Auditory Brainstem Response Characteristics of Children with Cerebral Palsy: Clinical Utility and Prognostic Significance
Received: 15-Jul-2016 / Accepted Date: 24-Aug-2016 / Published Date: 31-Aug-2016 DOI: 10.4172/2161-119X.1000259
Abstract
Background: Cerebral palsy affects body muscle and movement coordination due to organic complications in the peripheral and central nervous systems and therefore often accompanied by other disorders of cerebral function. CP with additional impairment of hearing results in severe developmental deficits in communication, speech and language and cognitive skills. Thus it is important to examine the auditory nervous system to identify the complications caused by the hidden hearing loss. Auditory Brainstem Responses (ABR) provides objective measure of auditory system function and can be an important adjunct to the clinical neurophysiologic examinations. However, there is scanty information about the neurophysiologic investigations in children with spastic cerebral palsy. Aim: To investigate whether the children affected with spastic CP exhibit distinct neural responses than the age matched normal hearing children. Methodology: ABR measures were obtained for 50 children with spastic CP in the age range 3 to 12 years. The results were subsequently correlated with birth weight, gestational age, etiology and type of CP, neuroradiological findings, additional impairments and disabilities (including the ability to walk independently). 50 typically normal hearing children served as reference group for comparisons of neurophysiologic measures of auditory brainstem responses. Results: A significant difference was found in the ABR latencies between the children with cerebral palsy and atypical children. Abnormal ABR measures in children with spastic CP demonstrated a correlation with the presence of moderate to severe developmental delay. Conclusion: It can be concluded that ABR measures of CP group revealed a statistical difference with that of the typically developing children and it has demonstrated a statistically significant correlation with the presence of neurological deficits. Therefore, Auditory Brainstem Response measurement being a non-invasive neurophysiologic investigation can serve as important tool in the diagnostic work up of spastic CP.
Keywords: Spastic cerebral palsy; Additional impairment; Hearing loss; Peripheral auditory system; Click evoked auditory brainstem response
252777Abbreviations
ABR: Auditory Brainstem Response; CP: Cerebral Palsy
Introduction
Cerebral palsy (CP) is a group of non-progressive neurological disorders [1,2] which affects body muscle tone and movements coordination [3]. CP occurs due to abnormal development of the cerebral motor cortex during fetal growth (embryonic period) or due to injury to the motor control centers of the developing brain during birth or after birth up to age about 2-3 years [4,5].
The four major subtypes of Cerebral palsy are spastic, athetoid, ataxic and mixed cerebral palsy. The spastic form of CP is the most commonly occurring in 65% of cerebral palsied population [5]. Prevalence of Cerebral palsy is increasingly encountered in neonatal clinics since more number of premature infants survives because of advance neonatal care and better medical facilities for treatment of perinatal infections.
Cerebral palsy is often accompanied by other disorders and problems of cerebral function, in particular intellectual impairment, speech and language deficits, epilepsy, vision and hearing disorders [6,7]. Recent studies have shown that hearing impairment occurs in 4 to 25% of children with CP [6]. These children with additional impairment of hearing presents ranges of special educational and psychological needs, to an even greater degree than for children with single disability [7].
Presence of reduced hearing acuity during infancy and early childhood in children with CP may have more deleterious effect on communication abilities, speech and language and cognitive development that can severely interfere with their psycho-, difficulties in parent-child and peer-child interactions, low self-esteem, linguistic and educational development [8]. However, a child's overall future and success can be improved greatly through the early identification of hearing loss, establishment of its site of lesion, and subsequent institution of intervention strategies may improve learning and language development [6,9].
Children with cerebral palsy have an organic complication in the peripheral and central nervous systems [7-9]. Thus it is important to examine the auditory nervous system to identify and reduce complications of hidden hearing loss. Although clinical evaluation may suggest hearing loss, a definitive diagnosis requires an audiological assessment [9].
Auditory Brainstem Response (ABR) measurements is an non invasive and objective method [8-10], can be used to assess hearing capabilities in infants younger than 6 months of age and in older children who are unable to perform conventional or conditioned play audiometry due to motor or intellectual problems [7-9].
ABRs are electrical potentials that are produced in response to a brief stimulus like click and are recorded from disk electrodes attached to the scalp. The early potentials reflect electrical activity at the cochlea, 8th cranial nerve, and brain stem levels and may be analyzed to estimate the magnitude of hearing loss and to differentiate among cochlea, 8th nerve, and brainstem lesions [7-9]. Therefore, ABR can be an important adjunctive diagnostic tool for the clinical neurophysiologic examinations of hearing loss in children with cerebral palsy.
In recent years, the neurophysiologic examination of children with Cerebral palsy has been of increasing interest to audiologists, otologists, pediatricians and other researchers to evaluate normal physiological maturation and integrity of the auditory system, in the screening of hearing impairments of infants, to diagnose and demonstrate brainstem damage and to provide prognosis for the patients with various neurological disorders [8,9].
In a retrospective study of 75 children with spastic cerebral palsy (CP), 17 (22.7%) had abnormal ABR waveform [10-12]. Another study concluded that one of the specific feature i.e., sensorineural hearing loss in athetoid CP caused by kernicterus can be identified by the ABR [13]. These authors concluded that ABR can provide new insights into mechanisms of brain damage and neural plasticity in children with cerebral palsy. In spite ABR being very promising diagnostic tool in assessment of hearing in difficult to test populations including the children with cerebral palsy, very fewer studies on auditory brainstem responses are available Indian literature.
Hence the present study was undertaken to characterize the electrophysiological findings in children with spastic cerebral palsy and correlate with their clinical features.
Materials and Methods
Research design
This prospective survey was done at our institute’s electrophysiological laboratory in accordance with Institutional ethical norms. The necessary informed consent by the parents of the children was obtained.
Participants
Total 100 participants of both the sexes in the age range 3–10 years (mean=6.6 years, SD=2.12 years) were recruited for the study. The subjects were divided into two groups. The Group A consisted of 50 subjects with spastic cerebral palsy (37 male and 23 females with mean age of 6.8 years, SD=1.92 years) diagnosed by pediatrician and Group B included 50 normal hearing children (33 male and 27 female with mean age of 5.9 years, SD=2.32 years) with no known history of neurological, psychiatric and otological disease or trauma.
Demographic data of confirmed spastic CP and typically developing children were collected through medical reports, parental interview, case histories about age, prenatal, perinatal and postnatal events, and history of epilepsy. Group B subjects evidenced normal peripheral hearing sensitivity, defined as pure-tone thresholds (≤ 25 dB HL, re: ANSI, 1996) for each ear at octave frequencies between 250 and 8000 Hz. Normal middle-ear admittance and presence of acoustic reflexes at 500 and 1000 Hz for all subjects were confirmed by means of immittance measures.
Stimuli and recording parameters
ABRs were elicited by an acoustic 100 μs click stimulus. Responses were recorded via four Ag-AgCl surface electrodes having absolute contact impedance of <5 kΩ with no more than 3 kΩ difference between each of the two electrodes. Before connecting the electrodes, the skin was cleaned thoroughly to ensure good contact between the skin and the electrode surfaces. Non inverting electrode was positioned centrally on the scalp at Cz, two inverting electrodes were placed behind the mastoid (A1 and A2) and ground electrode was attached at forehead (FPz).
Monaural auditory stimulus consisting of alternating polarity clicks were delivered into the ear at a rate of 11.1/s at intensity level of 80 dBnHL through electrically shielded insert earphones (ER-3). The sampling rate was 20000 Hz and responses were online band passed filtered from 100 to 3000 Hz. Artifacts greater than ± 35 μV were rejected online. Two traces of 2000 sweeps were collected at alternating polarity. Responses of alternating polarities were added together to minimize contributions from the cochlear micro phonic responses [12]. Responses to 2000 click presentations were averaged for 12 ms. During testing, the children were in the supine position with eyes closed.
Result analysis
In 50 children with spastic cerebral palsy (CP), Auditory Brainstem Responses (ABRs) were recorded and subsequently correlated with birth weight, gestational age, etiological factors and additional impairments and disabilities (including the ability to walk independently).
Identification of brainstem recorded wave by the click stimulus was done based on the conventional clinical analysis. The peaks were marked by the two independent observers. The identities and the diagnostic categorizations of the children were blinded to the observers. The observers were also requested to rate the individual wave morphology as poor, fair and good.
ABR measures like absolute peak latencies of I, III, V waves, interpeak latencies of I-III, III-V, and I-V were considered for comparison between the two groups. The mean, median, standard deviation, minimum, and maximum values for normal distribution of responses were calculated for the sample. An independent t-test was used to compare the mean value of the results. One way ANOVA was studied at significance levels of 5% (P<0.05) to find the association between ABR abnormalities and clinical features, additional impairment and risk factors. For statistical analysis, SPSS.16 software was used.
Results
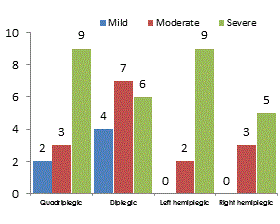
The current study was designed to assess and compare the brainstem responses to click stimuli in children with spastic cerebral palsy and typically normal hearing and healthy developing children. The Group A included 50 children suffering from spastic CP consisted of 14 spastic tetraplegic, 17 spastic diplegic, 11 left side hemiplegic and 8 right side hemiplegic (Figure 1).
| Absolute Latenciesmean (SD) in ms | Inter-peak Latencies mean (SD) in ms | |||||
|---|---|---|---|---|---|---|
| I | III | V | I-III | III-V | I-V | |
| Group A | 1.6 | 3.83 | 5.77 | 2.23 | 1.94 | 4.17 |
| ±.09 | ±.14 | ±.10 | ±.13 | ± .11 | ±.10 | |
| Group B | 1.5 | 3.61 | 5.54 | 2.11 | 1.91 | 4.04 |
| ±.06 | ±.13 | ±.20 | ± .15 | ±.20 | ± .18 | |
| p-value at <0.05 | 0.01* | .01* | .02* | .43* | 0.7 | 0.02* |
Table 1: Showing means and SD (in parenthesis) of absolute latency (ms) of wave peaks I, III, V and Inter-peaks latency (ms) I-III, III-V and I-V of click evoked ABR measures of Group A and Group B.
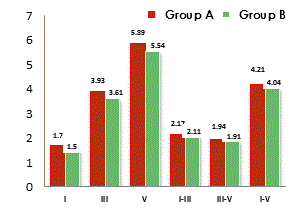
The ABR wave morphology in clinical population was rated as 67% poor, 31% fair and 2% good whereas normal hearing children were rated as having 3% poor 19% fair and 78% good wave morphology by the peak observers. The neurophysiologic responses to click generated waves I, III and V were analyzed based on the conventional clinical analysis of the absolute latencies and inter-peak latencies for both groups. Table 1 indicating mean and standard deviation of absolute and inter peak latencies and p-values between the groups.
The notable ABR abnormalities in CP children were prolongation of absolute latency of peak, III & V, inter-peak latencies of I-III and III–V. Figure 2 showing the absolute peak latency and inter-peak latency in both the groups. The means of Group A for ABR parameters were statistically significant from the Group B at significance level of p<0.05.
Out of the total 50 subjects, 31 children with spastic CP presented with associated clinical features and abnormalities such as microcephaly, mental retardation, delayed development and other risk factors. The percentage of abnormal auditory brainstem responses along with clinical feature of ABR findings are elucidated in Table 2.
| Clinical Features/Risk Factors | N | Abnormal ABR% |
|---|---|---|
| Normal Developmental Milestone | 7 | 2 (28%)* |
| Communication andSpeech delay | 31 | 31 (100%)** |
| Mental retardation/ Microcephaly | 11 | 4 (36%)* |
| Global delay | 12 | 10 (75%) ** |
| Developmental Delay | 7 | 4 (46%)* |
| Low birth weight | 9 | 2 (40%)* |
| Prematurity + LBW | 9 | 3(50%)* |
| BilateralSensorineural hg loss | 4 | 4(100%)** |
| Birth asphyxia , Seizures/Epilepsy | 8 | 4 (50%)* |
| Neonatal Jaundice/ Encephalitis | 16 | 8 (50%)* |
Table 2: Depicting clinical features, associated abnormalities and risk factors in children with spastic cerebral palsy, ** Statistically significant correlation at p value 0.01, * Statistically significant correlation at p value 0.05.
Abnormal ABR in children with spastic cerebral palsy demonstrated a statistically significant correlation at p value 0.001 with the presence of global developmental delay and additional disability of hearing loss, whereas prematurity, neonatal jaundice and encephalitis etc have statistically significant correlation at p value 0.05.
Discussion
The ABR results were primarily analyzed for the presence of the waves I, III and V. Wave I, or latency I is based on the transformation of tone-specific responses in the hair cells into impulses travelling along the auditory nerve, and after passing the cochlear nucleus of the brainstem, the impulse reaches the superior olivary complex, forming wave III or latency III [14,15]. Wave V is then produced in the inferior colliculus, and finally the temporal auditory cortex is reached [15-21].
The study faithfully characterizes the electrophysiological responses to click stimuli in children with spasticity and typically developing. It can be observed from Tables 1 and 2 that the children with CP who had other associated neurological symptoms showed statistically significant deviation from the regular pattern of ABR waveforms for absolute peak latencies I, III and V and inter-peak latency between IIII and III-V.
Most of the children with neurosensory disability, except one spastic CP with microcephaly, had bilaterally absent or delayed responses in ABR components. 31 of 50 CP children with clinical features, additional impairment and risk factors (Table 2), 17 had abnormality in wave I, 14 in wave III, 29 in wave V, 23 in IPL between wave I–V, 19 in IPL III–V (Table 1).
This is in consonance with the reports of previous works which reports that children with CP have abnormalities of the auditory sensory pathways at greater rates than found in the neurologically normal children [16,17]. These researches concluded that an abnormal ABR, manifested as an absence or prolongation of latencies have positive association with adverse neurological development in children [16,17].
The prolongation of the IPL I–V and IPL III–V conduction times has been found in high-risk preterm infants with a transient neurological abnormality [17,20,22]. A bilateral abnormality in a predischarge ABR examination of a VLBW infant has been shown to correlate with an adverse outcome in intelligent quotient, language and academic achievements [15,22].
Further, increased IPL III–V and IPL I–V conduction times have also been shown to be significant prognostic indicator for delayed motor development and abnormal neurological findings [15]. Abnormal or repeatedly absent ABR during the neonatal period have been correlated closely with the hearing impairment and psychomotor development deficits [15,22]. The studies also reported that abnormal ABRs in prematurely born children with spastic CP are indicative of poor prognosis and positive associated with a “multihandicap state” of the children [12,18,19].
The findings of the current study as elucidated in Table 2. Wherein 31 (62%) subjects had communication and speech problems, 12 (24%) had mental retardation, 19 (38%) had Global developmental delay, 13 (26%) were unable to walk independently and 4 (8%) had hearing loss. There was a statistically significant association between abnormal ABR recordings and preterm delivery, perinatal etiology of CP, hearing, speech and myoskeletal impairments, epilepsy and mental retardation (p<0.001). These findings are in concurrence with previous reports. Though, the exact etiology of abnormal ABR remains to be unleashed through this study. Hence, further research in this area is recommended.
However, it can be concluded that abnormal ABR recordings in children with spastic CP probably can be linked to the neurological deficits in the light of earlier reports. Thus, it is suggested that ABR testing should be incorporated in the diagnostic assessment of all children with spastic CP referred to Neurodevelopment Centers to plan holistic intervention strategies including amplification devices.
Conclusion
The study concludes that ABR measurement in spastic CP revealed marked differences with that of the typical children, delay in the absolute latency as well as inter-peak latency differences are indicative of neurological deficits. The clinical features, additional disabilities and risk factors have demonstrated a statistically significant correlation with the presence of abnormal ABR and thus neurological deficits.
ABR examinations can detect lesions that may be asymptomatic and subtle to preclude the optimal development of the child. The hearing impairment is frequent in children with cerebral palsy and causes severe deficits in communication, development of speech & language and cognitive skills. Thus it is important to examine the auditory nervous system to identify and reduce the complications caused by the hidden hearing loss.
Despite limitations of ABRs as time consuming tool, ABR is important tool to determinate the functional integrity of the auditory tract and evaluation of hearing thresholds in patients with cerebral palsy. Further, to conclude, since ABR measurement is non-invasive and objective neurophysiologic investigations, it can serve as important adjunct to the clinical examinations and diagnostic work up of spastic cerebral palsy.
References
- Morris C (2007) Definition and classification of cerebral palsy: A historical perspective.Dev Med Child NeurolSuppl 109: 3-7.
- Surveillance of Cerebral Palsy in Europe (2000) Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE).Dev Med Child Neurol 42: 816-824.
- Ghai OP (2004) Central nervous system. In: Ghai OP (eds.) Essentials paediatrics. New Delhi, pp: 540-549.
- Odding E, Roebroeck ME, Stam HJ (2006) The epidemiology of cerebral palsy: Incidence, impairments and risk factors.DisabilRehabil 28: 183-191.
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, et al. (2007) A report: The definition and classification of cerebral palsy April 2006.Dev Med Child NeurolSuppl 109: 8-14.
- Kolker IA (2004) Hearing function and auditory evoked potentials in children with spastic forms of cerebral palsy. Neurophysiology 36: 270-275.
- Sano M, Kaga K, Kitazumi E, Kodama K (2005) Sensorineural hearing loss in patients with cerebral palsy after asphyxia and hyperbilirubinemia.Int J PediatrOtorhinolaryngol 69: 1211-1217.
- Joint Committee on Infant Hearing (2007) Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. Suppl Audiology Today 120: 1-29
- Fowler KB, Boppana SB (2006) Congenital cytomegalovirus (CMV) infection and hearing deficit.J ClinVirol 35: 226-231.
- Kaplan M, Hammerman C (2005) American Academy of Pediatrics guidelines for detecting neonatal hyperbilirubinaemia and preventing kernicterus. Arch Dis Child Fetal Neonatal Ed 90: 448-449.
- Kohelet D, Arbel E, Goldberg M, Arlazoroff A (2000) Brainstem auditory evoked response in newborns and infants.J Child Neurol 15: 33-35.
- Zafeiriou DI, Andreou A, Karasavidou K (2000) Utility of brainstem auditory evoked potentials in children with spastic cerebral palsy.ActaPaediatr 89: 194-197.
- Morales Angulo C, Azuara Blanco N, Gallo Terán J, González Aledo A, Rama Quintela J (2006) [Sensorineural hearing loss in cerebral palsy patients].ActaOtorrinolaringolEsp 57: 300-302.
- Raj H (2004) Evoked potentials in preterm and term neonates with their relevance in hypoxic-ischemic insult. Journal of Neonatology 18: 34-39.
- Markand ON (1994) Brainstem auditory evoked potentials.J ClinNeurophysiol 11: 319-342.
- Majnemer A, Rosenblatt B, Riley PS (1990) Prognostic significance of multimodality evoked response testing in high-risk newborns.PediatrNeurol 6: 367-374.
- Kitamoto I, Kukita J, Kurokawa T, Chen YJ, Minami T, et al. (1990) Transient neurologic abnormalities and BAEPs in high-risk infants.PediatrNeurol 6: 319-325.
- Cox C, Hack M, Aram D, Borawski E (1992) Neonatal auditory brainstem response failure of very low birth weight infants: 8-year outcome.Pediatr Res 31: 68-72.
- O'Shea TM (2008) Diagnosis, treatment, and prevention of cerebral palsy.ClinObstetGynecol 51: 816-828.
- Pike AA, Marlow N (2000) The role of cortical evoked responses in predicting neuromotor outcome in very preterm infants.Early Hum Dev 57: 123-135.
- Kułak W, Sobaniec W, Kuzia JS, Boćkowski L (2006) Neurophysiologic and neuroimaging studies of brain plasticity in children with spastic cerebral palsy.ExpNeurol 198: 4-11.
- Venkateswaran S, Shevell MI (2008) Comorbidities and clinical determinants of outcome in children with spastic quadriplegic cerebral palsy.Dev Med Child Neurol 50: 216-222.
Citation: Ansari MS, Raghunathrao R, Ansari MAH (2016) Auditory Brainstem Response Characteristics of Children with Cerebral Palsy: Clinical Utility and Prognostic Significance. Otolaryngol (Sunnyvale) 6:259. DOI: 10.4172/2161-119X.1000259
Copyright: © 2016 Ansari MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13573
- [From(publication date): 9-2016 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 12621
- PDF downloads: 952