Bacteremia Associated with Community-Acquired Methicillin-Resistant Staphylococcus aureus (CA-MRSA) and Clindamycin Resistant in an Elderly Man with Carbuncle: A Case Report from Rio De Janeiro during the COVID-19 Pandemic
Received: 25-Jun-2021 / Accepted Date: 09-Jul-2021 / Published Date: 16-Jul-2021 DOI: 10.4172/2332-0877.1000465
Abstract
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infections have few treatment options in modern medicine. Nevertheless, according to the guidelines of the Infectious Diseases Society of America clindamycin is an option in managing this issue in outpatient clinics. The present report describes the case of a man with carbuncle during the COVID-19 pandemic in 2021, in which clindamycin-resistant S. aureus (mecA, Sccmec-IV) was identified in two blood samples.
Keywords: Carbuncle; Staphylococcus aureus; Bacteremia
Abbreviations
CA-MRSA: Community-Acquired Methicillin- Resistant Staphylococcus aureus; MIC: Minimal Inhibitory Concentration; SSTI: Skin and Soft Tissue Infections.
Introduction
A carbuncle is a cluster of several boils, which is typically, filled with purulent exudate (dead neutrophils, phagocytized bacteria, and other cellular components). The aim of this report to show complications of skin infection due to CA-MRSA resistant clindamycin in an elderly man with diabetes mellitus. In this work we identified community-acquired methicillin-resistant CA-MRSA of SSTIs resistant to clindamycin and our patient developed sepsis and renal failure during the treatment with vancomycin. Although initially the laboratory MIC by Etest of vancomycin was 2.0 μg/mL, notwithstanding, the MIC of vancomycin was=1 μg/mL by broth methodology in our MMD. The MIC by Etest of teicoplanin and daptomycin was 3.0 μg/mL and 1.5 μg/mL, respectively.
Case Presentation
A 62-year-old man living in an urban area of Rio de Janeiro who has hypertension and type 2 diabetes mellitus was admitted to our teaching hospital, Hospital Universitrio Pedro Ernesto (HUPE), with an itchy and painless head wound associated with local swelling 30 days prior. Approximately 15 days before admission, the patient reported discharge of purulent secretion out of the wound. Other constitutional symptoms, such as fever, nausea, vomiting and reduced level of consciousness, were observed. Furthermore, he reported that one week before seeking assistance at HUPE, he sought an Emergency Care Unit and the physician prescribed benzathine benzyl penicillin 1.200.000 IU and cephalexin 500 mg qid for 7 days. However, there was no improvement with this medicine and he was admitted to our department.
Upon admission, the patient had an acute Skin and Soft Tissue Infection (SSTIs) without signs of sepsis III (q-SOFA and SOFA<2) but notwithstanding there were two criteria of systemic inflammatory response syndrome (fever and leukocytosis).
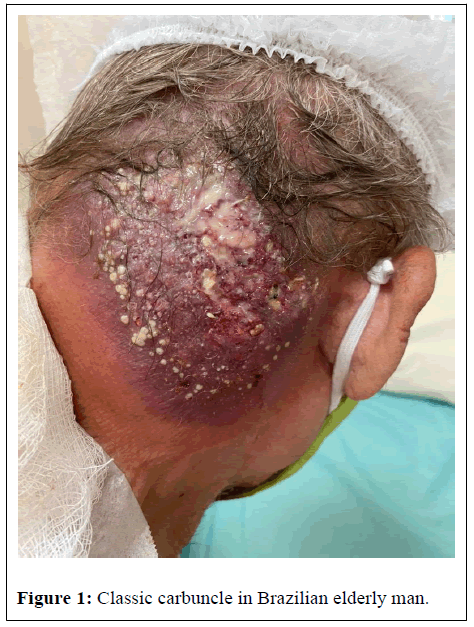
In dermatological semiology, we observed a plaque in the right occipito-temporal region of about 10 centimeters in diameter, erythematous-violet base, poorly delimited edges, associated with induration and fistulization with the release of purulent material (Figure 1). No other injuries were found. After collecting clinical material, vancomycin was prescribed.
The laboratory evaluation upon admission showed anemia, leukocytosis with left shift, lymphocytosis with predominance of typical lymphocytes and monocytes, in addition to a high level of Creactive protein. Bloodstream samples were withdrawn, placed in three aerobic bottles (Plus Aerobic), and incubated in a BACTEC 9240 blood culture instrument (BATEC/ALERT®, BioMérieux, Durham, NC, USA). After 24 hours, MRSA (methicillin-resistance gene detected mecA) was identified using Bio Fire Film Array Blood Culture ID assay.
Phenotypic identification and anti biogram were performed with the help of an automated VITEK 2 System (Bio Mérieux). The minimal inhibitory concentration (MIC) for vancomycin was 1.0 μg/mL by microdilution methodology, for daptomycin and teicoplanin it was MIC=1.5 μg/mL and MIC=3 μg/mL, respectively both by E-test. Additionally, PCR amplification was performed for the typing of the Sccmec [1] and was classified as type IV.
Magnetic resonance imaging of the skull revealed extensive damage to the skin and subcutaneous tissue with an infiltrative aspect, compromising the right posterior craniocervical region, in addition to heterogeneous areas that can characterize collections or cystic degeneration. This lesion, with ill-defined limits, compromised the muscles of the neck, including along the head and neck, trapezius and sternocleidomastoid, in addition to irregularities and/or ulcerations on the surface. There was no evidence of lesion extension to the intracranial compartment.
Furthermore, a CT scan was performed, which showed an increase in soft tissues, thickening of the musculoadipous planes and an increase in density that extended to the posterior cervical region, up to the C3 vertebral body, with imprecise and ill-defined limits. There were signs of remodeling of the skullcap and an aspect of inflammation or infectious process. An echocardiogram was also performed to rule out a possible infective endocarditis, which demonstrated cavity dimensions and wall thickness within normal limits. Thus, the patient was diagnosed with posterior cervical carbuncle due to the presence of folliculitis in the posterior region of the neck and scalp and diabetes, which was difficult to control.
Our medical microbial department searched for the bacteria and identified it as community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) with vancomycin MIC of 2 μg/ mL, daptomycin MIC=1.5 μg/mL, teicoplanin MIC=3 μg/mL by by Etest® and clindamycin resistance and sensitivity to cotrimoxazole, Hence, vancomycin was replaced by daptomycin.
In relation to therapy, daptomycin was administered for 20 days, along with daily cleaning and dressings. After this first course of antibiotics, the patient developed fever, tachycardia and hypotension with SOFA>2. Thus, linezolid and cefepime were introduced, but discontinued after 5 days. Throughout hospitalization, the lesion developed to hemorrhagic phenomena, requiring plastic surgery to address the wound. Due to the suspicion of neoplasia, a biopsy was performed, whose histopathological result was descriptive of benign disease (compatible with carbuncle). The microscopic evaluation revealed the formation of abscesses containing numerous polymorphonuclear cells in the deep dermis, a dense infiltrate of mature plasma cells, eosinophils and histiocytes, in addition to a smaller number of small B and T cells. Neoplasia was not found. In immunohistochemistry, CD20 positive was detected in B lymphocytes, CD3 positive in T lymphocytes, MUM-1 positive in most cells and CD30 was negative.
Results and Discussion
Our elderly patient during the coronavirus pandemic developed bacteremia associated with CA-MRSA identified with mecA and Sccmec-IV. MICs for S. aureus were determined using Etest®, which showed vancomycin MIC=2 μg/mL, daptomycin MIC=1.5 μg/mL, teicoplanin MIC=3 μg/mL, clindamycin resistance and sensitivity to cotrimoxazole but vancomycin MIC was 1 μg/mL by micro dilution methods.
Overall, the patient had a good clinical and laboratory evolution, having been discharged after 45 days of hospitalization. Bacterial SSTIs have been affecting millions of people worldwide, and skin infections leading to cutaneous pathology have a considerable impact on the quality of life and longevity of people affected [2-4]. Important risk factors, such as age and colonization with microorganisms, are associated with the host integrity and the acquisition of these diseases [2,5]. Nevertheless, diabetic patients tend to have a high probability of infection or complication [6,7]. This is because, due to hyperglycemia, these usually evolve with reduced leukocyte and nutrients circulation, neuropathic and vascular damage and delayed healing. The risk increases in cases of poorly controlled diabetes [8].
Among the agents involved, Gram-positive cocci are primarily responsible [2]. Nonetheless, S. aureus is the major etiological agent of ABSSI, including impetigo, boils, carbuncles, cellulite with or without an abscess, necrotizing soft tissue infection [6]. MRSA is mainly conferred by the mecA gene carried in the staphylococcal cassette chromosomes (Sccmec) [9].
Traditionally, most of all MRSA infections are hospital-adquired. However, after first report, MRSA infection outside the hospital setting due to CA-MRSA has been found widespread around the world [9]. Currently, the distinction between HA-MRSA and CA-MRSA is still confusing [10]. The Centers for Disease Control and Prevention Active Bacterial Core Surveillance Program defined a CA-MRSA case as a patient with an MRSA infection and no history of the following: surgery, hospitalization, or residence in a long-term care facility within the year before infection, presence of a percutaneous [11].
The Infectious Diseases Society of America (IDSA) has published guidelines for ABSSSI [12]. Despite the availability of these guidelines, guideline-discordant antibiotic use for SSTIS is commonly reported [13]. Theo’s investigated the antibiotic susceptibility profiles of S. aureus isolates in an outpatient dermatology office in Hawaii in which the susceptibility of MRSA to clindamycin was less at 78% [13]. A similar retrospective study was performed from 2005 to 2011 in a dermatology clinic at the University of Miami where the prevalence of resistance to clindamycin was 70% [3].
Recently, Sutton et al. developed a retrospective study where they identified a non-purulent SSTI in 1299 (71%) patients and a purulent SSTI was identified in 529 (20%) patients. Only 203 (38%) patients with purulent skin infections received IDSA guideline-concordant empirical therapy with clindamycin as adequate MRSA [4]. S. aureus is a major inhabitant of the skin and mucosa and principal agent of SSTIs [2]. In cases of loss of integrity of the skin barrier or decreased immunity of the individual, this microorganism can become pathogenic [2,6,7,9,12,14]. Acute bacterial SSTI resulting from S. aureus, such as impetigo, boils, carbuncles, cellulite with or without an abscess, necrotizing soft tissue infections are still a concern in the antibiotic era [2]. ABSSSI is an important triggering factor for bacterial sepsis. Nevertheless, in the medical literature there are few reports of carbuncle with secondary of sepsis due to CA-MRSA with resistance to clindamycin after our review at this moment [2,4,5,7,13]. The inpatient management of carbuncle requires a multidisciplinary team at the hospital. According to IDSA guidelines, the options for treatment of MRSA are: Ceftaroline, Daptomycin, Lipoglycopeptides, Oxazolidinones, Tetracyclines, Trimethoprim-Sulfamethoxazole, Clindamycin, Vancomycin. Nevertheless, clindamycin was not recommended as empiric therapy in IDSA guidelines for inpatient management of SSTIs [15].
Middle-aged and elderly men are the most affected by this condition, being diabetes one of the main risk factors [2,5,7,16]. This may occur because these patients usually present metabolic changes that facilitate staphylococcal colonization and, consequently, the development of infection [2]. In this case report, we showed the concern of neck carbuncle associated sepsis due to CA-MRSA clindamycin-resistant in Rio de Janeiro during the COVID-19 pandemic.
Conclusion
We observed a good outcome with daptomycin therapy in bacteremia associated CA-MRSA and resistant clindamycin in an elderly man with carbuncle. This report reinforces that clindamycin is not an option in anti-MRSA antibiotic therapy for inpatient management of SSTIs. Our patient developed sepsis due to “CA- MRSA” during vancomycin therapy in which MIC was 1 μg/ml by broth methodology.
Future studies should assess whether the identification of MRSA clindamycin-resistant may be a risk factor for therapeutic failure with vancomycin in carbuncle.
References
- Boye K, Bartels MD, Andersen IS, Møller JA, Westh H (2007) A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCC mec types I–V. Clin Microbiol and Infect 13:725-727.
- Watkins RR, David MZ (2021) Approach to the patient with a skin and soft tissue infection. Infect Dis Clin 35:1-48.
- Zabielinski M, McLeod MP, Aber C, Izakovic J, Schachner LA (2013) Trends and antibiotic susceptibility patterns of methicillin-resistant and methicillin-sensitive Staphylococcus aureus in an outpatient dermatology facility. JAMA Dermatol 149:427-432.
- Sutton JD, Carico R, Burk M, Jones MM, Wei X, et al. (2020) In patient management of uncomplicated skin and soft tissue infections in 34 veterans affairs medical centers: A medication use evaluation. In Open forum infectious diseases US: Oxford University Press. 7:554.
- Pacheco RL, Lobo RD, Oliveira MS, Farina EF, Santos CR, et al. (2011) Methicillin-resistant Staphylococcus aureus (MRSA) carriage in a dermatology unit. Clinic 66:2071-2077.
- Magalhães MMM, Nascimento NG, de Rezende GVS, Rocha LA, Penatti VS, et al. (2020) Bacterial skin infection: a case report of furunculosis in a diabetic patient. Braz J of Develop 6:68487-68495.
- Venkatesan R, Baskaran R, Asirvatham AR, Mahadevan S (2017) ‘Carbuncle in diabetes’: a problem even today!. BMJ Case Rep 19:1.
- Falcone M, Meier JJ, Marini MG, Caccialanza R, Aguado JM, et al. (2021) Diabetes and acute bacterial skin and skin structure infections. Diab res and clinic pracT 174:108732.
- Gelatti LC, Bonamigo RR, Inoue FM, Carmo MS, Becker AP, et al. (2013) Community-acquired methicillin-resistant Staphylococcus aureus carrying Sccmec type IV in southern Brazil. Revista da Sociedade Brasileira de Medicina Tropical 46:34-38.
- Kateete DP, Bwanga F, Seni J, Mayanja R, Kigozi E, et al. (2019) CA-MRSA and HA-MRSA coexist in community and hospital settings in Uganda. Anti microbial Res & Infec Cont 3:8-9.
- Buck JM, Como-Sabetti K, Harriman KH, Danila RN, Boxrud DJ, et al. (2005) Community-associated methicillin-resistant Staphylococcus aureus, Minnesota, 2000–2003. Emer infect dis 11:1532-1538.
- Hirabayashi M, Takedomi H, Ando Y, Omura K (2018) Neck carbuncle associated with methicillin-susceptible Staphylococcus aureus bacteraemia. BMJ Case Rep 25:1.
- Theos KR, Johnson KM, Johnson DW (2019) Staphylococcus aureus Antibiotic Susceptibilities in Infections in an Outpatient Dermatology Office on O ‘ahu. Hawai'i Journal of Medicine & Public Health. 78:163-168.
- Meurehgâ€Haik C, Garciaâ€Velasco J Carbuncle (1974) Discussion and a report of two cases. Int J of Derm 13:63-68.
- Zabielinski M, McLeod MP, Aber C, Izakovic J, Schachner LA (2013) Trends and antibiotic susceptibility patterns of methicillin-resistant and methicillin-sensitive Staphylococcus aureus in an outpatient dermatology facility JAMA derm 149:427-432.
- Stenstrom R, Grafstein E, Romney M,JFahimi J, Harris D, et al. (2009) Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus skin and soft tissue infection in a Canadian emergency department. CIEM 11:430-438.
Citation: Passos GAB, Zuma AAP, Navarro NB, Silva RS, Oliveira Ferreira DBC, et al. (2021) Bacteremia Associated with Community-Acquired Methicillin-Resistant Staphylococcus aureus (CA-MRSA) and Clindamycin Resistant in an Elderly Man with Carbuncle: A Case Report from Rio De Janeiro during the COVID-19 Pandemic. J Infect Dis Ther 9:465. DOI: 10.4172/2332-0877.1000465
Copyright: © 2021 Passos GAB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3015
- [From(publication date): 0-2021 - Apr 29, 2025]
- Breakdown by view type
- HTML page views: 2309
- PDF downloads: 706