Research Article Open Access
Association of Inflammation and Possible Mild Cognitive Decline Measured by the Stroop Cognitive Function Test
Fabrègue F1,2, Butkowski E2, Voigt A2,3, Mouquot G1,2, de Jong B2, Stachowiak FJ2,4 and Jelinek HF2,5*
1Faculty of Sciences, University of Poitiers, Poitiers, France
2School of Community Health, Charles Sturt University, Albury, Australia
3Institute of Chemistry and Biochemistry, Free University of Berlin, Germany
4SRH University of Applied Health Sciences, Gera, Germany
5Australian School of Advanced Medicine, Macquarie University, Sydney, Australia
- *Corresponding Author:
- Jelinek HF
School of Community Health, Charles Sturt University, Albury, Australia
Tel: 61427681754
E-mail: hjelinek@csu.edu.au
Received date: May 11, 2016; Accepted date: May 20, 2016; Published date: May 27, 2016
Citation: Fabrègue F, Butkowski E, Voigt A, Mouquot G, de Jong B, et al. (2016) Association of Inflammation and Possible Mild Cognitive Decline Measured by the Stroop Cognitive Function Test. J Alzheimers Dis Parkinsonism 6:237. doi:10.4172/2161-0460.1000237
Copyright: © 2016 Fabrègue F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease and dementia have been shown to be associated with various inflammatory markers. However the association between mild cognitive decline (MCI) and inflammation is not conclusive. Determining MCI requires a battery of tests of which the Stroop battery is one that is often used to assess cognitive function. The current study investigated the level of inflammation and the correlation to the RIT and NIT assessment of MCI in a rural cohort attending a health screening clinic.
Ninety-six participants free of diagnosed MCI undertook the Stroop testing battery and were divided into a short reaction time (SRT) and a long reaction time (loRT) group based on the RIT and NIT results. Serum Interleukin (IL-10, IL-6, IL1-β), Insulin-like Growth Factor-1 (IGF-1), C-Reactive Protein (CRP) and the Monocyte Chemotactic Protein-1 (MCP-1) levels were measured using commercial ELISA kits.
CRP was significantly higher in the hiRT group based on RIT (p=0.022). IL-1 β was associated with NIT (p=0.039). MCP-1 was significant only for NIT (p<0.01). The IL-10 (p<0.01) was significantly lower (p=0.0096) and the IL-6/IL-10 ratio significantly higher in the hiRT group regardless whether RIT or NIT was used (p<0.03).
The study extends previous work indicating an association between cognitive function measured by the Stroop battery and inflammation. IL-10 and the IL-6/IL-10 ratio are the most appropriate markers to use to assess the level of inflammation associated with reaction time and hence cognitive function.
Keywords
Inflammation; Mild cognitive impairment; Stroop test; Reading interference test; Naming interference test
Abbreviations
IL: Interleukin; IGF-1: Insulin-like Growth Factor-1; CRP: C-Reactive Protein; MCP-1: Monocyte Chemotactic Protein-1; NIT: Naming Interference Test; RIT: Reading Interference Test
Introduction
Many factors such as cerebrovascular accidents, hypothyroidism, heart failure, vitamin deficiencies, hepatic impairment, depression, drug and alcohol abuse, toxins, infections, metabolic and structural causes influence cognitive function that can lead to mild cognitive impairment, dementia and Alzheimer’s disease. Furthermore MCI is also linked to obesity, vascular disease, dyslipidemia, inflammation and oxidative stress [1-4]. Mild cognitive impairment (MCI) is a common age related phenomenon, which may be a pre-clinical stage of dementia in general and Alzheimer´s Disease (AD) specifically. Episodic memory is most often affected by cognitive impairment, which can lead to AD dementia [5]. How MCI is identified and whether it is affected by dyslipidemia, inflammation and oxidative stress is important in clinical practice as it can determine treatment priorities.
Oxidative stress and inflammation associated with vascular disease have been proposed to be early markers of cognitive decline and one study suggested that the level of oxidative markers is directly related to the severity of cognitive impairment [6]. Previous research has also shown a possible link between oxidative stress and cytokine production [7]. Expression of inflammatory cytokines in neuronal cells suggests that inflammation and the production of ROS are closely related in the progression of cognitive decline [8]. Cholesterol has also been implicated with a causative role in MCI [9]. However controversy still exists whether inflammation and MCI are related [10].
Cognitive tests are commonly used to determine the presence of MCI. Scores on cognitive tests for individuals with MCI are typically 1 to 1.5 standard deviations below the mean for their age and education matched peers on culturally appropriate normative data [5]. Examples of such test batteries include the Differential Aptitude Test Battery, Cambridge Neuropsychological Test Automated Battery (CANTAB®) [11] and the Vienna Cognitive Test Battery [12].
The Vienna Cognitive Test Battery includes a number of tests for attention, memory and visiospatial skills of which the color-word interference tendency, (Stroop) test has been extensively investigated and often used as part of a test battery to diagnose MCI and vascular disease [13]. The principle of the Stroop test [14] is that it is harder for an individual, to name the color of the writing if the word is written in a different color than if naming the color of the word written in the same color. Test results are interpreted by the median reaction time (seconds) and/or the number of incorrect responses made in relation to congruent and incongruent words. The interference between congruent and incongruent words can then be a predictor of MCI as interference deficits may be present before cognitive decline symptoms reach clinical levels [15]. The results of the Stroop test can then be applied to determine whether certain inflammatory markers are raised or deficient in MCI.
Cytokines are inflammation biomarkers, which play a role in enhancing or preventing disease progression. An imbalance of pro and anti-inflammatory cytokines, which may be caused by dyslipidemia and leading to inflammatory cascades is related to changes in the levels of interleukin-6 (IL-6) and increases in other cytokines such as interleukin-1β (IL1-β) production [16]. The pro-inflammatory IL1-β is a crucial factor contributing to MCI and is a possible tool to predict neurodegenerative diseases [17].
Much of the current published research on MCI investigated only one specific biomarker and reported associations with either the Stroop RIT or NIT scores but did not apply both Stroop tests. C-reactive protein (CRP) [18], Interleukin-12 (IL-12) [19], Interleukin-1 (IL-1) [20], tumor necrosis factor-α (TNF-α) [21] and monocyte chemotactic protein-1 (MCP-1) [22] have been shown to significantly increase or decrease in MCI. The link between pro-inflammatory and antiinflammatory biomarkers and cognitive decline has only been shown in one study of patients with early or late onset Alzheimer’s disease (LOAD) [23], reporting a significant higher serum IL-6 level in the LOAD group and a significant correlation with high serum IL-10 levels. In addition results of previous research investigating the relationship between MCI and inflammation are not conclusive and may be nonspecific [10,24]. The role of anti-inflammatory biomarkers and their interaction with pro-inflammatory cytokines has not been investigated in the context of Stroop results [25,26]. The current study therefore aimed at investigating the association of pro- and anti-inflammatory biomarkers using both RIT and NIT to determine whether an association exists between various inflammatory markers and the Stroop RIT and NIT score.
Methods
The study was approved by the University Ethics in the Human Research Committee (Ethics approval number: 2006/042). Informed consent was obtained from each participant after providing a comprehensive outline of the project and methods followed by answering any questions participants may have had.
Study population
For the present study, 132 participants were recruited attending a rural health screening clinic for cognitive function testing using the Stroop test battery and analysis of blood biomarkers. We divided the results into a low reaction time (loRT) and high reaction time (hiRT) group based on the RIT or NIT results. Twenty-nine patients were excluded because blood samples were not available, while seven others did not complete the cognitive function tests. The final number of participants for the study was 96. The cut-off values for hiRT using RIT or NIT were determined from 25 patients attending the health screening clinic who reported no hypertension (HT), cardiovascular disease (CVD), diabetes, psychiatric disease or cognitive difficulty and presented the baseline values. The cut-off values for the reading interference test (RIT) and naming interference test (NIT) were then determined by setting the T-score value of the Stroop test results for RIT and NIT equal to 60, which represents one standard deviation from the normalized mean of the baseline group. This provided a cut-off for RIT of >240 milliseconds (ms), and for NIT of >680 ms. Scores below the cut-off were deemed as loRT and represent the 0-85th centile for the current cohort.
Comorbidities presenting in patients such as diabetes were defined as fasting plasma glucose levels (FPG) ≥ 126 mg/dL (7 mmol/l) [27]. Hypertension was defined as a systolic blood pressure (SBP ≥ 140 mmHg or self-reported hypertension with or without medication use. CVD was identified by 12-lead ECG, self-reported clinical history and use of medication.
Chemicals
The blood samples were analyzed using ELISA kits (Elisakit.com, Melbourne, Australia) (Interleukin-6 (human) Elisa kit Lot No. 120925, Interleukin-10 (human) Elisa kit No. 13426, Interleukin-1β (human) Elisa kit Lot No. 141208, Insulin-like growth factor-1 (IGF-1) (human) Elisa kit Lot No. 130807, and Monocyte chemotactic protein (MCP-1) Elisa kit Lot No. 150413.
Sample preparation
Fasting plasma glucose (FPG) levels were determined using the Accu-Chek® system (Roche Australia Pty Ltd). All centrifugation procedures for the blood preparation were performed with a Universal 32R (Hettich Zentrifugen, Germany). The photometric measurements to determine the levels of biomarkers in blood were carried out with a Thermo Scientific Multiskan FC (Fisher, China) [28]. Venous blood was collected into serum-separating-tubes (SST) and stored at -80ºC until analysis. All samples and standards were measured in duplicates. Blank and seven standards were used for each Elisa assay. The sample concentrations were calculated by four parameter logistic ELISA curve fitting software provided online by elisaanlaysis.com software (Thermo Scientific Multiskan FC).
Vienna cognitive test battery
All participants completed the Stroop test included with the Vienna Cognitive Test Battery. Two baseline conditions and two interference conditions were included in the Stroop test. First, a color-word is displayed in grey and the patients had to read and then choose the correct color field with a light-pen (for example, the color-word “blue” written in grey, the answer is the blue-color). In a second case, the patients have to choose the correct color of a color-line by saying the color of the banner aloud (if the banner is “red”, the answer is the red color). Third, for reading-color interference, the patients have to read the color word aloud and disregard the color the word is written in. (for example, the color-word “yellow” written in green, the patients have to read aloud yellow and choose the yellow color. The last test addresses naming-word interference. The patients have to name aloud the color, in which the word is written and choose this color (for example, the color-word “red” written in green, the patients have to say aloud “green” and choose the green-color. The Stroop test is based on how relevant and irrelevant information are processed by the brain in parallel. As the processing speed for reading a word is faster than naming a color and more strongly automatized, it interferes with the task of naming the color, when the color stimulus presented on the screen and the color in which the color word is written are different.
Statistical analysis
Data were collected using Microsoft Excel (Office 2007, Microsoft) and descriptive data is expressed as mean ± standard deviation (x ± SD). Statistical analysis was performed with SPSS (Version 22, IBM Co). To determine if there were significant differences in biomarker levels between the control and mild cognitive impairment, two samples Wilcoxon Rank Sum Test was used. A p-value of p ≤ 0.05 was considered as significant.
Results
Anthropometric data for the 96 adults who completed the cognitive function tests and for whom blood samples were available is shown in Table 1. All participants were older than 40 years. Sixty-two per cent of the study participants in the low reaction time Stroop group were women versus 35.7% in the high reaction time Stroop group. The mean age of the loRT Stroop group was 67 years and not significantly different to the hiRT Stroop group. Only CVD and HDL-Cholesterol were significantly different between the loRT and the hiRT group (p<0.05).
| Low Stroop | High Stroop | |
|---|---|---|
| Size (n) | 82 | 14 |
| Gender (women) % | 62.2 | 35.7 |
| Age (years) | 66.98 ± 9.5 | 69.71 ± 10.7 |
| Glucose (mmol/L) | 6.86 ± 2.8 | 6.81 ± 1.6 |
| BMI (kg/m2) | 28.55 ± 5.8 | 30.57 ±5.3 |
| Waist (cm) | 97.25 ± 14.8 | 102.89 ± 9.8 |
| CVD (%) | 22.78 | 53.85* |
| HT (%) | 54.32 | 78.57 |
| Type 2 Diabetes (%) | 50.65 | 50 |
| Tchol (mmol/L) | 5 ± 1.1 | 4.49 ± 1.3 |
| LDL-Chol (mmol/L) | 2.85 ± 1.1 | 2.54 ± 1.2 |
| HDL-Chol (mmol/L) | 1.55 ± 0.5 | 1.26 ± 0.3* |
| HbA1c (mmol/L) | 6.17 ± 0.9 | 6.17 ± 0.7 |
| Tchol/HDL ratio | 3.48 ± 1.3 | 3.71 ± 1.2 |
| TG (mmol/L) | 1.25 ± 1.5 | 1.51 ± 0.7 |
| SBP (mmHg) | 131.29 ± 17.9 | 130.86 ± 13.4 |
| DBP (mmHg) | 76.01 ± 7.6 | 76.14 ± 8.1 |
Abbreviations: HT: Hypertension; CVD: Cardiovascular Disease. TChol: Total Cholesterol; LDL-Chol: Low-Density Lipoprotein Cholesterol; HDL-Chol: High- Density Lipoprotein Cholesterol; HbA1c: Glycated Haemoglobin; TG: Triglycerides; SBP: Systolic Blood Pressure; DBP: Diastolic blood pressure *: p<0.05
Table 1: The demographics of high Stroop patients and low Stroop patients.
For the RIT our cut-off was 240 ms and the cut-off for NIT was 668 ms. Reaction times were determined based on the RIT and NIT Stroop results and presented separately.
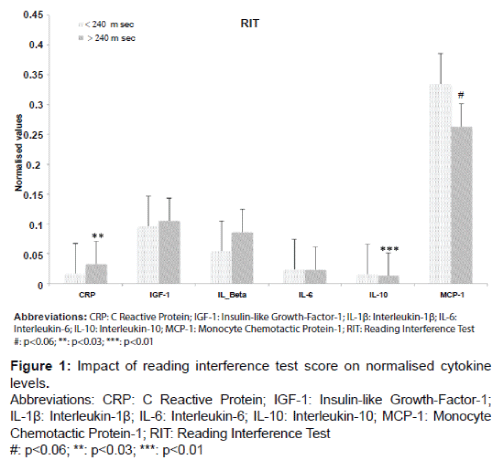
Mean cytokines levels were determined with respect to the RIT and NIT test results. For RIT, serum CRP levels were significantly higher in the hiRT group compared with the loRT group (increase of 50%, p=0.022) (Figure 1) and IL-10 was significantly lower with a decrease of 16.7% (p=0.0096).
Values shown in the bar plots are the normalized means ± SD of all inflammatory biomarkers. The white bar plots represents the participants, who have a RIT value below the cut-off (<240 ms). The grey bar plots represents the participants, who have a RIT value above the cut-off (>240 ms).
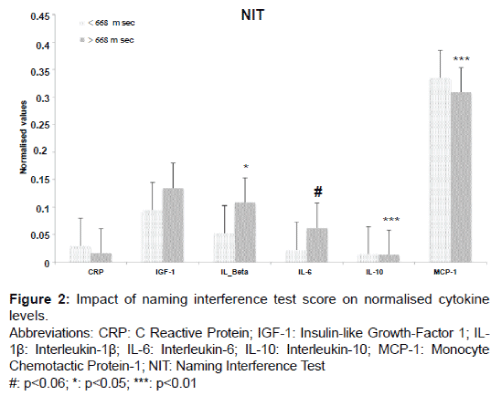
MCP-1 was significantly lower in the hiRT Stroop group when the NIT classification was applied, (7.8%, p=0.003). The hiRT group also demonstrated a significant increase in IL-1β levels of 49% (p=0.039) but IL-1β levels were not significantly increased on the basis of RIT (increase of 36% compare Figures 1 and 2).
Only IL-10 levels decreased significantly regardless of the Stroop test (RIT: p=0.0096 and NIT: p=0.003).
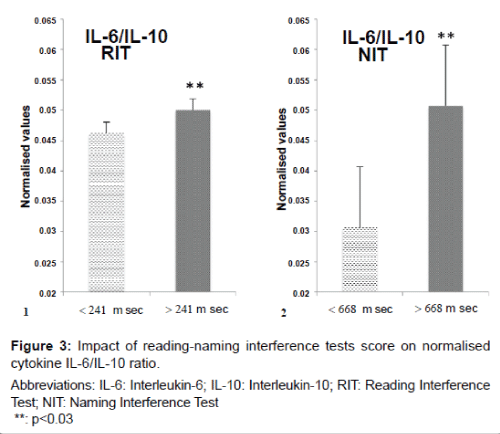
The interactions between pro- and anti-inflammatory markers are an important component of disease progression. To obtain an understanding of this balance we used the ratio between IL-6, a proinflammatory cytokine and IL-10, an anti-inflammatory cytokine. Figure 3 indicates an increase in the IL-6/IL-10 ratio when applying RIT of 8% (p=0.016) and an increase of 40% for NIT (p=0.019).
Values shown in bar plots are the normalised means ± SD of the ratio IL-6/IL-10.
Discussion
Cognitive decline due to vascular impairment without dementia as a disease entity has gained increased recognition in clinical practice. MCI can be diagnosed by a battery of tests including the Stroop test. Our results indicate that whether a difference in inflammation markers is observed between the low reaction time compared to the high reaction time group based on the Stroop test is observed depends on whether the RIT or NIT is used.
An elevated Stroop reaction time score and therefore a possible risk of developing MCI were linked with a higher mean CRP level and a lower IL-10 level when the RIT score was used. Higher reaction time scores with the NIT test were significantly associated with lower MCP-1, and IL-10 but higher IL-1β levels. Previous studies demonstrated higher level of IL-1β in serum samples of MCI patients [17], and AD patients [29,30], with the AD group having a mean age similar to our study. Similarly, Koziorowski et al. [31] detected higher IL-1β levels, but no statistically significant change was demonstrated in Parkinson’s disease patients. We found a significant increase in IL-1β only in the hiRT group based on the NIT results. The difference in whether IL-1β was significantly increased in the hiRT group depended on which of the two Stroop tests was used (RIT versus NIT) for defining the high reaction time group. This highlights the need to be aware that cognitive function test results may reflect different neurological functions as suggested by the current results following the reading and naming interference tests.
Mean levels of the anti-inflammatory marker MCP-1 were significantly lower for the NIT. Our results in this case are in disagreement with some previous studies that have shown MCP-1 to be increased in MCI patients [22]. However Galimberti et al. [22] demonstrated that MCP-1 elevation is a very early event in AD pathogenesis and decreases with AD progression. The findings of Galimberti et al. [22] suggest that NIT may be a sensitive test for early AD. However the current study did not include MCI nor AD patients but investigated the association between inflammation and low or high reaction times determined with the Stroop battery. Therefore the significantly lower MCP-1 level observed in the high reaction time group suggests a possibly different pathophysiology compared to that of developing AD.
We also studied the association of the anti-inflammatory IL- 10 and reaction times on the Stroop test. IL-10 acts to diminish pro-inflammatory cytokines [32]. What the role of IL-10 is in MCI pathology is not clear. Previous results have shown an increase and a decrease in IL-10 in association with Parkinson’s disease and multiple sclerosis that may have an MCI component [33]. Experiments with lipopolysaccharide (LPS) activated macrophages increased the level of IL-10 in AD patients [34]. In our study, participants with known Parkinson’s disease and multiple sclerosis were excluded and thus the significantly lower IL-10 levels may be due to IL-10 acting as an antiinflammatory cytokine in the hiRT group and is decreased due IL-10 binding to certain transcription factors that decrease inflammatory activity or the increased reaction time observed in this group may be linked to IL-10 hypo-responsiveness.
IGF-1 was shown to be increased in association with the NIT in a cohort of 61 MCI patients, suggesting that IGF-1 is part of a complex response pattern [35]. We found no significant increase in IGF-1 in the hiRT group but according to the literature, IGF-1 can also be a protecting factor against MCI suggesting that in our study IGF-1 may be on the rise or already decreased due to its protective function in the high reaction time group [36]. A future study investigating progression of MCI may be able to clarify whether IGF-1 increases as MCI progresses.
IL-6 was not significantly increased in our hiRT group regardless of whether MCI was defined on the basis of a high RIT or NIT score. However IL-6 was close to being significantly increased when hiRT was determined by NIT (p=0.052), supporting an increased IL-6 level reported previously in MCI patients [23,37,38]. Further, Dursun et al. [23] was the first to show a significant relationship between IL-6 and IL-10 where high IL-6 levels led to an increased IL-10 level in an Alzheimer’s disease study. Our study consolidates this finding and shows that a similar pathophysiological relationship between IL-6 and IL-10 is found with hiRT. In our study the hiRT group had an elevated IL-6/IL-10 ratio, suggesting that anti and pro-inflammatory biomarkers interact as reaction times to the Stroop battery increase. CRP is one of the most common inflammatory cytokines studied in pathological processes. CRP was increased in MCI [39], AD, and T2DM [40], but there are two studies with no correlation between CRP levels and cognitive decline [41,42]. We found a significant increase in CRP when reaction time was increased based on the RIT score.
According to the literature, NIT and RIT could be used together and can act in parallel to determine cognitive responses [43]. However the majority of studies found in the literature used only NIT for determining cognitive function or correlating with inflammation processes. We are the first study to report on the use the RIT and NIT to define reaction time to a cognitive function test battery and compare the test results with multiple inflammatory biomarker levels. We found that there were slight differences between the RIT and NIT tests with respect to inflammation levels in the hiRT group compared to the loRT group. Only IL-10 and the ratio IL-6/IL-10 were significantly different between the hiRT and loRT group independent of whether reaction time was determined by the RIT or NIT. These current results are more reliable as the use of two simultaneous tests can improve the specificity and sensitivity if we consider that a patient is classified as positive when both tests are positive and negative otherwise. Reliance on accurate results that reflect the clinical profile of the patient is necessary to provide timely and effective treatment.
Conclusion
Our study demonstrated that reaction time by either the NIT or RIT Stroop test results led to some discrepancies in terms of measured inflammatory differences between the loRT and hiRT groups. Only IL- 10 and IL-6/IL-10 were consistent between the two Stroop tests. It is the first study to demonstrate a connection between basic inflammatory biomarkers and behavioral data. Early cognitive neuropsychological testing and intervention can benefit patients, but the type of Stroop test used to determine reaction times needs to be considered if neuroinflammatory processes are investigated.
Acknowledgement
The authors wish to acknowledge Roche Australia Ltd for providing blood glucose test strips and Glucose Reader. Simon McDonald from Spatial Data Analysis Network (SPAN) provided statistical support.
References
- Darby DG, Pietrzak RH, Fredrickson J, Woodward M, Moore L, et al. (2012) Intra-individual cognitive decline using a brief computerized cognitive screening test. Alzheimer's and Dementia 8: 95-104.
- Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, et al. (2015)Vascular disease and risk factors are associated with cognitive decline in the alzheimer disease spectrum. Alzheimer Dis AssocDisord29: 18-25.
- Marlatt MW, Lucassen PJ, Perry G, Smith MA, Zhu X (2008) Alzheimer’s disease: Cerebrovascular dysfunction, oxidative stress and advanced clinical therapies. J Alzheimers Dis 15: 199-210.
- Jason CD Nguyen, Simon Killcross, Trisha A Jenkins (2014) Obesity and cognitive decline: Role of inflammation and vascular changes. Front Neurosci 8: 375.
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, et al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 270-279.
- Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, et al. (2012) Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer's disease. Int J ImmunopatholPharmacol 25: 345-353.
- Elmarakby AA, Sullivan JC (2012) Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. CardiovascTher 30: 49-59.
- Madeo J, Elsayad C (2013) The Role of Oxidative Stress in Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 3:116.
- Michikawa M (2003) Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer's disease? J Neurosci Res 72: 141-146.
- Schram MT, Euser SM, de Craen AJ, Witteman JC, Frölich M, et al. (2007) Systemic markers of inflammation and cognitive decline in old age. J Am GeriatrSoc 55: 708-716.
- Egerházi A, Berecz R, Bartók E, Degrell I (2007) Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer's disease. ProgNeuropsychopharmacolBiol Psychiatry 31: 746 - 751.
- Pusswald G, Moser D, Gleiss A, Janzek-Hawlat S, Auff E, et al. (2013) Prevalence of mild cognitive impairment subtypes in patients attending a memory outpatient clinical comparison of two modes of mild cognitive impairment classification. Results of the Vienna Conversion to Dementia Study. Alzheimer's and Dementia 9: 366-376.
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, et al. (2005) Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 128: 2034-2041.
- Stroop JR (1935) Studies of interference in serial verbal reactions. J ExpPsychol 18: 643-662.
- Bélanger S, Belleville S, Gauthier S (2010) Inhibition impairments in Alzheimer's disease, mild cognitive impairment and healthy aging: Effect of congruency proportion in aStroop task. Neuropsychologia48: 581-590.
- Fearon WF, Fearon DT (2008) Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation 117: 2577-2579.
- Forlenza OV, Diniz BS, Talib LL, Mendonça VA, Ojopi EB, et al. (2009) Increased serum IL-1beta level in Alzheimer's disease and mild cognitive impairment. Dement GeriatrCognDisord 28: 507-512.
- Laurin D, David Curb J, Masaki KH, White LR, Launer LJ (2009) Midlife C-reactive protein and risk of cognitive decline: A 31-year follow-up. Neurobiol Aging 30: 1724-1727.
- Rentzos M, Paraskevas GP, Kapaki E, Nikolaou C, Zoga M, et al. (2006) Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer's disease and frontotemporal dementia. J NeurolSci 249: 110-114.
- Yucesoy B, Peila R, White LR, Wu KM, Johnson VJ, et al. (2006) Association of interleukin-1 gene polymorphisms with dementia in a community-based sample: The Honolulu-Asia Aging Study. NeurobiolAging 27: 211-217.
- Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonça VA, et al. (2010) Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer's disease. J Alzheimers Dis 22: 1305-1311.
- Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, et al. (2006) Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer's disease. Neurobiol Aging 27: 1763-1768.
- Dursun E, Gezen-Ak D, HanaÄÂ?ası H, Bilgiç B, Lohmann E, et al. (2015) The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer's disease, mild cognitive impairment or Parkinson's disease. J Neuroimmunol 283: 50-57.
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, et al. (2004) The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292: 2237-2242.
- Banchereau J, Pascual V, O'Garra A (2012) From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 13: 925-931.
- Wilson CJ, Finch CE, Cohen HJ (2002) Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am GeriatrSoc 50: 2041-2056.
- American Diabetes, Association (2012) Diagnosis and classification of diabetes mellitus. Diabetes Care35(Suppl 1): S64-S71.
- Maschirow L, Khalaf K, Al-Aubaidy HA, Jelinek HF (2015) Inflammation, coagulation, endothelial dysfunction and oxidative stress in pre-diabetes — Biomarkers as a possible tool for early disease detection for rural screening. ClinBioch 48: 581-585.
- Angelopoulos P, Agouridaki H, Vaiopoulos H, Siskou E, Doutsou K, et al. (2008) Cytokines in Alzheimer's disease and vascular dementia. Int J Neurosci 118: 1659-1672.
- Khemka VK, Ganguly A, Bagchi D, Ghosh A, Bir A, et al. (2014)Raised serum pro-inflammatory cytokines in Alzheimer's disease with depression. Aging Dis 5: 170-176.
- Koziorowski D, Tomasiuk R, Szlufik S, Friedman A (2012) Inflammatory cytokines and NT-proCNP in Parkinson's disease patients. Cytokine 60: 762-766.
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683-765.
- Patanella AK, Zinno M, Quaranta D, Nociti V, Frisullo G, et al. (2009) Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res 88: 1106-1112.
- Lombardi VR, García M, Rey L, Cacabelos R (1999) Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer's Disease (AD) individuals. J Neuroimmunol 97: 163-171.
- Baker LD, Barsness SM, Borson S, Merriam GR, Friedman SD, et al. (2012) Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: Results of a controlled trial. Arch Neurol 69: 1420-1429.
- Sonntag WE, Ramsey M, Carter CS (2005) Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev 4: 195-212.
- Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, Loba J (2015) Serum levels of inflammatory markers in depressed elderly patients with diabetes and mild cognitive impairment. PLoS One 10: e0120433.
- Kálmán J, Juhász A, Laird G, Dickens P, Járdánházy T, et al. (1997) Serum interleukin-6 levels correlate with the severity of dementia in Down syndrome and in Alzheimer's disease. ActaNeurolScand 96: 236-240.
- Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, et al. (2014) Trajectories of inflammatory markers and cognitive decline over 10 years. Neurobiol Aging 35: 2785-2790.
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327-334.
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, et al. (2005) Serum inflammatory proteins and cognitive decline in older persons. Neurology 64: 1371-1377.
- Weuve J, Ridker PM, Cook NR, Buring JE, Grodstein F (2006) High-sensitivity C-reactive protein and cognitive function in older women. Epidemiology 17: 183-189.
- Lindsay DS, Jacoby LL (1991) Stroop Process Dissociations: The relationship between facilitation and interference. J ExpPsychol 20: 219-234.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 12169
- [From(publication date):
June-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11261
- PDF downloads : 908