Autoimmune Pancreatitis Mimicking Pancreatic Cancer: A Case Report
Received: 02-Nov-2018 / Accepted Date: 07-Nov-2018 / Published Date: 12-Nov-2018
Keywords: Pancreatitis; Radiograph; Jaundice; Cancer; Plasma cells
Introduction
Autoimmune pancreatitis (AIP) is a particular form of recently individualized chronic pancreatitis that was sometimes confused with the diagnosis of pancreatic cancer. It was from single cases reporting cases of pancreatitis associated with hypergammaglobulinemia, primary biliary cirrhosis, or Sjögren’s syndrome [1-3] that the hypothesis of an autoimmune process was born. In the pathogenesis of certain pancreatitis [4,5]. The term PAI was introduced for the first time in 1995 by Yoshida et al. [6,7]. Since then, PAI has been individualized as a separate disease entity. It is very important for clinicians to distinguish between AIP and pancreatic cancer because of the treatments and prognoses are significantly different. Although the diagnosis of AIP has been improved, it remains a practical strategy to differentiate between AIP and pancreatic cancer.
Case Reports
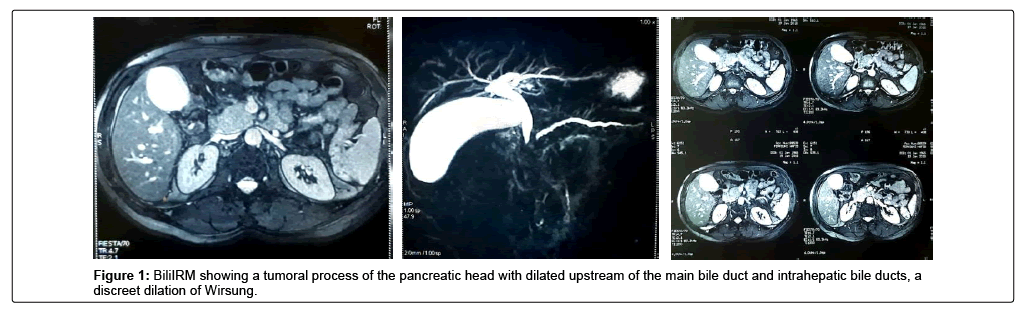
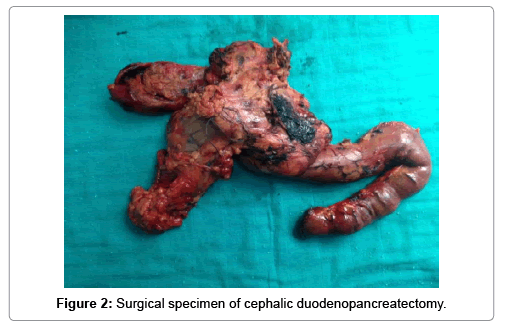
It is a patient of 53 years, chronic smoking, admitted for a cholestatic jaundice, without fever nor digestive haemorrhage, which evolves for 15 days in a context of apyrexia and conservation of the general state. The biological assessment showed: Hb: 14.4 g/dl; TP: 100%; Total bilirubin: 18 mg/l; Direct bilirubin: 10 mg/l; Indirect bilirubin: 8 mg/l ASAT: 177 IU/l; ALT: 312 IU/l; PAL: 336 IU/l; GGT: 511 IU/l; ACE: 3.26 ng/ml; Ca19-9: 54.3 IU/ml. Abdominal ultrasound and BiliIRM showed a tumoral process of the pancreatic head 30 × 30 mm in diameter with dilatation upstream of the main bile duct and intrahepatic bile ducts, a discreet dilation of the Wirsung without secondary locations (Figure 1). Given the malignancy of the lesion, an exploratory laparotomy was performed, revealing a mass of the head of the pancreas that is resectable and a Cephalic duodenopancreatectomy according to Child was performed (Figure 2). The postoperative period was uneventful. Anatomo-pathological examination of the surgical specimen revealed that it is a polymorphic infiltration rich in lymphoplasma cells, which made it possible to establish the diagnosis of type II autoimmune pancreatitis. The patient started oral corticosteroid therapy, with normalization of the biological balance and improvement of the general condition.
Discussion
PAI is currently defined as a pancreatic manifestation of an inflammatory and fibrotic systemic disease that may affect not only the pancreas but also various other viscera, particularly the bile ducts, salivary glands, retroperitoneum and lymph nodes [8-10]. All organs affected by the disease have a dense lymphoplasmocytic infiltrate rich in cells expressing IgG4 [11,12]. PAI is a rare disease. Its prevalence remains poorly specified. A national Japanese study estimated in 2002 the annual number of PAIs at 900, a calculated prevalence of 0.82 cases per 100,000 individuals [13-15]. This prevalence, derived from a Mayo Clinic study [16,17], did not represent an absolute prevalence of PAI. It was only its diffuse form and the prevalence was estimated from pancreatectomies performed for benign pancreatic disease, a rare condition in chronic pancreatitis [18-20].
PAI is at least twice as common in men than in women [18,21]. The disease most often affects adults over the age of 50 [21], but the age range of PAI is broad (16-83 years) [22-24]. There are two types of autoimmune pancreatitis (PAI) identified to date [7]:
- Autoimmune type I pancreatitis, which corresponds to the pancreatic localization of an autoimmune systemic inflammatory disease recognized in the early 2000s. It represents at least 80% of PAI cases. In 2012, the name IgG4-related disease was adopted by the 1st international consensus on this systemic disease [8-10].
- Type II autoimmune pancreatitis, less common, of more recent discovery, which is an autoimmune disease more classical in its pathophysiology and which is associated in 20 to 30% of cases with chronic inflammatory bowel disease (MICI) [7].
Histologically, it is chronic inflammatory pancreatitis with precise criteria that must all be observed to confirm the diagnosis [25-28]:
Four criteria for AIP type I
• Marked lympho-plasmocyte infiltration without neutrophil infiltration.
• Storiform fibrosis (arciform).
• Phlebitis obliterans.
• Abundance of IgG4 plasma cells (>10 IgG4 plasma cells per large field). This lesion called LPSP for Lympho-Plasmocytic Sclerosing Pancreatitis is the signature of the type I PAI.
Two criteria for type II AIP
• Destruction of the interlobular and intralobular channels by neutrophils (called GEL for granulocytic epithelial lesions), lesions that are surrounded by lymphoplasmocytic infiltration and less abundant fibrosis than in the LPSP.
• No or few IgG4 plasma cells (
The clinical presentations of AIP are very variable. The most common acute clinical presentation is painful obstructive jaundice. It was observed in 65% of the cases of the study by Kim et al. [15], in 86% of cases that of Takvama et al. [29]. This presentation may closely resemble that of pancreatic cancer with, in particular, a tumor aspect of the pancreas head, as the case of our patient other symptoms have been described during IBD, such as abdominal pain. [15] On the other hand, true acute pancreatitis is exceptional [18]. This table would be more common in young people [24]. Elsewhere, it may be recurrent vomiting or weight loss [21,30]. Occasionally, extra-pancreatic manifestations may be present, such as salivary gland hypertrophy, hydronephrosis associated with peritoneal fibrosis, and lymphadenopathy [31]. Lipasemia is moderately or very moderately elevated in 50% of cases. It is very rarely >3N. Cholestasis (sometimes fluctuating, which must then attract attention) is in the foreground, observed in 60 to 85% of cases. It is rarely due to pancreatic disease itself (by bile compression). It almost always reports the sclerosing cholangitis associated with IgG4, and 15% of asymptomatic patients all free from cholestasis. Diabetes is observed in 65% of cases. It precedes the diagnosis of autoimmune pancreatitis in 1/3 of cases, is synchronous every other time and appears under corticosteroid therapy in 15% of cases [32,33]. The elevation of serum IgG4 levels is the key to diagnosis. At the threshold of 2.70 g/l, the criterion retained by the international consensus, the specificity is 99% but the sensitivity is only 53% [34]. In other words, half of type I AIPs are seronegative or not associated with IgG4 levels high enough to rule out the main differential diagnosis of pancreatic adenocarcinoma. No imaging technique makes it possible to carry out the diagnosis of AIP in an absolute way, however each makes it possible to bring additional arguments. The combination of several types of imaging (including tomodensitometry [CT] and MRI) is essential. The most typical abnormalities visualized in CT are an overall increase of the entire pancreatic gland associated with loss of lobulation. The smooth appearance of the contours gives an image in saussice “sausage pancreas”. We can also note:
- A decrease in peripheral contrast enhancement at the origin of a halo or a peripheral ring;
- An involution of the tail of the pancreas;
- A contrast enhancement of the wall of the bile ducts thickened in the form of cockade;
- Tiered and suspended stenoses of the Wirsung canal without upstream dilatation;
- Focal pseudo-tumor forms not enhancing after injection of contrast enhancement (hypodense mass) [35].
In the case of PAI that has been evolving for many years, calcifications and vascular abnormalities are possible [30,36]. MRI provides essential additional data. There is also a loss of intensity in the T1 phase and T2 hyperintense of the parenchyma correlated with inflammation of the gland [37]. The interest of the Petscan has been evaluated in special cases: response to treatment with corticosteroids and diagnostic tool in case of suspicion of cancer and it can detect the involvement of other organs (indirect argument) and it would “monitor” the activity of the disease [38,39]. There is no effective diagnostic tool to differentiate PAI from cancer with a sensitivity of 100% apart from a biopsy showing carcinomatous cells. A negative puncture does not formally eliminate the diagnosis of cancer and it is essential to know how to repeat the biopsy puncture once or twice in case of strong suspicion [40-42]. In practice, in case of diagnostic doubt in the presence of a pancreatic mass associated with a dilation of the bile ducts, it is possible to propose an assay of serum IgG4, an abdominopelvic CT with thin sections centered on the pancreas, an MRI pancreatic with Wirsungo- MRI sequences and diffusion sequences, endoscopic ultrasound with puncture-biopsy of the mass. The value of serum Ca 19-9 is limited because a non-specific elevation of the marker is noted in cases of uncompensated diabetes or cholestasis.
Corticotherapy is the treatment of choice, consensual of the AIP. The evolution of symptoms may be so dramatic after a few days of treatment that the response to corticosteroids is an integral part of the Asian diagnostic criteria and HISORt [43]. Clinical and morphologic remission was obtained in 98% of patients treated versus 74% of untreated patients (p<0.001) [44]. The initial dose of oral prednisolone is 40 mg/day for 4 weeks and a decrease of 5 mg/week is recommended from the beginning of the improvement of symptoms. This equates to a total treatment duration of 12 weeks on average [29,35,45]. Because of the high rate of recurrence, especially in cases of biliary disease, monitoring is recommended: hepatic assessment (transaminases, alkaline phosphatase and total/conjugated bilirubin) and serum IgG4 assay every 3 months for 2 years and achievement of Pancreatic and biliary MRI every year for 2 years. In our case, the patient had certain clinical and radiological features that could impose a malignant tumor process. According to the diagnostic algorithms of Shimosegawa et al. [46], the absence of a characteristic characteristic of the PAI with CT or MRI in our patient would have required the search for another organ affected, the realization of an IgG4 serology, even a pancreatic biopsy whose results might have to guide diagnosis and therapeutic management.
Conclusion
Autoimmune pancreatitis is a rare but not exceptional disease. It is a real challenge for gastroenterologists and surgeons, because we must both avoid unnecessary pancreatectomies, and conversely do not delay a salvating surgery with sterile corticotherapy, whose inefficiency is masked by the pose of a biliary prosthesis. His diagnosis is difficult. Only perfectly interpreted imagery can evoke it.
References
- Sarles H, Sarles JC, Muratore R, Guien C (1961) Chronic inflammatory sclerosis of the pancreas an autonomous pancreatic disease? Am J Dig Dis 6: 688-698.
- Epstein O, Chapman RW, Lake-Bakaar G, Foo AY, Rosalki SB, et al. (1982) The pancreas in primary biliary cirrhosis and primary sclerosing cholangitis. Gastroenterology 83: 117-182.
- Montefusco PP, Geiss AC, Bronzo RL, Randall S, Kahn E, et al. (1984) Sclerosing cholangitis, chronic pancreatitis, and SjS: A syndrome complex. Am J Surg 147: 822-826.
- Lankisch PG, Koop H, Seelig R, Seelig HP (1981) Antinuclear and pancreatic acinar cell antibodies in pancreatic disease. Digestion 21: 65-68.
- Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, et al. (1991) Lymphoplasmacytic sclerosing pancreatitis with cholangitis: A variant primary sclerosing cholangitis extensively involving pancreas. Hum Pahtol 22: 387-395.
- Â Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, et al. (1995) Chronic pancreatitis caused by an autoimmune abnormality.Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci 40: 1561-1568.
- Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, et al. (2011) International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of PancreaÂtology. Pancreas 40: 352-358.
- Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, et al. (2001) High serum IgG 4 concentrations in patients with sclerosing pancreatitis. New Engl J Med 344: 1328.
- Kamisawa T, Funata H, Hayashi Y, Eishi Y, Koike M, et al. (2003) A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 38: 982-984.
- Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, et al. (2012) Recommandations for the nomenclature of IgG 4-related disease and its individual organ system manifestations. Arthritis Rheum 64: 3061-3067.
- Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi, et al. (2006) Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience. Clin Gastroenterol Hepatol 4: 1010-1016.
- Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, et al. (2006) IgG4-related sclerosing disease. Intern Med 45: 125-126.
- Nishimori I, Tamakoshi A, Otsuki M (2007) Research Committee on Intractable Diseases of the Pancreas, Ministry of Health, Labour, and Welfare of Japan. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol 42: 6-8.
- Â Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, et al. (2003) Controversies in clinical pancreatology. Autoimmune pancreatitis: does it exist? Pancreas 27: 1-13.
- Â Kim KP, Kim MH, Song MH, Lee SS, Seo DW, et al. (2004) Autoimmune chronic pancreatitis. Am J Gastroenterol 99: 1605-1616.
- Weber SM, Cubukcu-Dimopulo O, Palesty JA, Suriawinata A, Klimstra D, et al. (2003) Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg 7: 129-137.
- Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, et al. (2005) A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 138: 618-628.
- Finkelberg DL, Sahani D, Deshpande V, Brugge WR (2006) Autoimmune pancreatitis. N Engl J Med 355: 2670-2676.
- Yadav D, Notahara K, Smyrk TC, Clain JE, Pearson RK, et al. (2003) Idiopathic tumefactive chronic pancreatitis: clinical profile, histology, and natural history after resection. Clin Gastroenterol Hepatol 1: 129- 35.
- Â Raina A, Yadav D, Krasinskas AM (2007) Autoimmune pancreatitis. N Engl J Med 356: 1587.
- Church NI, Pereira SP, Deheragoda MG, Sandanayake N, Amin Z, et al. (2007) Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 102: 2417-2425.
- Kamisawa T, Yoshiike M, Egawa N, Nakajima H, Tsuruta K, et al. (2004) Chronic pancreatitis in the elderly in Japan. Pancreatology 4: 223-237.
- Blejter J, Weller S, Pace R, Cusumano H, Giambini D (2008) Autoimmune pancreatitis: an adolescent case and review of literature. J Pediatr Surg 43: 1368-1372.
- Kamisawa T, Wakabayashi T, Sawabu N (2006) Autoimmune pancreatitis in young patients. J Clin Gastroenterol 40: 847-850.
- Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, et al. (2002) HLA DRB1* 0405-DQB1* 0401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterol 122: 1264-1269.
- Kountouras J, Zavos C, Gavalas E, Tzilves D (2007) Challenge in the pathogenesis of autoimmune pancreatitis: potential role of helicobacter pylori infection via molecular mimicry. Gastroenterol 133: 368-369.
- Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, et al. (2010) Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreatology 10: 664-672.
- Ravi K, Chari ST, Vege SS, Sandborn WJ, Smyrk TC, et al. (2009) Inflammatory bowel disease in the setting of autoimmune pancreatitis. Inflamm Bowel Dis 15: 1326-1230.
- Hirano K, Tada M, Isayama H, Yagioka H, Sasaki T, et al. (2007) Long-term prognosis of autoimmune pancreatitis without and with corticosteroid treatment. Gut 56: 1719-1724.
- Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, et al. (2004) Autoimmune pancreatitis: imaging features. Radiology 233: 345-352.
- Kamisawa T, Nakajima H, Egawa N, Hayashi Y, Funata N (2004) Autoimmune pancreatitis can be confirmed with gastroscopy. Dig Dis Sci 49: 155-156.
- Okazaki K, Kawa S, Kamisawa T, Ito T, Inui K, et al. (2014) Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol 49: 567-588.
- Kanai K, Maruyama M, Kameko F, Kawasaki, K., Asano, J, et al. (2016) Autoimmune pancreatitis can transform into chronic features similar to advanced chronic pancreatitis with functional insufficiency Following Severe Calcification. Pancreas 45: 1189-1195.
- Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, et al. (2007) Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 102: 1646-1653.
- Pannala R, Chari ST (2009) Corticosteroid treatment for auto-immune pancreatitis. Gut 58: 1438-1439.
- Bodily KD, Takahashi N, Fletcher JG, Fidler JL, Hough DM, et al. (2009) Autoimmune pancreatitis: pancreatic and extrapancreatic imaging findings. AJR Am J Roentgenol 192: 431-437.
- Carbognin G, Girardi V, Biasiutti C, Camera L, Manfredi R, et al. (2009) Autoimmune pancreatitis: imaging findings on contrastenhanced MR, MRCP and dynamic secretinenhanced MRCP. Radiol Med 114: 1214-1231.
- Matsubayashi H, Furukawa H, Maeda A, Matsunaga K, Kanemoto H, et al. (2009) Usefulness of positron emission tomography in the evaluation of distribution and activity of systemic lesions associated with autoimmune pancreatitis. Pancreatology 9: 694-699.
- Lee TY, Kim MH, Park do H, Seo DW, Lee SK, et al. (2009) Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol 193: 343-348.
- Moon SH, Kim MH, Park DH, Hwang CY, Park SJ, et al. (2008) Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut 57: 1704-1712.
- Fattahi R, Balci NC, Perman WH, Hsueh EC, Alkaade S, et al. (2009) Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC) and normal pancreas. J Magn Reson Imaging 29: 350-356.
- Kartalis N, Lindholm TL, Aspelin P, Permert J, Albiin N (2009) Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol 19: 1981-1990.
- Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, et al. (2006) Diagnosis of auto-immune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 4: 1010–1016.
- Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, et al. (2009) Standard steroid treatment for auto-immune pancreatitis. Gut 58: 1504-1507.
- Ito T, Nishimori I, Inoue N, Kawabe K, Gibo J, et al. (2007) Treatment for auto-immune pancreatitis: consensus on the treatment for patients with auto-immune pancreatitis in Japan. J Gastroenterol 42: 50-58.
- Kamisawa T, Yoshiike M, Egawa N, Nakajima H, Tsuruta K, et al. (2005) Treating patients with auto-immune pancreatitis: results from a long-term follow-up study. Pancreatology 5: 234-238.
Citation: Yassine H, Chakiri A, Elmouatassim Z, Azzaoui F, Lafrouji S, et al. (2018) Autoimmune Pancreatitis Mimicking Pancreatic Cancer: A Case Report. J Oncol Res Treat 3: 125.
Copyright: © 2018 Yassine H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4640
- [From(publication date): 0-2018 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 3605
- PDF downloads: 1035