103: 77-84.
- Muller S, Barnes L (1996) Basal cell adenocarcinoma of the salivary glands. Report of seven cases and review of the literature. Cancer 78: 2471-2477.
- Kim KI, Oh HE, Mun JS, Kim CH, Choi JS (1997) Basal cell adenocarcinoma of the salivary gland--a case report. J Korean Med Sci 12: 461-464.
- Ikeda K, Watanabe M, Oshima T, Nakabayashi S, Kudo T, et al. (1998) A case of basal cell adenocarcinoma of the parotid gland. Tohoku J Exp Med 186: 51-59.
- Golz A, Goldenberg D, Ben-Arie Y, Keren R, Netzer A, et al. (2000) Basal cell adenocarcinoma of the parotid gland presenting as a retroauricular abscess. Am J Otolaryngol 21: 421-426.
- Jingu K, Hasegawa A, Mizo JE, Bessho H, Morikawa T, et al. (2010) Carbon ion radiotherapy for basal cell adenocarcinoma of the head and neck: preliminary report of six cases and review of the literature. RadiatOncol 5: 89.
- Michael H (2011) Metastasis of parotid basal cell adenocarcinoma to the handâ€â€Âa case report. Hand 6: 321-323.
- Hamamoto M (2012) A case of basal cell adenocarcinoma of the parotid gland, practicaoto rhino laryngological. 105: 441-446
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15471
- [From(publication date): 1-2015 - Jul 06, 2025]
- Breakdown by view type
- HTML page views: 10841
- PDF downloads: 4630