Association Between Neutrophil To-Lymphocyte Ratio (NLR) and Outcome of Septic Patients with Atrial Fibrillation (AF): A Retrospective Observational Study Based on Medical Information Mart for Intensive Care IV
Received: 01-Apr-2024 / Manuscript No. JIDT-24-131162 / Editor assigned: 04-Apr-2024 / PreQC No. JIDT-24-131162 (PQ) / Reviewed: 18-Apr-2024 / QC No. JIDT-24-131162 / Revised: 25-Apr-2024 / Manuscript No. JIDT-24-131162 (R) / Published Date: 02-May-2024 DOI: 10.4172/ 2332-0877.1000590
Abstract
Background: This study aimed to evaluate the association between the Neutrophil to-Lymphocyte Ratio (NLR) and outcome of septic patients with atrial fibrillation.
Methods: Patients with sepsis and AF from the Medical Information Mart For Intensive Care-IV (MIMIC-IV) database had their baseline data and in-hospital prognosis retrieved. Multivariable logistics regression analyses were applied to calculate adjusted Odds Ratios (OR) with 95% Confidence Intervals (CI). Survival curves were plotted, and subgroup analyses were stratified by relevant covariates. To address the linearity relationship, curve fitting were performed.
Results: Of the 7,241 patients, 5,864 patients with sepsis and AF were included. The overall in-hospital mortality rate was 21.1% (1,235/4,629). Adjusted for confounding factors in the multivariable logistics regression analysis models, when NLR was used as a categorical variable, patients in the highest NLR tertile had increased in-hospital mortality compared to patients in the lowest NLR tertile (HR=1.31, 95% CI: 1.09–1.58). A linear relationship between NLR and in-hospital mortality was found in patients with sepsis and AF. K-M curves showed the in-hospital mortality rate was highest in group 3 (NLR 8.4) than in the other two groups. Stratified analyses indicated that the correlation between the NLR and in-hospital mortality was stable.
Conclusion: There is a linear relationship between NLR and in-hospital mortality in intensive care of septic patients with atrial fibrillation. A higher NLR in ICU patients is associated with in-hospital mortality in the United States. However, further research is needed to confirm the findings.
Keywords: Intensive care unit; Infection; Platelet-lymphocyte ratio; Red cell distribution width; Massachusetts institute of technology
Introduction
Sepsis is a clinical syndrome characterized by an overactive immune response to infection, posing a grave threat to the lives and well-being of patients [1-3] . Around 20% to 30% of patients admitted to an Intensive Care Unit (ICU) are diagnosed with sepsis. Of these, approximately 25% to 40% do die before being discharged from the hospital despite the decline in hospital mortality rates associated with sepsis [4,5].
Atrial Fibrillation (AF) refers to the rapid and irregular beating of the heart’s atria. AF is the most common arrhythmia among patients admitted to the ICU, the incidence of new-onset AF ranges from 23% to 40% [6]. Due to the irregular heart rhythm, blood clots may form in the atria or atrial appendages, increasing the risk of bacterial or viral infection and exacerbating sepsis. On the other hand, patients with sepsis may experience changes in cardiac function and electrical activity due to systemic inflammatory responses, which in turn can lead to the occurrence of atrial fibrillation, and compared to patients without AF, sepsis patients with AF have worse clinical outcomes [7-9].
The nature of sepsis is a host-mediated systemic inflammatory response to infection [10,11]. The impairment and dysfunction of immune cells are believed to be the primary contributing factors to secondary infections and unfavorable outcomes in sepsis patients. Previous studies have indicated that some systemic inflammatory biomarkers were associated with sepsis and poor prognosis, including Platelet-Lymphocyte Ratio (PLR), Lymphocyte-Monocyte Ratio (LMR), Red Cell Distribution Width (RDW), and Neutrophil to Lymphocyte Ratio (NLR) [12-19]. However, few studies have explored the prognostic correlation between biomarkers in sepsis patients with concomitant atrial fibrillation and neutrophil and lymphocyte counts. The objective of this study was to investigate the association between the NLR and hospital outcomes in sepsis patients with AF, aiming to provide a new foundation and reference for the clinical management of sepsis.
Materials and Methods
Data source
We enrolled patients with sepsis and atrial fibrillation from the MIMIC-IV database of the Massachusetts Institute of Technology (MIT). More than 70,000 adult patients were admitted to the ICU of Beth Israel Deaconess Medical Center in Boston between 2008 and 2019. The requirement for informed consent or ethical approval was waived because the data were obtained from publicly available sources with de-identified information. The first author, Kui Tang, completed the training on “Human Subject Protection”and gained full access to the database (certification number 59259021). Raw data were extracted using Structured Query Language with PostgreSQL 13.0 and Navicat Premium 15.
Participants
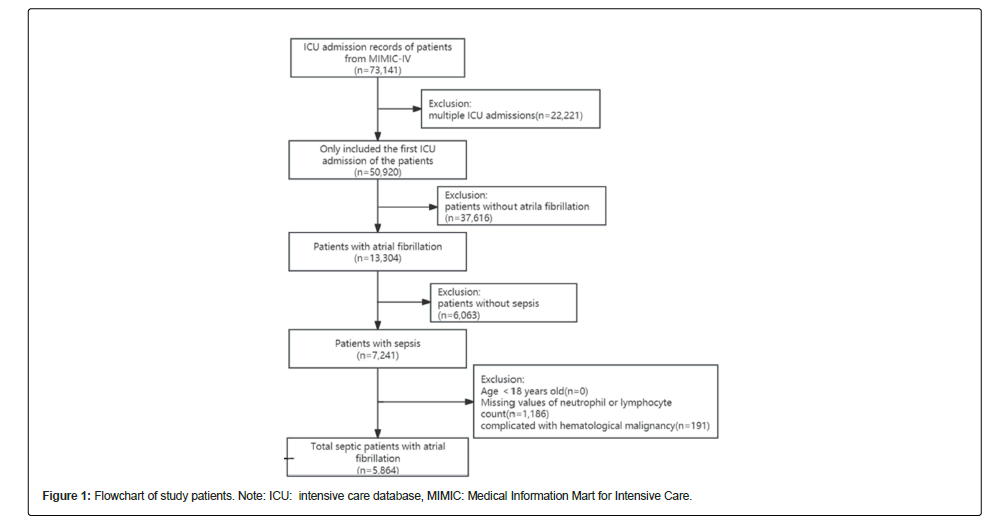
Patients aged >18 years who fulfilled the Sepsis-3 criteria were eligible for our study. Sepsis was defined as an increase of ≥ 2 points in the Sequential Organ Failure Assessment (SOFA) score, plus documented or suspected infection [20,21]. Septic was defined as (ICD) code 78552 (9th revision) and ICD code R6521 (10th revision). The diagnosis of atrial fibrillation was based on ICD-9. If patients were admitted to the ICU more than once, we only adopted the date of their first ICU admission [22].
Variates
Variables considered confounders of sepsis outcomes based on existing literature and clinical judgment were included [23,24]. Demographic and admission information: Age, sex, race, insurance, language, marital status, height, weight, BMI, Charlson and severity at admission, as measured by the Acute Physiology Score (APS) III score and Sequential Organ Failure Assessment (SOFA) score.
Vital signs: Heart rate, Mean Arterial Pressure (MAP), and SPO2 at ICU admission.
Interventions: Invasive ventilation.
Laboratory results: Glucose, neutrophil count, lymphocyte count, hemoglobin, White Blood Cell (WBC) count, Red Blood Cell (RBC), Red Cell Distribution Width (RDW), hematocrit, albumin, chlorid, aniongap, PCO2, PO2, total CO2, free calcium, Partial Thromboplastin Time (PTT), plasma Prothrombin Time (PT), creatinine, urea nitrogen, platelet count, lactate, and pH. NLR was calculated using the neutrophil count (K/uL)/lymphocyte count (K/uL). If the above data were tested multiple times within 24 h, we chose the first set of parameters.
Outcome
The outcome was in-hospital mortality, which is defined as survival status at hospital discharge. Patients without any outcome information were excluded from the final cohort.
Statistical analysis
Descriptive analysis was performed for categorical variables to assess the significance of differences between groups stratified by NLR tertile (<4.0; 4.0–8.4; ≥ 8.4) using the Kruskal– Wallis test or one-way analysis of variance. Baseline characteristic data are presented as proportions (%) and were compared using chi-square tests for categorical variables. Normally distributed continuous data are presented as mean ± Standard Deviation (SD) and compared using Student’s t-test between groups, while skewed distribution data are presented as the median and Inter Quartile Range (IQR) and compared using the Wilcoxon rank-sum test.
A multivariate logistics proportional hazard model was used to assess the independent association between the NLR and in-hospital mortality. We constructed three models: Model 1, no covariance were adjusted. Model 2 adjusted only for age and sex. Model 3 was additionally adjusted for weight, height, BMI, HR, SBP, DBP, WBC, neutrophil count, RDW, Albumin, Glucose, Aniongap, PH, PO2, lactate, free calcium, PTT, invasive ventilation, MI, hypertriglyceridemia, and AKI. In all models, linear trends were tested using NLR tertile as categorical variables by assigning the median values of the tertile to the variable.
A logistics proportional hazards regression model was used to assess the linear relationship between NLR and the outcome of sepsis and AF. Based on the curve fitting (restricted cubic spline), we conducted a two- piecewise linear regression model to identify threshold effects.
A sensitivity analysis was performed to ensure the robustness of the data analysis. NLR was transformed into a categorical variable and a p-value for the trend was calculated. The purpose of this test was to validate the results of treating the NLR as a continuous variable and to determine the possibility of linearity.
Hospital survival was assessed using Kaplan-Meier survival curves according to NLR tertile and evaluated using the logrank test. Stratified and interaction analyses were applied based on sex (male or female), age (<65 or ≥ 65 years), BMI (<25,25-30,>=30),MI (yes or no), CKD (yes or no),and hypertension (yes or no). Subgroup analyses were adjusted for age, sex, weight, height, BMI, HR, SBP, DBP, WBC, neutrophilcount, RDW, Albumin, Glucose, Aniongap, PH, PO2, lactate, freecalcium, PTT, invasive ventilation, MI, hypertriglyceridemia, and AKI.
The percentages of covariates with missing data were less than 25% for all analyses. The missing values of the covariates were imputed via multiple imputations. We created and analyzed three datasets together. Data analyses were performed using packages R 4.1.2 (The R Foundation)1 software and Free Statistics software versions 1.5. P-values < 0.05 were considered significant.
Results
Baseline characteristics of the study population
According to the screening criteria, 5,864 patients were included whose demographic characteristics were shown in Table 1. As previously mentioned, participants were divided into three groups based on NLR<4.0 (n=1,244); 4.0 ≤ NLR<8.4 (n=1,537); and NLR ≥ 8.4 (n=3,083). The average age of the patients was 76.2 ± 11.4 years old and approximately 57.8% of them were men. There were no significant differences among the different groups concerning SBP, BMI, charlson score, SPO2, PCO2, temperature, RDW, PTT, hypertension, DM, MI, CKD and COPD (all p>0.05). Patients with a higher NLR tended to be male, have a heavier weight, higher SOFA score, glucose and aniongap, more WBC count, with invasive ventilation, heart failure, MI, CKD, but with lower PO2, neutrophil count and platelet count.
| V+C3:J47ariables | Total (n=5,864) | <4.0 (n=1,244) | 4.0-8.4 (n=1,537) | ≧ 8.4 (n=3,083) | p | statistic |
|---|---|---|---|---|---|---|
| NLR | 8.9 (4.5, 18.5) | 2.4 (1.2, 3.3) | 5.9 (5.0, 7.1) | 17.7 (11.7, 31.0) | <0.001 | 4849.415 |
| Age, years | 76.2 ± 11.4 | 76.5 ± 11.5 | 75.8 ± 11.4 | 76.3 ± 11.4 | 0.279 | 1.277 |
| Gender (%) | _ | _ | _ | _ | 0.691 | 0.739 |
| Male | 3389 (57.8) | 712 (57.2) | 879 (57.2) | 1798 (58.3) | _ | _ |
| Female | 2475 (42.2) | 532 (42.8) | 658 (42.8) | 1285 (41.7) | _ | _ |
| Weight (Kg) | 82.5 ± 23.7 | 80.8 ± 22.1 | 82.6 ± 23.0 | 83.2 ± 24.7 | 0.01 | 4.56 |
| Height (cm) | 168.9 ± 10.9 | 168.4 ± 10.8 | 168.5 ± 10.7 | 169.3 ± 11.0 | 0.013 | 4.371 |
| BMI | 28.8 ± 7.4 | 28.4 ± 6.8 | 29.0 ± 7.3 | 28.9 ± 7.7 | 0.06 | 2.815 |
| HR (bpm) | 86.9 ± 16.0 | 85.5 ± 16.6 | 85.9 ± 15.4 | 87.9 ± 16.1 | <0.001 | 13.563 |
| SBP (mmHg) | 114.9 ± 14.9 | 118.9 ± 16.7 | 115.2 ± 13.6 | 113.1 ± 14.5 | < 0.001 | 67.664 |
| DBP (mmHg) | 56.6 ± 9.7 | 57.8 ± 10.5 | 56.8 ± 9.5 | 56.0 ± 9.4 | <0.001 | 14.919 |
| Temperature (°C) | 36.7 ± 2.2 | 36.7 ± 1.7 | 36.7 ± 2.0 | 36.8 ± 2.5 | 0.281 | 1.27 |
| SOFA | 5.0 (3.0, 8.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 8.0) | 6.0 (4.0, 9.0) | <0.001 | 39.682 |
| Charlson | 6.4 ± 2.6 | 6.5 ± 2.7 | 6.4 ± 2.5 | 6.4 ± 2.6 | 0.389 | 0.945 |
| WBC (K/uL) | 13.6 ± 9.7 | 11.1 ± 13.3 | 12.5 ± 5.8 | 15.1 ± 9.3 | <0.001 | 89.956 |
| RBC (K/uL) | 3.5 ± 0.6 | 3.3 ± 0.6 | 3.4 ± 0.6 | 3.5 ± 0.7 | < 0.001 | 29.809 |
| Hemoglobin (g/dL) | 10.4 ± 1.9 | 10.2 ± 1.9 | 10.3 ± 1.7 | 10.5 ± 1.9 | <0.001 | 12.995 |
| RDW,n (%) | 15.5 ± 2.3 | 15.6 ± 2.4 | 15.4 ± 2.3 | 15.6 ± 2.3 | 0.069 | 2.68 |
| Hematocrit,n (%) | 31.7 ± 5.5 | 31.2 ± 5.7 | 31.5 ± 5.2 | 31.9 ± 5.6 | <0.001 | 9.7 |
| Albumin (g/dL) | 3.1 ± 0.6 | 3.2 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.6 | <0.001 | 26.451 |
| Glucose (mq/dL) | 142.1 ± 53.2 | 135.8 ± 47.6 | 137.6 ± 47.5 | 146.8 ± 57.4 | < 0.001 | 26.355 |
| Aniongap (mEq/L) | 14.9 ± 4.0 | 14.3 ± 3.7 | 14.5 ± 3.8 | 15.3 ± 4.2 | <0.001 | 35.846 |
| PH | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | <0.001 | 28.253 |
| PCO2 (mmHg) | 41.7 ± 9.6 | 41.9 ± 11.1 | 41.8 ± 9.3 | 41.6 ± 9.1 | 0.531 | 0.633 |
| PO2 (mmHg) | 137.7 ± 79.6 | 152.2 ± 87.5 | 146.4 ± 82.2 | 127.5 ± 73.2 | <0.001 | 56.423 |
| Free calcium (mmol/L) | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.001 | 6.523 |
| PTT (sec) | 40.4 ± 19.2 | 39.7 ± 18.7 | 40.7 ± 19.4 | 40.6 ± 19.2 | 0.292 | 1.231 |
| Neutrophil count (K/uL) | 9.7 (6.4, 14.2) | 4.8 (2.3, 7.2) | 8.8 (6.5, 11.8) | 12.6 (9.2, 17.6) | <0.001 | 2013.531 |
| Lymphocytes (K/uL) | 1.1 (0.6, 1.7) | 2.1 (1.4, 2.9) | 1.5 (1.1, 2.0) | 0.7 (0.4, 1.0) | <0.001 | 2530.875 |
| Platelet count (K/uL) | 175.3 (128.0, 240.0) | 148.8 (112.0, 201.8) | 171.0 (126.0, 232.0) | 190.4 (140.5, 258.5) | <0.001 | 230.822 |
| Lactate (mmol/L) | 1.9 (1.4, 2.6) | 1.7 (1.3, 2.4) | 1.8 (1.4, 2.5) | 1.9 (1.4, 2.7) | <0.001 | 43.467 |
| Hypertension | _ | _ | _ | _ | 0.619 | 0.959 |
| No | 3389 (57.8) | 724 (58.2) | 872 (56.7) | 1793 (58.2) | _ | _ |
| Yes | 2475 (42.2) | 520 (41.8) | 665 (43.3) | 1290 (41.8) | _ | _ |
| DM | _ | _ | _ | _ | 0.86 | 0.301 |
| No | 3974 (67.8) | 846 (68) | 1033 (67.2) | 2095 (68) | _ | _ |
| Yes | 1890 (32.2) | 398 (32) | 504 (32.8) | 988 (32) | _ | _ |
| Heart failure | _ | _ | _ | _ | 0.037 | 6.578 |
| No | 3047 (52.0) | 686 (55.1) | 792 (51.5) | 1569 (50.9) | _ | _ |
| Yes | 2817 (48.0) | 558 (44.9) | 745 (48.5) | 1514 (49.1) | _ | _ |
| Myocardial infarction | _ | _ | _ | _ | 0.681 | 0.767 |
| No | 5311 (90.6) | 1133 (91.1) | 1395 (90.8) | 2783 (90.3) | _ | _ |
| Yes | 553 ( 9.4) | 111 (8.9) | 142 (9.2) | 300 (9.7) | _ | _ |
| CKD | _ | _ | _ | _ | 0.694 | 0.731 |
| No | 4253 (72.5) | 914 (73.5) | 1113 (72.4) | 2226 (72.2) | _ | _ |
| Yes | 1611 (27.5) | 330 (26.5) | 424 (27.6) | 857 (27.8) | _ | _ |
| COPD | _ | _ | _ | _ | 0.554 | 1.18 |
| No | 5262 (89.7) | 1123 (90.3) | 1385 (90.1) | 2754 (89.3) | _ | _ |
| Yes | 602 (10.3) | 121 (9.7) | 152 (9.9) | 329 (10.7) | _ | _ |
| AKI | _ | _ | _ | _ | 0.005 | 10.688 |
| No | 1005 (17.1) | 250 (20.1) | 263 (17.1) | 492 (16) | _ | _ |
| Yes | 4859 (82.9) | 994 (79.9) | 1274 (82.9) | 2591 (84) | _ | _ |
| Invasive ventilation, n (%) | _ | _ | _ | _ | <0.001 | 25.34 |
| No | 4436 (75.6) | 997 (80.1) | 1183 (77) | 2256 (73.2) | _ | _ |
| Yes | 1428 (24.4) | 247 (19.9) | 354 (23) | 827 (26.8) | _ | _ |
| In hospital mortality | _ | _ | _ | _ | <0.001 | 46.355 |
| No | 4629 (78.9) | 1036 (83.3) | 1265 (82.3) | 2328 (75.5) | _ | _ |
| Yes | 1235 (21.1) | 208 (16.7) | 272 (17.7) | 755 (24.5) | _ | _ |
Table 1: Patient characteristics according to tertiles of neutrophil to lymphocyte ratio.
Association between NLR and in hospital mortality
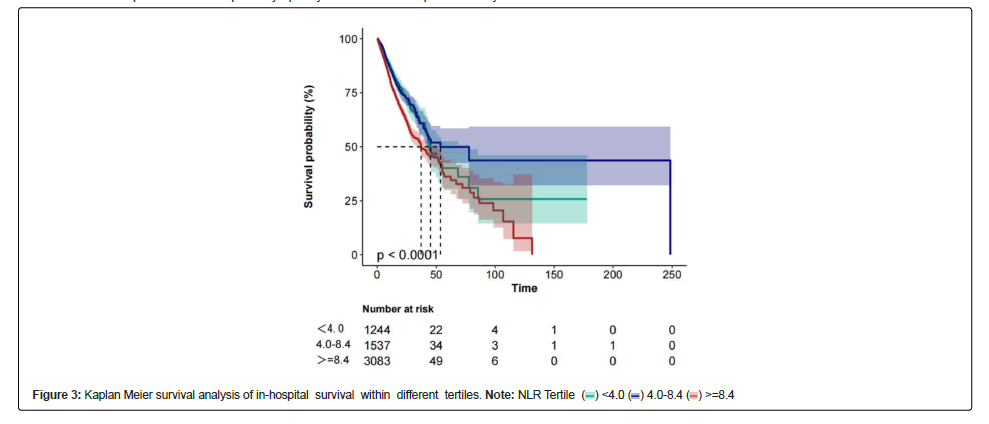
A univariable logistics regression analysis was used to select the variables of prognostic value for in-hospital mortality Figure 1. Age, gender, weight, height, BMI, SOFA, charlson, SBP, WBC, neutrophil count, RDW, glucose, aniongap, PH, lactate, free calcium, PTT, invasive ventilation, and MI were all signifcantly associated with in-hospital mortality in patients with sepsis and AF (P<0.05, Table 2). Figure 2 displayed the K-M curves of three groups. The in-hospital mortality rate was highest in group 3 (NLR ≧ 8.4) than in the other two groups.
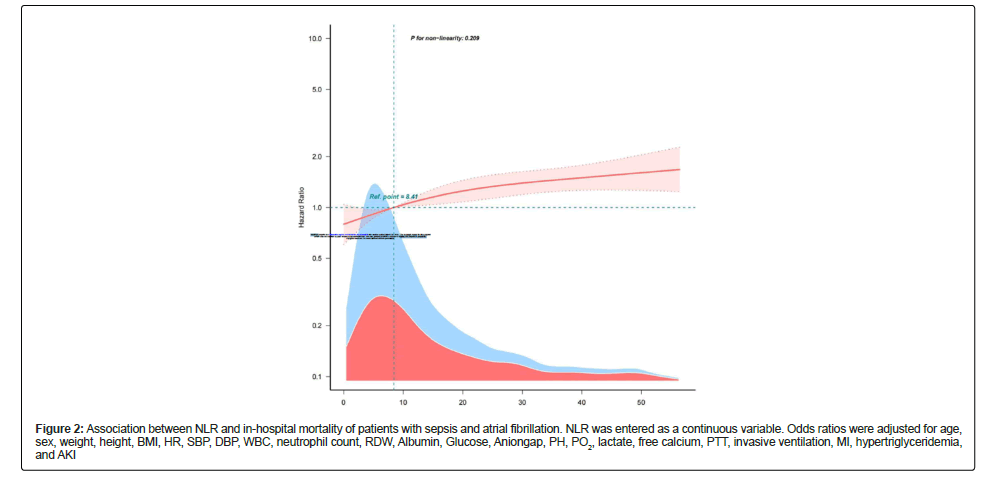
Figure 2: Association between NLR and in-hospital mortality of patients with sepsis and atrial fibrillation. NLR was entered as a continuous variable. Odds ratios were adjusted for age, sex, weight, height, BMI, HR, SBP, DBP, WBC, neutrophil count, RDW, Albumin, Glucose, Aniongap, PH, PO2, lactate, free calcium, PTT, invasive ventilation, MI, hypertriglyceridemia, and AKI
| Item | HR (95%CI) | P (Wald's test) | P (LR-test) |
|---|---|---|---|
| Age | 1.02 (1.01,1.03) | <0.001 | <0.001 |
| Gender( Female vs. Male) | 1.19 (1.04,1.37) | 0.012 | 0.012 |
| Weight (Kg) | 0.9956 (0.9928,0.9985) | 0.003 | 0.002 |
| Height (cm) | 0.9927 (0.9865,0.999) | 0.023 | 0.023 |
| BMI | 0.99 (0.98,1) | 0.021 | 0.018 |
| SOFA | 1.11 (1.09,1.13) | <0.001 | <0.001 |
| Charlson | 1.08 (1.05,1.11) | <0.001 | <0.001 |
| HR (bpm) | 1.0091 (1.005,1.0132) | <0.001 | <0.001 |
| SBP (mmHg) | 0.9921 (0.9875,0.9966) | <0.001 | <0.001 |
| DBP (mmHg) | 0.9954 (0.9885,1.0023) | 0.186 | 0.182 |
| Temp (°C) | 0.98 (0.95,1) | 0.094 | 0.113 |
| WBC (K/uL) | 1.0058 (1.0021,1.0095) | 0.002 | 0.01 |
| RBC (K/uL) | 1.03 (0.93,1.14) | 0.584 | 0.585 |
| Neutrophil count (K/uL) | 1.02 (1.01,1.03) | <0.001 | <0.001 |
| Lymphocytes (K/uL) | 0.99 (0.9616,1.0193) | 0.501 | 0.309 |
| NLR | 1.0008 (0.9995,1.0021) | 0.208 | 0.251 |
| Platelet count (K/uL) | 1.0001 (0.9995,1.0008) | 0.656 | 0.657 |
| Hemoglobin (g/dL) | 1.0076 (0.9727,1.0439) | 0.672 | 0.673 |
| RDW | 1.09 (1.06,1.12) | <0.001 | <0.001 |
| Hematocrit | 1.01 (1,1.02) | 0.061 | 0.063 |
| Albumin (g/dL ) | 0.85 (0.76,0.95) | 0.004 | 0.004 |
| Glucose (mg/dL) | 1.0033 (1.0022,1.0044) | <0.001 | <0.001 |
| Aniongap (mEq/L) | 1.09 (1.08,1.1) | <0.001 | < 0.001 |
| PH | 0.01 (0,0.02) | <0.001 | <0.001 |
| PCO2 (mmHg) | 0.9995 (0.9926,1.0065) | 0.895 | 0.895 |
| PO2 (mmHg) | 0.9952 (0.994,0.9963) | <0.001 | <0.001 |
| Lactate (mmol/L) | 1.14 (1.12,1.17) | <0.001 | <0.001 |
| Free calcium (mmol/L) | 0.15 (0.07,0.32) | <0.001 | <0.001 |
| PTT (sec) | 1.009 (1.0061,1.0118) | <0.001 | <0.001 |
| Invasive ventilation(Yes vs. No) | 1.21 (1.05,1.39) | 0.008 | 0.008 |
| Hypertension(Yes vs. No) | 0.88 (0.76,1.01) | 0.067 | 0.065 |
| DM(Yes vs. No) | 0.97 (0.84,1.12) | 0.649 | 0.648 |
| Heart failure(Yes vs. No) | 1.06 (0.92,1.21) | 0.425 | 0.425 |
| MI(Yes vs. No) | 1.4 (1.15,1.71) | <0.001 | 0.001 |
| CKD(Yes vs. No) | 1.06 (0.91,1.23) | 0.464 | 0.466 |
| COPD(Yes vs. No) | 1.05 (0.85,1.29) | 0.662 | 0.664 |
| AKI(Yes vs. No) | 1.31 (0.86,2) | 0.21 | 0.192 |
Note: BMI: Body Mass Index; SOFA: Sequential Organ Failure Assessment; HR: Heart Rate; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; WBC: White Blood Cell; RBC: Red Blood Cell; NLR: Neutrophil to-Lymphocyte Ratio; RDW: Red Cell distribution Width; DM: Diabetes Mellitus; MI: Myocardial Infarction; PTT: Partial Thromboplastin Time; CKD: Chronic Kidney Disease; COPD: Chronic Obstructive Pulmonary Disease; AKI: Acute Kidney Injury.
Table 2: Univariable Cox regression analysis of in hospital mortality of septic patients with atrial fibrillation.
Table 3 shows an unadjusted and a multivariable-adjusted correlation between NLR and in-hospital mortality. When NLR was analyzed as categorical variables, the highest NLR (model 3 vs. model 1) was associated with a higher risk of in-hospital mortality after adjusting all covariates (adjusted odds ratio, 1.31; 95% Confidence Interval (CI), 1.09–1.58; p<0.001). No covariate were adjusted in Model 1, age and sex were adjusted in Model 2, and all covariates were adjusted in Model 3. A linear relationship was observed between the NLR and in-hospital mortality in patients with sepsis and AF (Figure 3).
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Exposure | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| In Hospital Mortality | ||||||
| <4.0 | 1(Ref.) | 1(Ref.) | 1(Ref.) | |||
| 4.0-8.4 | 0.98 (0.82~1.17) | 0.829 | 1 (0.83~1.2) | 0.99 | 0.99 (0.80~1.23) | 0.784 |
| ≧ 8.4 | 1.43 (1.22~1.66) | <0.001 | 1.44 (1.23~1.67) | <0.001 | 1.31(1.09~1.58) | <0.001 |
| P for trend | 1.24 (1.15~1.34) | <0.001 | 1.24 (1.15~1.33) | <0.001 | 1.04 (0.95~1.13) | <0.001 |
Table 3: Relationship between neutrophil to lymphocyte ratio and in hospital mortality in different models.
Results of subgroup analyses
The subgroup analysis was conducted to reveal the correlation between NLR and in-hospital mortality across age, gender, BMI, comorbidities, and the results were shown in Figure 4. No significant interactions were observed between NLR and age, gender, BMI, MI, CKD and hypertension (p>0.05).
Discussion
Our study demonstrated that high NLR was related to increased in-hospital mortality of septic patients with atrial fibrillation, and there was a linear relationship between NLR and in-hospital mortality, stratified analyses indicated stable correlation between the NLR and in-hospital mortality. The findings of this retrospective observational study based on the MIMIC-IV database provide compelling evidence of a significant association between the Neutrophil to-Lymphocyte Ratio (NLR) and the outcome of septic patients with Atrial Fibrillation (AF).
Sepsis is a host-mediated systemic inflammatory response to infection, and evidence of immune cell activation and host response dysfunction has been observed in severe sepsis patients[10,11,25,26]. There are existing literature that have identified the cell ratio as an important indicator for predicting mortality rates. The Platelet-to- Lymphocyte Ratio (PLR) has been embraced by medical professionals as a widely used and cost-effective tool for diagnosing and predicting mortality rates. Additionally, PLR has been found to enhance the diagnostic role of the Neutrophil-to-Lymphocyte Ratio (NLR) [12]. Kriplani et al. [13] evaluated the Lymphocyte-Monocyte Ratio (LMR) in patients undergoing Percutaneous Nephro Lithotomy (PNL) and found that patients who developed Systemic Inflammatory Response Syndrome (SIRS) had a lower LMR (2.5 ± 1.7 vs. 3.2 ± 1.8, OR 1.18, 95% CI 1.04-1.34, p=0.006). They suggested that LMR could serve as an independent, easily obtainable, and cost-effective predictive marker for early identification of SIRS/sepsis after PNL, particularly patients with LMR<3.23 should be closely monitored for early detection of postoperative infections and complications. Increased Red Cell Distribution Width (RDW) also has been found to be significantly associated with higher in-hospital mortality rates and 4-year mortality rates. Importantly, the prognostic value of RDW for 4-year mortality rates is independent of traditional severity scoring, suggesting that RDW can predict in-hospital and 4-year mortality rates in adult critically ill patients and provide additional prognostic value beyond traditional severity scoring [27]. Besides, Daniel Dankl found an association of RDW with mortality in septic patients and proposed he optimal RDW cutoff for the prediction of hospital mortality was 16% [16].
NLR can be calculated by dividing the absolute neutrophil count by the absolute lymphocyte count obtained from a complete blood count of peripheral blood. Neutrophils are white blood cells primarily involved in inflammatory and infection defense. An increase in neutrophil count usually indicates an ongoing inflammatory response or infection. Lymphocytes, on the other hand, are a type of immune cell. They play a crucial role in recognizing and attacking pathogens, as well as maintaining immune balance. The quantity and function of lymphocytes are closely related to the immune status of the body. The NLR is used in assessing the severity of inflammation, predicting disease progression, and prognosis. Higher NLR values typically indicate immune system dysregulation or intensified inflammatory response [26,28-31]. Numerous studies have reported associations between NLR and adverse outcomes in critically ill patients. Zahorec [25] mentioned that the NLR is useful in distinguishing the severity of diseases and predicting mortality rates. Patients with an NLR value greater than 30 are considered to be in critical condition, with a higher mortality rate. On the other hand, patients with an NLR value lower than7 have significantly reduced probability of sepsis occurrence, leading to a lower mortality rate as well [32-34]. In this study, we analysed NLR as a continuous and categorical variable based on the literature. Numerous covariates were found to be associated with in- hospital mortality, including age, gender, weight, height, BMI, SOFA, charlson, HR, SBP, WBC, neutrophilcount, RDW, albumin, glucose, aniongap, PH, PO2, lactate, freecalcium, PTT, and MI. After adjusting for all covariates, the highest NLR group had a 31% increase in in- hospital mortality compared to the lowest group, which is consistent with previous research findings. Atrial FIbrillation (AF) is a common cardiac arrhythmia characterized by rapid and irregular electrical activity in the atria of the heart. Clinical data consistently show an increased incidence of AF in patients with sepsis, shedding light on their association and clinical implications. Although NLR is a commonly used indicator to assess the severity of inflammation in sepsis patients, it is rarely used as a biomarker in AF population. Confirmed NLR has been associated with adverse prognosis in various cardiovascular diseases, including acute coronary syndrome and other coronary artery diseases, coronary artery bypass grafting, hospitalization for heart failure [35-39]. In a randomized, double-blind trial involving 19,697 non-severe atrial fibrillation patients, Fagundes et al. found that a high proportion of 72.9% had NLR distribution between 1.5-4.0, while only 15.8% had NLR greater than 4.0. However, higher NLR values were consistently associated with a higher risk of adverse clinical outcomes, including stroke, cardiovascular death, myocardial infarction, and all- cause mortality [40]. In the present study, we included patients with sepsis complicated by AF. According to the literature, we divided the NLR into three groups: <4.0, 4.0-8.4, and ≧ 8.4. The results showed that the majority (3,083/5,864) of patients had NLR values in the third group (NLR ≧ 8.4), which indirectly reflects the severity of inflammation in septic patients. Besides, a higher NLR was found to be significantly associated with increased mortality rates and prolonged hospital stays in septic shock patients with concurrent atrial fibrillation.
Several mechanisms have been proposed to explain the link between sepsis and AF. Firstly, sepsis induces a pro-inflammatory state through the release of cytokines, such as interleukin-6 (IL-6) and Tumor Necrosis Factor-Alpha (TNF-α). This excessive inflammatory response can disrupt the normal electrical conduction pathways of the heart, leading to the development of AF [41]. In addition, sepsis can cause myocardial dysfunction, myocardial ischemia, and oxidative stress, which further contribute to the occurrence and persistence of AF [42-44]. Furthermore, sepsis-related alterations in autonomic tone and neurohormonal activation may also play a role in the development of AF [45]. The sympathetic over activation and parasympathetic withdrawal associated with sepsis can lead to an imbalance in the autonomic nervous system, promoting the initiation and maintenance of AF [46]. Additionally, elevated levels of circulating catecholamines and abnormal calcium handling in cardiomyocytes during sepsis have been implicated in the pathogenesis of AF [47]. The presence of AF in septic patients has been associated with worse outcomes, including increased mortality, longer hospital stays, and a higher incidence of stroke [41].
In the present study, we recruited 5,864 septic patients with AF. Both logistics regression and Kaplan–Meier survival curves showed that higher NLR is related to increased mortality, which is consistent with previous literature, and it’s a linear relationship between NLR and in-hospital mortality in septic patients with AF. Moreover, the subgroup analysis results indicated that the correlation between the NLR and in- hospital mortality was stable. The linear relationship between NLR and in-hospital mortality observed in our study suggests that NLR could serve as a valuable prognostic marker for septic patients with AF in the intensive care setting. This information holds potential clinical relevance for risk stratification and treatment decision-making in this patient population.
Nonetheless, it is essential to acknowledge the limitations of our study, including its retrospective design and reliance on data from a single database. Future research, incorporating prospective designs and data from multiple sources, is warranted to validate our findings and explore the clinical utility of NLR in septic patients with AF.
Conclusion
In conclusion, our study contributes to the growing body of evidence supporting the association between NLR and clinical outcomes in septic patients with AF. The wealth of literature on this topic, including the studies cited above and others, underscores the importance of further investigation into the prognostic value of NLR in critical care settings, ultimately aiming to improve patient care and outcomes.
Author Contributions
All authors contributed to: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Denning NL, Aziz M, Gurien SD, Wang P (2019) DAMPs and NETs in sepsis. Front. immunol 10:2536.
[Crossref] [Google Scholar] [PubMed]
- Hoffman M, Kyriazis ID, Lucchese AM, de Lucia C, Piedepalumbo M, et al. (2019) Myocardial strain and cardiac output are preferable measurements for cardiac dysfunction and can predict mortality in septic mice. J Am Heart Assoc 8: e012260.
[Crossref] [Google Scholar] [PubMed]
- Razazi K, Boissier F, Surenaud M, Bedet A, Seemann A, et al. (2019) A multiplex analysis of sepsis mediators during human septic shock: a preliminary study on myocardial depression and organ failures. Ann Intensive Care 9:64.
[Crossref] [Google Scholar] [PubMed]
- Zampieri FG, Bagshaw SM, Semler MW (2023) Fluid therapy for critically ill adults with sepsis: A review. JAMA 329:1967-1980.
[Crossref] [Google Scholar] [PubMed]
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, et al. (2016) Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 193:259-272.
[Crossref] [Google Scholar] [PubMed]
- Jesus Aibar SS (2020) New-onset atrial fibrillation in sepsis: A narrative review. Semin Thromb Hemost 47:18-25.
[Crossref] [Google Scholar] [PubMed]
- Ananthaseshan S, Bojakowski K, Sacharczuk M, Poznanski P, Skiba DS, et al. (2022) Red blood cell distribution width is associated with increased interactions of blood cells with vascular wall. Sci Rep 12:13676.
[Crossref] [Google Scholar] [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G (2015) Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 52:86-105.
[Crossref] [Google Scholar] [PubMed]
- Walkey AJ (2018) Atrial fibrillation in the ICU. Chest 154:1424-1434.
[Crossref][Google Scholar] [PubMed]
- Ankawi G, Neri M, Zhang J, Breglia A, Ricci Z, et al. (2018) Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: the promises and the pitfalls. Crit Care 22:262.
[Crossref] [Google Scholar] [PubMed]
- Zhang YY, Ning BT (2021) Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther 6:407.
[Crossref] [Google Scholar] [PubMed]
- Spoto S, Lupoi DM, Valeriani E, Fogolari M, Locorriere L, et al. (2021) Diagnostic accuracy and prognostic value of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in septic patients outside the intensive care unit. Medicina (Kaunas) 57(8).
[Crossref] [Google Scholar] [PubMed]
- Kriplani A, Pandit S, Chawla A, de la Rosette J, Laguna P, et al. (2022) Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and lymphocyte-monocyte ratio (LMR) in predicting systemic inflammatory response syndrome (SIRS) and sepsis after percutaneous nephrolithotomy (PNL). Urolithiasis 50:341-348.
[Crossref] [Google Scholar] [PubMed]
- Fan YW, Liu D, Chen JM, Li WJ, Gao CJ (2021) Fluctuation in red cell distribution width predicts disseminated intravascular coagulation morbidity and mortality in sepsis: a retrospective single-center study. Minerva Anestesiol 87:52-64.
[Crossref] [Google Scholar] [PubMed]
- Han YQ, Zhang L, Yan L, Li P, Ouyang PH, et al. (2018) Red blood cell distribution width predicts long-term outcomes in sepsis patients admitted to the intensive care unit. Clin Chim Acta 487:112-116.
[Crossref] [Google Scholar] [PubMed]
- Dankl D, Rezar R, Mamandipoor B, Zhou Z, Wernly S, et al. (2022) Red cell distribution width is independently associated with mortality in sepsis. Med Princ Pract 31(2):187-194.
[Crossref] [Google Scholar] [PubMed]
- Hou SK, Lin HA, Chen SC, Lin CF, Lin SF (2021) Monocyte distribution width, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio improves early prediction for sepsis at the emergency. J Pers Med 11(8).
[Crossref] [Google Scholar] [PubMed]
- Liu S, Li Y, She F, Zhao X, Yao Y (2021) Predictive value of immune cell counts and neutrophil-to-lymphocyte ratio for 28-day mortality in patients with sepsis caused by intra-abdominal infection. Burns Trauma 9:040.
[Crossref] [Google Scholar] [PubMed]
- Moisa E, Corneci D, Negoita S, Filimon CR, Serbu A et al. (2021) Dynamic changes of the neutrophil-to-lymphocyte ratio, systemic inflammation index, and derived neutrophil-to-lymphocyte ratio independently predict invasive mechanical ventilation need and death in critically ill COVID-19 patients. Biomedicines 9:1656.
[Crossref] [Google Scholar] [PubMed]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801-810.
[Crossref] [Google Scholar] [PubMed]
- Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA (2018) The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med 24:1716-1720.
[Crossref] [Google Scholar] [PubMed]
- Yang Q, Zheng J, Chen W, Chen X, Wen D, et al. (2021) Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: A cohort study. Front Med (Lausanne) 8:640785.
[Crossref] [Google Scholar] [PubMed]
- Mitsuyama Y, Shimizu K, Komukai S, Hirayama A, Takegawa R, et al. (2022) Sepsis-associated hypoglycemia on admission is associated with increased mortality in intensive care unit patients. Acute Med Surg 9: e718.
[Crossref] [Google Scholar] [PubMed]
- Feng M, McSparron JI, Kien DT, Stone DJ, Roberts DH, et al. (2018) Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 44:884-892.
[Crossref] [Google Scholar] [PubMed]
- Zahorec R (2000) Definition for septic syndrome should be re-evaluated. Intensive Care Medicine 26:1870-1870.
[Crossref] [Google Scholar] [PubMed]
- Socorro Faria S, Fernandes Jr PC, Barbosa Silva MJ, Lima VC, Fontes W, et al. (2016) The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience 12:702.
[Crossref] [Google Scholar] [PubMed]
- Yan-Qiu Han LY, Lei Zhang, Pei-Heng Ouyang, Peng Li, Hemant Goyal, et al. (2019) Red blood cell distribution width provides additional prognostic value beyond severity scores in adult critical illness. Clin Chim Acta 498:6.
[Crossref] [Google Scholar] [PubMed]
- Ha YJ, Hur J, Go DJ, Kang EH, Park JK, et al. (2018) Baseline peripheral blood neutrophil-to-lymphocyte ratio could predict survival in patients with adult polymyositis and dermatomyositis: A retrospective observational study. PLoS One 13: e0190411.
[Crossref] [Google Scholar] [PubMed]
- Giede-Jeppe A, Bobinger T, Gerner ST, Sembill JA, Sprugel MI, et al. (2017) Neutrophil-to-lymphocyte ratio is an independent predictor for in-hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovasc Dis 44:26-34.
[Crossref] [Google Scholar] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, et al. (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 88:218-230.
[Crossref] [Google Scholar] [PubMed]
- Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, et al. (2010) Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 106:470-476.
[Crossref] [Google Scholar] [PubMed]
- R Z (2021) Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Med J 122:14.
[Crossref] [Google Scholar] [PubMed]
- Lee JS, Kim NY, Na SH, Youn YH, Shin CS (2018) Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 97.
[Crossref] [Google Scholar] [PubMed]
- Domen Ribnikar IS, Philippe L. Bedard (2021) The prognostic value of neutrophil-to-lymphocyte ratio in metastatic testicular cancer. Curr Oncol 28:7.
[Crossref] [Google Scholar] [PubMed]
- Park JJ, Jang H-J, Oh I-Y, Yoon C-H, Suh J-W, et al. (2013) Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 111:636-642.
[Crossref] [Google Scholar] [PubMed]
- Ahmet Arif Yalcin, Mustafa Topuz, Ibrahim Faruk Akturk (2014) Is there a correlation between coronary artery ectasia and neutrophil–lymphocyte ratio? Clin Appl Thromb Hemost 21:5.
[Crossref] [Google Scholar] [PubMed]
- Irfan Tasoglu OT, Yunus Nazli (2013) Preoperative neutrophil–lymphocyte ratio and saphenous vein graft patency after coronary artery bypass grafting. Clin Appl Thromb Hemost 20:1-6.
[Crossref] [Google Scholar] [PubMed]
- Kim SC, Sun K-H, Choi D-H, Lee Y-M, Choi S-W, et al. (2016) Prediction of long-term mortality based on neutrophil-lymphocyte ratio after percutaneous coronary intervention Am J Med Sci 351:467-472.
[Crossref] [Google Scholar] [PubMed]
- Cho JH, Cho H-J, Lee H-Y, Ki Y-J, Jeon E-S, et al. (2020) Neutrophil-lymphocyte ratio in patients with acute heart failure predicts in-hospital and long-term mortality. J Clin Med 18:557.
[Crossref] [Google Scholar] [PubMed]
- Fagundes A, Ruff CT, Morrow DA, Murphy SA, Palazzolo MG, et al. (2023) Neutrophil-lymphocyte ratio and clinical outcomes in 19,697 patients with atrial fibrillation: Analyses from ENGAGE AF-TIMI 48 trial. International Journal of Cardiology 386:118-124.
- Corica B, Romiti GF, Basili S, Proietti M (2022) Prevalence of new-onset atrial fibrillation and associated outcomes in patients with sepsis: a systematic review and meta-analysis. J Pers Med 12:547.
[Crossref] [Google Scholar] [PubMed]
- McManus AJWD (2017) When rhythm changes cause the blues: new-onset atrial fibrillation during sepsis. Am J Respir Crit Care Med 195.
[Crossref] [Google Scholar] [PubMed]
- Boos CJ (2020) Infection and atrial fibrillation: inflammation begets AF. Eur Heart J 41:1120-1122.
[Crossref] [Google Scholar] [PubMed]
- Isuru Induruwa EH, James Hennebry (2022) Sepsis-driven atrial fibrillation and ischaemic stroke. Is there enough evidence to recommend anticoagulation? Eur J Intern Med 98:32-36.
[Crossref] [Google Scholar] [PubMed]
- Tralhão A, Póvoa P (2020) Cardiovascular events after community-acquired pneumonia: A global perspective with systematic review and meta-analysis of observational studies. J Clin Med 9:414.
[Crossref] [Google Scholar] [PubMed]
- Walkey AJ, Quinn EK, Winter MR, McManus DD, Benjamin EJ (2016) Practice patterns and outcomes associated with use of anticoagulation among patients with atrial fibrillation during sepsis. JAMA Cardiol 1:682-90.
[Crossref] [Google Scholar] [PubMed]
- Bosch NA, Rucci JM, Massaro JM, Winter MR, Quinn EK, et al. (2021) Comparative effectiveness of heart rate control medications for the treatment of sepsis-associated atrial fibrillation. Chest 159:1452-1459.
Citation: Tang W, Shi W, Li L, Tang K (2024) Association Between Neutrophil To-Lymphocyte Ratio (NLR) and Outcome of Septic Patients with Atrial Fibrillation (AF): A Retrospective Observational Study Based on Medical Information Mart for Intensive Care IV. J Infect Dis Ther 12: 588. DOI: 10.4172/ 2332-0877.1000590
Copyright: © 2024 Tang W et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1045
- [From(publication date): 0-2024 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 840
- PDF downloads: 205