ATP4a Expression during Pesticide Induced Lung Damage
Received: 12-Dec-2022 / Manuscript No. JCEP-23-83061 / Editor assigned: 15-Dec-2022 / PreQC No. JCEP-23-83061 / Reviewed: 29-Dec-2022 / QC No. JCEP-23-83061 / Revised: 02-Jun-2023 / Manuscript No. JCEP-23-83061 / Published Date: 30-Jun-2023 DOI: 10.4172/2161-0681.1000446 QI No. / JCEP-23-83061
Abstract
Lung is vtial organ that contains more than 40 different cell types localised regionally and spatially with varied number of mitochondria. It is evident that every cell depends on the mitochondrial oxidative phosphorylation for the production and supply of ATP. Among the cells, one type of cell is bronchial epithelial cells that possess cila. These specialized cells act as a line of defense against the entered pathogen or antigen. These cells with the help of mucous secreted by goblet cells expel out the entered pathogen by its cilary beating movement.
Keywords: Lung, ATP, Phosphorylation, Mitochondria, Goblet cells
Keywords
Lung; ATP; Phosphorylation; Mitochondria; Goblet cells
Introduction
Lung of an organism comes in contact with numerous pollutants present in atmosphere. The occupational health hazards due to such pollutants is one of the emerging problem that farmers and farm workers suffering from, despite the use of the protection kit. Since green revolution, pesticides utilisation has significantly minimized the pest menifested crop destruction and has increased the crop yield worldwide. With the passage of time, the unstrained use of pesticides have caused resistance of pesticides in pests. Consequenlty, farmers started using the cocktail of pesticide that shows synergistic effect on the pest. As result of this trend, the farmer are likely to get more respiratory problems than before. A recent report have shown 79% correlation between the repiratory health and pesticide exposure [1].
In the present study, mice has been exposed chronically to chlorpyriphos and/or cypermethrin through oral route to explore its potential to dysregulate the molecular cascade system of the lung. Chlorpyriphos is a organophosphate and cypermethrin is a class II pyrethroid pesticide, globally used to control pests in agriculture, to reduce pest population around the residential areas. The oxon metabolite of chlorpyriphos bind and irreversibly inhibit Acetyl Cholinesterase (AChE) in target tissues that halts synaptic function of the pest, on the other hand cypermethrin crosses the blood-brain barrier and extends the hyper-excitation of the central nervous system of the pest by interfering with the sodium channel [2]. Nowadays, organophosphates are being used in combination with pyrethroids to increase the toxicity. In combined form the chemical cocktail inhibit the detoxification of pyrethroid and increase overal toxicity. The available data suggest that prolonged exposure to these contaminants cause chronic or persistent neurological developmental deformaties and other reproductive failures. Uncontrolled administration of pesticides in agricultural lands has proven to be toxic to targeted and nontargeted organism. Similarly, pesticide exposure has found to be associated with immunomodulation that makes organism more susceptible to bacterial, viral and parasitic infections and cause to promote the tumors development. However, the molecular data on the effect of these pesticides on pulmonary molecular profile is scanty [3].
ATP4a is an integral component of ATP transmembrane proton pump that become functional when coordinately work with other catalytic ATP4b. Earlier, it was known that this ATP4 is highly functional in vertebrate’s stomach for the acidification of the gastric lumen. In a recent study, it has also been found to be expressing in the mammalian airways, though due to lack of other supporting data, these reports are questionable.
In our recent reports we found that prolong dietary exposure to various chlorpyrifos and/or cypermethrin changes the histomorphology and transcription profile of lung of mice [4]. Similarly, it was observed that Apaf1 gene that play an important role in apoptosis was found to be suppressed leading to impairment of apoptotic pathway in mice lung during pesticide induced lung pathology. However, this current study may set up a base to understand the molecular role of ATP4a during the pesticide induced lung damage which will further help us elucidating its potential as biomarker for the pesticide induced lung damage [5].
Materials and Methods
In vivo experimental design
The experiment has been completed as per permitted etiquette of institutional animal ethics committee, Guru Angad Dev veterinary and animal sciences university, Ludhiana. Twenty-eight disease-free albino mice (6-8 weeks old) were purchased from the small animal colony maintained by Lala Lajpat Rai university of veterinary and animal sciences university, Hisar Haryana. Mice were weighed, randomly divided into 4 groups each containing 6 mice in polypropylene cages and acclimatized for a period of a week at the institutional small animal house under controlled condition with 12 h light and dark cycle. The treatment groups received cypermethrin (2 mg kg-1 body weight), chlorpyriphos (2.76 mg kg-1 body weight) and the mixture of both pesticides dissolved in corn oil at the same concentration orally for continuous 90 days. The fourth group served as the negative control that received normal diet [6]. Throughout the experiment, mice were fed synthetic pelleted mice feed obtained from Ashirward feed industries Chandigarh, along with water ad libitum. Immediately, on 90th day of trial each animal was anaesthetized with 1/10th dose of xylazine and ketamine combination intraperitoneally (xylazine: 0.5 ml; 20 mg/ml mixed with ketamine: 2 ml; 50 mg/ml).
Tissue collection
The lung was cut and tissues was immersed in RNA later and stored at -80°C which were then subjected to RNA extraction for transcriptomic analysis. The second lung was suspended in 4% paraformaldehyde for 12 hours and processed for immuno-histopathology [7].
Microarray gene expression and analysis
Approximately, 50 mg of lung tissues from each animal of all groups were used to extract RNA using trizol method. The purity and concentration were evaluated in nanodrop spectrophotometer (thermofisher) followed by quality check in agilent 2100 bio-analyzer. The RNA sample that passed the RNA Integrity Number (RIN) ≥ 7 was utilized for microarray. RNA (100 ng) was labeled with low input quick amp WT labeling kit and then pooled into two samples that served as biological replicates and technical replicates in which one color based exon analysis was carried out using two mouse microarray slides (8 × 60 K: agilent-028005). The quality check of the labeled cRNA along with its yield was done using nanodrop. After the generation of microarray scan images, the signals intensities were obtained using feature extraction software version 10.7.3. The data generated were analyzed using gene spring software version 14.9 (agilent technologies) to identify the Differentially Expressed Genes (DEG’s) [8].
Bioinformatics analysis and gene ontology analysis of DEG’s
The differentially expressed genes symbol (cut off 7 log 2-fold change) were then uploaded onto the online DAVID bioinformatics tool in order to obtain the topmost dysregulated molecular pathway. Furthermore, on the basis of biological process, molecular function, cellular component, protein class gene ontology was checked using panther databases. A venn diagram was also generated using online tool for the identification of differentially and uniquely expressed genes following exposure to chlorpyriphos, cypermethrin and mixture of both pesticides [9].
Construction of the Protein-Protein Interaction (PPI) network
To study the interaction of ATP4a with other genes of same or different molecular pathways were used to generate the PPI network (>0.4 combined score) using search tool for the retrieval of interacting genes (string) database.
RNA extraction and cDNA synthesis for RT qPCR
Lung tissue (50 mg) from all animal was used to extract the RNA following trizol method. Before using the extracted RNA for cDNA preparation, the quality and its quantification test was performed in nanodrop (A260/A280 ratio of RNA was ≅ 2.0). The passed samples, were used (100 ng/μl of RNA) was used for cDNA synthesis using RT2 first strand kit (Cat: 330401) from Qiagen in which RNA was first denatured at 42°C for 5 minutes in the presence of buffer GE (Genomic DNA Elimination) followed by immediate cooling for at least one minute. An additional step to eliminate the genomic DNA was also performed using genomic DNA elimination mixture followed by addition of reverse transcription mix. Finally, the cDNA was prepared at 42°C for 15 and 90°C for 5 minutes, respectively [10].
Real Time quantitative Polymerase Chain Reaction (RT qPCR)
RT qPCR was performed on the same lung that were used for the microarray assay. The expression profile of Atp4a F: CATTGCCTTTGCCATCCAGGR:CTGGTAGTAGCCAAAACAGCC was validated against the results obtained from microarray assay. The qPCR reaction was run in duplicates using SYBR green chemistry with β-actin as an endogenous control. The relative expression of each gene was calculated using the ΔΔCT method [11].
Immunohistochemistry
The immunohistochemistry was performed as per the standardized method by Sethi et al. In brief, the deparaffinized tissues were dehydrated and then incubated with 3% H2O2 for 20 min to quench endogenous peroxidase followed by boiling in tris-borate EDTA and 1X PBS for antigen retrieval. Then 1% BSA was added to the sections and incubated in dark chamber. The sections were then stained with primary antibodies against mouse ATP4a (CAT: E-AB-13943; dilution 1:50) and kept overnight at 4°C. The tissues were then washed with 1X PBS and incubated with secondary anti-rabbit antibody (ATP4a-1:400). The reaction was visualized using a color development kit (SK4100, vector laboratories and USA). The sections were counterstained with methyl green. Controls consisted of staining without primary antibody or secondary antibody or both [12].
Grading for immunohistochemistry
Immuno-positive stained slides were then subjected to quantification of the number of positive ATP4a cells in the complete 10 cross section.
Enzyme-Linked Immunosorbent Assay (ELISA)
Sandwich ELISA was performed according protocol provided by the manufacturer to compare the concentration of ATP4a (Immunotag ELISA kit: 02603; dilution: 1:10) in serum following the exposure to chlorpyriphos, cypermethrin and their mixture. Optical Density (OD) was measured at wavelength of 450 nm on ELISA reader. The standard curve was created using the given formula on the manual to calculate the protein content in the blood [13].
Statistical analysis
A one-way Analysis of Variance (ANOVA) was conducted to determine the level of significance among the data of RT qPCR, immuno-positive cells and protein concentration obtained by Enzyme-Linked Immunosorbent Assay (ELISA) in different pesticide treated groups with comparison to control group at p<0.05 using SPSS 20.0.
Results
Bioinformatics analysis
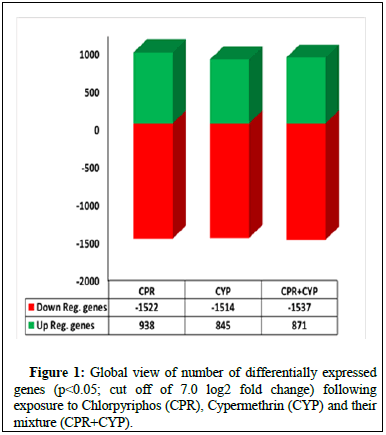
The bioinformatics analysis of microarray based transcriptomic data of the lung samples (2500 genes; p<0.05; minimum cut off of 7.0 log2 fold change) exposed to chlorpyriphos and/or cypermethrin with respect to healthy unexposed mice reflected an upregulation of 938 and 845 genes and downregulation 1522 and 1514 genes following the exposure to chlorpyriphos and cypermethrin alone, respectively. Furthermore, combination of cypermethrin and chlorpyriphos dissolved in corn oil, upregulated 871 genes and downregulated 1537 genes (Figure 1).
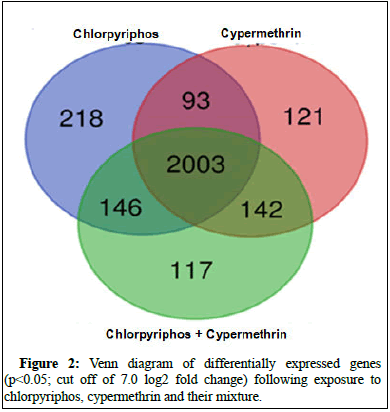
When extracted the data on commonly expressing genes of all three groups, a total 2003 genes were obtained whereas 218, 121 and 117 genes were found to be uniquely expressing in case of chlorpyriphos, cypermethrin individual and in case of exposure to their mixture respectively (Figures 2 and 3).
Validation in RT qPCR
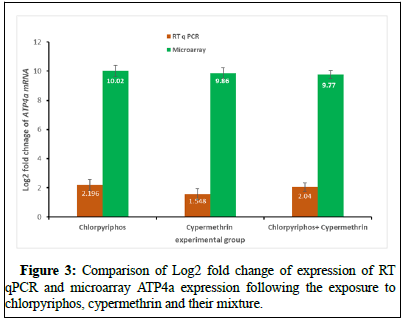
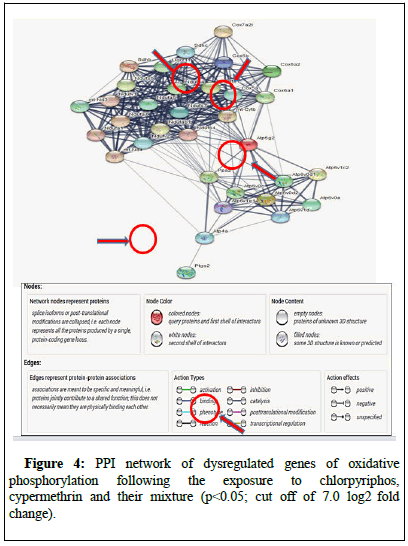
The microarray data when analyzed for any change in expression profile, it was found that ATP4a was overexpressing (10.02, 9.86 and 9.77) in the mice lung following exposure to chlorpyriphos, cypermethrin and their mixture. These expressions of genes were later on validated using SYBR green chemistry-based RT qPCR in which exposure to chlorpyriphos, cypermethrin and their mixture showed similar pattern of expression for ATP4a (2.19, 1.54 and 2.04). Similarly, the molecular interaction of ATP4a was observed by producing a protein-protein interaction where its molecular position was also determined in molecular pathways such as oxidative phosphorylation (Figure 4).
Validation through immunohistochemistry
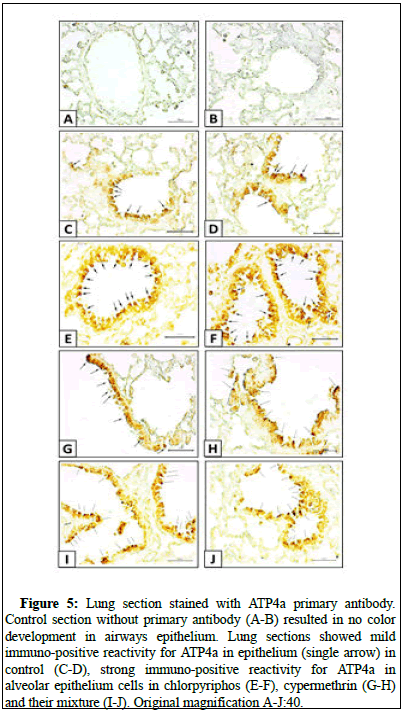
In the immunohistochemistry, the lung sections incubated without primary antibody did not show any immuno-positive reactivity for ATP4a. The expression of the ATP4a in the alveolar epithelial lining was moderate in biological control group and had higher number of positive cells in the mice lungs exposed to chlorpyriphos and/or cypermethrin [14].
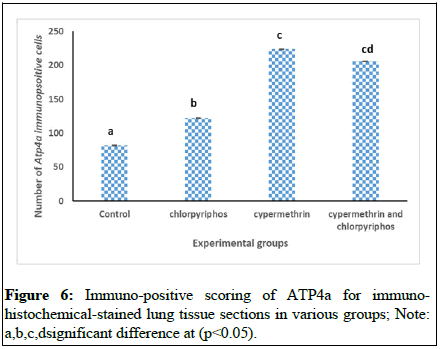
To correlate the data statistically, the data of positive cells count were statistically analyzed and compared to each other which showed that ATP4a expression was statistically higher in pesticide exposed group compared to unexposed control group. The intergroup comparison depicted that the comparative toxicity posed by cocktail of chlorpyriphos and cypermethrin was equal but was more than single exposure to chlorpyriphos demonstrated by the number of ATP4a positive cells (Figures 5 and 6).
Figure 5: Lung section stained with ATP4a primary antibody. Control section without primary antibody (A-B) resulted in no color development in airways epithelium. Lung sections showed mild immuno-positive reactivity for ATP4a in epithelium (single arrow) in control (C-D), strong immuno-positive reactivity for ATP4a in alveolar epithelium cells in chlorpyriphos (E-F), cypermethrin (G-H) and their mixture (I-J). Original magnification A-J:40.
Validation through ELISA assay
Assuming that this selected gene can be used as the biomarker to predict the lung health of the mice, sandwich ELISA was conducted using mice serum to estimate the presence of the protein in the blood. The exposure to chlorpyriphos, cypermethrin and their mixture did not show any significant alteration in the concentration of ATP4a as compared to control group. The difference in concentration of the targeted protein in the blood control, chlorpyriphos, cypermethrin and their cocktail was found to be insignificant 0.41 ± 0.006a 0.427 ± 0.009a 0.43 ± 0.014a 0.43 ± 0.027a, respectively.
Discussion
Several research studies have suggested that chemical toxicants such as pesticides if inhaled or ingested causes respiratory problems such as pulmonary edema in which fluid accumulates in the lung. Usually, respiratory airways are covered by beating cilia (mucocilary epithelium) which protects the passage from pathogenic invasions. In brief, the fluid accumulated are expelled out by the beating movement of the cilia present on the cilia containing cells. On the other hand, the inhaled pathogens are removed from the airway passage in which goblet cells assist them with secretion of mucous, that is why, the relationship between mucus secretion and respiratory problems has been discussed in several studies that depicts the role of ciliary cells during the elimination of trapped pathogen through its back and forth beating movement. However, the available molecular data is not sufficient to elucidate the missing behind the same, thus this present effort has been made to understand and generate a possible relationship between ATP4a and lung pathology [15].
ATP synthase (ATPase) or complex V is a trans-membranous proton pump and consists of catalytic α (ATP4a) and β (ATP4b) subunits. It is known that vertebrates have high ATP4a is expression in their stomach which they use to acidify the gastric lumen. Recently, a study conducted in xenopus embryonic epidermis reported the expression of this ATP4a protein and its role in ciliogenesis. In our study we have observed enhanced expression of ATP4a in lung exposed to chlorpyriphos, cypermethrin and their cocktail at same concentration administered through oral route. As mentioned earlier, usually, respiratory airways are covered by beating cilia (mucocilary epithelium) which protects the passage from pathogenic invasions. In the present study, one of the first attempt has been made to report the pulmonary expression of this ATP4a during pesticide induced lung damage. We can assume a correlation between the pesticide exposure and lung damage. In addition to this, it has also been seen that the presumption that cocktail will have higher ill effect compared to the single exposures was also not seen in this study, rather, the cocktail exposure had increased the mRNA expression of ATP4a more than cypermethrin but less than chlorpyriphos. The positive scoring of when done for the epithelial cells, found that cocktail of pesticides enhanced the mRNA expression of ATP4a in the airway epithelia of mice lungs equal to cypermethrin but more than chlorpyriphos. The reason behind this result could be techniques only, where immune-positive scoring cannot be that efficient as the RT qPCR. However, the pattern of the immuno-positive are in favor of the microarray and RT qPCR in which compared to control the exposed groups have shown up reregulation of ATP4a gene in lungs. In addition to this, a few of the genes responsible for the mucus production has also been found to be upregulated (Muc4: 3.08, 3.38 and 3.16; Muc19: 7.02, 5.89, 5.63; Muc3: 6.21, 6.44, 6.24; Muc15: 13.39, 13.83, 13.79; Muc19: 7.02, 5.89, 5.63). Several previous studies have also enlightened the role of this ATP4a gene in cilia-genesis.
Similarly, it has been reported that ATP4a malfunctioning may alter motile cilia function which may further result in elevated airway infections leading to a diversity of human diseases such as Primary Cilia Dyskinesia (3PCD), Chronic Obstructive Pulmonary Disease (COPD) and Meckel-Gruber syndrome. On the other hand, it has been presented that any disruption in this airway epithelial layer enhances the chance of pulmonary infection, when we connected the links of ATP4a and mucous secreting cells with respect to cilio-genesis as per the previous molecular studies, found that ATP4a activates foxj1, a master transcription factor, for motile cilia-genesis and if halted the expression of ATP4a resulted in defected motile cilia formation. Similarly, non-canonical Wnt/Planar Cell Polarity (PCP) requires ATP4a function for the polarization of cilia at the gastrocoel roof plate during gastrulation. Thus, it can be assumed that the increased mRNA expression of ATP4a will activate the foxj1 transcription factor will regulate the cilia growth which is otherwise found normal in case of control group.
The extract of microarray data on mucus producing gene such as Muc4, Muc19, Muc3, Muc15, Muc19 which were found to be upregulated, according to earlier studies suggests that mucin production is essential for the clearance of respiratory tract and for normal functioning of the lungs. From the above discussion, it becomes evident that mucin and cilia work together and the shows elevated functionality upon any external pathogen entry into the lung as a defense mechanism against pathogen or to remove the deposited bronchiolar fluid interrupting in lungs functionality following to some other external pollutant that may contain any harmful chemical such as pesticides.
Earlier we have reported that mice exposed to chlorpyriphos, cypermethrin and their mixture in same concentration found to showed lung inflammation characterized by the increase in the TNF-alpha overexpression. Similarly, the APAF1, a regulator in apoptosis was also found to be downregulated following the same pesticide exposure. In the present attempt we have found that mice exposed to chlorpyriphos, cypermethrin and their mixture in same concentration increases the ATP4a expression which activates foxj1 gene which promotes the cilia formation around the airways to remove the alveolar congestion produced in lung due to long term dietary exposure to chlorpyriphos and/or cypermethrin. The enhanced mucin production facilitates the easy clearance of lung fluid which is otherwise also required for the normal functioning of the lung.
Conclusion
Overall, it can be assumed that against pesticide induced inflammation, congestion or pulmonary edema lung enhances the ATP4a expression which in turn activates the lung’s natural phenomenon of fluid clearance in which ATP4a further activates the foxj1, the major cilia-related transcription factor. Together, the data suggest that components of chlorpyriphos, cypermethrin and their mixture are responsible for lung inflammation, congestion and edema, but by stimulating cilio-genesis with the transcription factor foxj1, activated via ATP4a overexpression, it may be possible to maintain lung health despite the stress of pesticides. However, more mechanistic approach such as gene knockout mice and siRNA in vitro methods will further help us elucidating the whole phenomenon.
Conflict of Interest
The authors declare no conflict of interest.
References
- AlavanjaMC, Dosemeci M, Samanic C, Lubin J, Lynch CF, et al. (2004) Pesticides and lung cancer risk in the agricultural healthstudy cohort. Amer J Epidemiol 160: 876-885.
[Crossref][Google Scholar] [PubMed]
- AltmanKW, Waltonen JD, Tarjan G, Radosevich JA, Haines III GK (2007) Human lung mucous glands manifest evidence of the H+/K+-ATPase proton pump. AnnOtol Rhinol Laryngol 116: 229-234.
[Crossref] [Google Scholar] [PubMed]
- ArdhanariA, Srivastava U, Kumar A, Saxena S (2011) Management of a case of prallethrinpoisoning-an unusual agent for suicidal ingestion. Sri Lankan J Anaesthesiol 19: 51-52.
- BhaskarEM, Moorthy S, Ganeshwala G, Abraham G (2010) Cardiac conduction disturbance due to prallethrin(pyrethroid) poisoning. J Med Toxicol 6: 27-30.
[Crossref][Google Scholar] [PubMed]
- BretveldRW, Hooiveld M, Zielhuis GA, Pellegrino A, van Rooij IA, et al. (2008) Reproductive disorders among male and female greenhouseworkers. Reprod Toxicol 25: 107-114.
[Crossref] [Google Scholar] [PubMed]
- BuralliRJ, Dultra AF, Ribeiro H (2020) Respiratory and allergic effects in children exposed to pesticides-Asystematic review. Intern J Env Res Publ Health 17: 2740.
[Crossref] [Google Scholar] [PubMed]
- Caron A,Xu X, Lin X (2012) Wnt/β-catenin signaling directly regulates Foxj1expression and ciliogenesis in zebrafish Kupffer’s vesicle. Development139: 514-524.
[Crossref] [Google Scholar] [PubMed]
- CloonanSM, Choi AM (2016) Mitochondria in lung disease. J Clin Inves 126: 809-820.
[Crossref] [Google Scholar] [PubMed]
- CrystalRG, Randell SH, Engelhardt JF, Voynow J, Sunday ME (2008) Airway epithelial cells: Current concepts and challenges. Proc Amer Thor Soc 5: 772-777.
[Crossref] [Google Scholar] [PubMed]
- EatonDL, Daroff RB, Autrup H, Bridges J, Buffler P, et al. (2008) Review of the toxicology of chlorpyrifos with an emphasison human exposure and neurodevelopment. Crit Rev Toxicol 38: 1-125.
[Crossref] [Google Scholar] [PubMed]
- FischerH, Widdicombe JH (2006) Mechanisms of acid and base secretion by the airwayepithelium. J MembBiol 211: 139-150.
[Crossref][Google Scholar] [PubMed]
- FliegaufM, Benzing T, Omran H (2007) When cilia go bad: Cilia defects and ciliopathies. Nat Rev Molec Cell Biol 8: 880-893.
[Crossref] [Google Scholar] [PubMed]
- Gupta B,Kerai S, Khan I (2018) Organophosphate-pyrethroid combined poisoning may beassociated with prolonged cholinergic symptoms compared to either poison alone. Ind J Anaes 62: 903.
[Crossref] [Google Scholar] [PubMed]
- IyyaduraiR, Peter JV, Immanuel S, Begum A, Zachariah A, et al. (2014) Organophosphate-pyrethroid combination pesticides may beassociated with increased toxicity in human poisoning compared to eitherpesticide alone. ClinToxicol 52: 538-541.
[Crossref] [Google Scholar] [PubMed]
- KumarSingh A, Nath Tiwari M, Prakash O, Pratap Singh M (2012) A current review of cypermethrin-induced neurotoxicity andnigrostriatal dopaminergic neurodegeneration. Curr Neuropharmacol 10: 64-71.
[Crossref] [Google Scholar] [PubMed]
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1522
- [From(publication date): 0-2023 - Dec 19, 2024]
- Breakdown by view type
- HTML page views: 1422
- PDF downloads: 100