Association between Cytokine Cycling Levels and Sjogren's Syndrome: Genetic Correlation and Bidirectional Mendelian Randomization Study
Received: 20-Mar-2024 / Manuscript No. DPO-24-130105 / Editor assigned: 22-Mar-2024 / PreQC No. DPO-24-130105 / Reviewed: 05-Apr-2024 / QC No. DPO-24-130105 / Revised: 12-Apr-2024 / Manuscript No. DPO-24-130105 / Accepted Date: 12-Apr-2024 / Published Date: 19-Apr-2024 DOI: 10.4172/2476-2024.9.1.225
Abstract
Background: Sjogren's Syndrome (SS) is a complex autoimmune disease influenced by genetics, yet its genetic underpinnings remain elusive. This study investigates the genetic correlation and potential causative link between cytokine cycling levels and SS.
Methods: Genome-Wide Association Studies (GWAS) were conducted with 8,293 and 14,824 European participants to identify cytokines. The GWAS dataset for SS, comprising 368,028 individuals of European ancestry (2,495 cases and 365,533 controls), was sourced from the Finnish biological sample library. Single Nucleotide Polymorphisms (SNPs) associated with SS were identified using Linkage Disequilibrium Score (LDSC) regression for Mendelian Randomization (MR) analysis. The Inverse Variance Weighted (IVW) method was the primary analytical approach. Additional methods including MR Egger, weighted median, and weighted mode were employed for robustness assessment. Heterogeneity testing, horizontal pleiotropy testing and steiger testing were conducted for sensitivity analysis. Reverse MR analysis was performed to assess the potential for a reverse causal relationship between SS and cytokines.
Results: LDSC regression analysis identified 46 cytokines for bidirectional MR analysis with SS. The IVW method revealed significant associations of genetically predicted cytokines IL10RB (P=0.019, OR=1.138, 95% CI: 1.021-1.267) and CXCL11 (P=0.015, OR=1.269, 95% CI: 1.048-1.537) with increased SS risk. The absence of heterogeneity and horizontal pleiotropy in sensitivity analysis underscores the robustness of these findings.
Conclusion: The study suggests a potential causal relationship between genetically predicted cytokines and SS, particularly through IL10RB and CXCL11 cycles. Further research is warranted to elucidate the biological mechanisms by which cytokine cycling levels influence SS.
Keywords: Cytokines; Sjogren's syndrome; Mendelian randomization; Bidirectional; Linkage disequilibrium score regression analysis
Abbreviations
CD5: T-Cell Surface Glycoprotein CD5; CXCL11: C-X-C Motif Chemokine 11; GWAS: Genome-Wide Association Study; 10RB: Interleukin-10 Receptor Subunit Beta; IV: Instrumental Variable; IVW: Inverse Variance Weighted; OR: Odds Ratio; SNP: Single Nucleotide Polymorphism; SS: Sjogren's Syndrome
Highlights
• Genetic evidence suggests a causal relationship between cytokines and Sjogren's syndrome.
• Linkage disequilibrium score regression analysis revealed a causal association between cytokines and Sjogren's syndrome.
• The circulating levels of IL10RB and CXCL11 are risk factors for the onset of Sjogren's syndrome.
Introduction
Sjogren's Syndrome (SS) is a chronic autoimmune disorder marked by T and B cell dysfunction and lymphocyte infiltration, causing damage to various organs. It frequently affects secretory glands, musculoskeletal, respiratory and blood systems [1,2]. The prevalence of SS, influenced by environmental, gender, and genetic factors, ranges from 0.1% to 4.8% globally [3-5]. SS is associated with an increased risk of cardiovascular disease, adverse pregnancy outcomes, and cancer [6-9], posing significant health and safety concerns. Current understanding of SS pathogenesis remains limited, and treatment primarily involves symptomatic relief, lacking curative options [10]. Investigating the pathogenic factors of SS could offer new perspectives for its management.
Cytokines, encompassing Interleukins (IL), Tumor Necrosis Factors (TNF), and growth factors, are critical in signal transduction and immune regulation via autocrine and paracrine mechanisms [11,12].The link between SS and cytokines is well-established [13,14]. Being an autoimmune disease, SS's pathogenesis involves immune cells regulated by cytokines in their development and function [15]. Research indicates cytokines contribute to SS pathogenesis through pathways including cell differentiation, apoptosis signaling, immune response regulation, and epigenetic mechanisms. This leads to immune cell dysregulation, inflammatory responses, and abnormal B cell-mediated lymph node proliferation [16-18]. However, the specific impact of cytokines on SS pathogenesis and identifying the influential cytokines require further exploration.
A whole Genome Wide Association Study (GWAS) is an approach used to investigate and validate the relationship between genetic variation and specific traits or diseases using extensive gene datasets [19]. Linkage Disequilibrium Score (LDSC) regression is widely employed in GWAS to assess the genetic correlation between genes and disease activity, offering robust evidence for GWAS findings [20]. Mendelian Randomization (MR) is an epidemiological technique that utilizes GWAS comprising Single Nucleotide Polymorphisms (SNPs) as genetic Instrumental Variables (IV) to elucidate complex causal relationships between genes and diseases or traits [21,22]. The random allocation of these genetic variations during gestation minimizes the effects of confounding factors and reverses causality, enhancing the reliability of results. MR adheres to the principle of "single gene, single phenotype," bolstering the credibility of research findings [23]. Over 100 GWAS datasets exist for cytokines and a substantial sample size for SS [3,24,25], yet MR studies examining the causal relationship between cytokines and SS are scarce.
Therefore, this study aims to utilize public GWAS data, integrating LDSC regression and bidirectional MR analysis, to explore the correlation between cytokine cycling levels and SS. This approach may provide novel insights into the impact of cytokine cycling levels on SS.
Materials and Methods
Study design
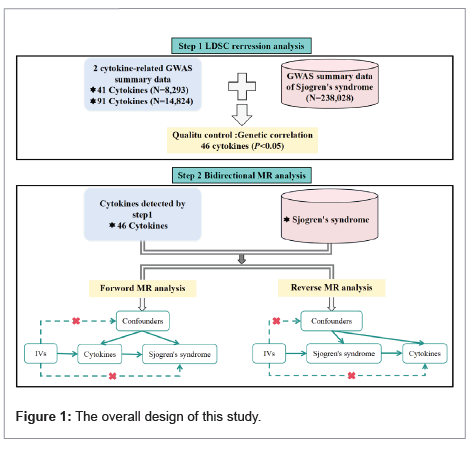
Figure 1 illustrates the study's design. Initially, LDSC regression analysis is used to identify the genetic contribution of SNPs to cytokines and SS. Subsequently, bidirectional MR analysis on cytokines and SS was conducted to investigate their causal relationship. Data for this study was sourced from a public database, negating the need for additional ethical approval.
GWAS data source for cytokines
The GWAS dataset for 41 cytokines was derived from 8, 293 Finnish subjects (aged 25 to 74) between 1997 and 2002 [24]; data for 91 cytokines came from 11 cohorts comprising 14,824 participants of European ancestry (25). Instrumental Variable (IV) SNPs were selected based on significant correlation with cytokines (P<5 × 10^-6) and independence criteria (r^2<0.001 within 10 Mb). Accordingly, 104 cytokine-related SNPs totaling 1,677 were retained (Supplementary Table 1).
GWAS data source for Sjogren's syndrome
The GWAS dataset for SS included 368,028 individuals (2,495 cases and 365,533 controls) of European ancestry from the Finnish biological sample library. SS diagnoses were obtained using ICD-10 diagnostic codes [26]. Following screening, 18 SNPs significantly correlated with SS were identified as independent IVs (P<5 × 10^-6, r^2<0.001 within 10 Mb) (Supplementary Table 2). Reverse MR analysis utilizing these SNPs was conducted to assess potential causality between SS and cytokine cycling levels.
LDSC regression analysis
Linkage Disequilibrium (LD) score quantifies the extent of LD association between SNPs and adjacent SNPs. LDSC, a prevalent method in GWAS and MR analysis, evaluates genetic correlation between complex diseases and traits based on LD [27,28]. In this study, the LDSC regression analysis threshold was set at P<0.05 to investigate the genetic correlation between cytokines and SS. The final GWAS data for SS met this criterion, identifying 46 candidate cytokines for subsequent MR analysis (Supplementary Table 3).
Instrumental variable selection
The selection of IVs involved identifying SNPs with significant genetic correlation and independence (r2<0.001 within 10 Mb) from exposure factors, as per LDSC regression analysis. Forward MR analysis identified 829 independent SNPs from 46 cytokine GWAS studies as IVs (Supplementary Table 4), while reverse MR analysis utilized 18 independent SNPs from SS GWAS studies as IVs (Supplementary Table 2).
Mendelian randomization analysis
Bidirectional MR analyses were performed using the "two sample MR" software package. The Inverse Variance Weighting (IVW) method was employed as the primary, more accurate, and unbiased analysis approach. Supplementary methods, including MR Egger, weighted median, and weighted mode, were utilized to test the robustness of the results [29]. The Steiger test (P<0.05) helped ascertain the correctness of the research direction. To explore a causal relationship between SS and cytokine cycling levels, Steiger tests and reverse MR analysis were also conducted.
MR analysis adheres to three key assumptions, outlined in step 2 of Figure 1, depicting the fundamental principles of MR. First; the IV SNPs must exhibit significant correlation with cytokines. This was assessed using F-statistics, where a value greater than 10 indicates sufficient IV strength for robust results [21,22,30]. The F-statistic is calculated as follows:
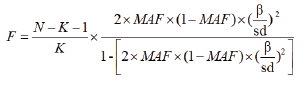
Among them, N represents the number of samples in the GWAS study, K represents the number of SNPs, and MAF represents minor allel frequency, β represents the effect size of SNP on exposure, and SD represents the Standard Deviation. The F-statistic of SNP used in this study ranges from 18 to 1510, indicating that the IVs used are sufficiently powerful.
Second, the chosen IVs should be independent of confounding factors. Third, IVs must not influence outcomes via pathways other than the exposure factors. Sensitivity analysis on these hypotheses included forest maps and Leave-One-Out (LOO) analyses to assess SNP impacts on outcomes. Heterogeneity testing, MR Egger level pleiotropy testing, and MRPRESSO testing were also performed. A Cochran Q test value greater than 0.05 indicates no heterogeneity, while consistent MR Egger intercept values and MRPRESSO results above 0.05 suggest the absence of level pleiotropy in SNPs.
In the concluding phase of the MR analysis, a causal association between cytokine SNPs and SS was established. This finding was further scrutinized using the phenoscanner website (http://www. phenoscanner.medschl.cam.ac.uk/). A subsequent MR analysis was conducted, excluding SNPs not directly related to SS or potentially exhibiting horizontal pleiotropy. This refined approach ensured a more precise evaluation of the causal relationship.
Results
MR analysis of the association between cytokines and Sjogren's syndrome
In the MR analysis investigating the causal relationship between 46 cytokines and SS, suggestive causal associations were observed using the Inverse Variance Weighted (IVW) method (Supplementary Table 5). A positive causal relationship was identified for cytokines CD5 (P=0.015, OR=1.245, 95% CI: 1.043-1.486), CXCL11 (P=0.003, OR=1.293, 95% CI: 1.089-1.536), and IL10RB (P=0.019, OR=1.138, 95% CI: 1.021-1.267) with SS. An examination of SNPs related to these cytokines on the phenoscanner website helped to further exclude potential pleiotropy. It was discovered that CD5 (rs3184504, rs4939490) and CXCL11 (rs10733789, rs3184504) are linked to three SNPs associated with other traits (Table 1). After removing these pleiotropic SNPs, a subsequent MR analysis revealed the associations for IL10RB and CXCL11 (P=0.015, OR=1.269, 95% CI: 1.048-1.537) remained consistent, whereas CD5 (P=0.506) showed no causal link to SS risk upon exclusion of potential pleiotropy (Table 2).
| SNPs | Pleiotropic trait | P | PMID |
|---|---|---|---|
| rs3184504 | Juvenile idiopathic arthritis including oligoarticular and rheumatoid factor negative polyarticular JIA | 2.60E-09 | 23603761 |
| Primary biliary cirrhosis | 1.11E-08 | 22961000 | |
| Rheumatoid arthritis and celiac disease | 1.40E-11 | 21383967 | |
| Lymphocyte count | 6.93E-134 | 27863252 | |
| Type 1 diabetes | 3.00E-27 | 19430480 | |
| White blood cell count | 9.00E-70 | 27863252 | |
| Inflammatory bowel disease | 1.00E-09 | 26192919 | |
| rs10733789 | Myeloid white cell count | 7.80E-13 | 27863252 |
| White blood cell count | 4.91E-12 | 27863252 | |
| Platelet count | 2.96E-70 | 27863252 | |
| rs4939490 | Multiple sclerosis | 1.00E-09 | 21244703 |
Table 1: Details of the genetic variants with potential pleiotropy among instrumental variables used for Sjogren's syndrome.
| Cytokine | nSNPs | Methods | P | OR | 95% CI |
|---|---|---|---|---|---|
| CD5 | 17 | Inverse variance weighted | 0.506 | 1.073 | 0.872-1.321 |
| MR Egger | 0.881 | 0.96 | 0.571-1.616 | ||
| Weighted median | 0.535 | 1.095 | 0.823-1.457 | ||
| Weighted mode | 0.721 | 1.09 | 0.685-1.733 | ||
| CXCL11 | 20 | Inverse variance weighted | 0.015 | 1.269 | 1.048-1.537 |
| MR Egger | 0.814 | 1.058 | 0.669-1.673 | ||
| Weighted median | 0.167 | 1.222 | 0.919-1.625 | ||
| Weighted mode | 0.227 | 1.21 | 0.897-1.634 |
Table 2: MR analysis of genetic variation SNPs with potential pleiotropy excluded.
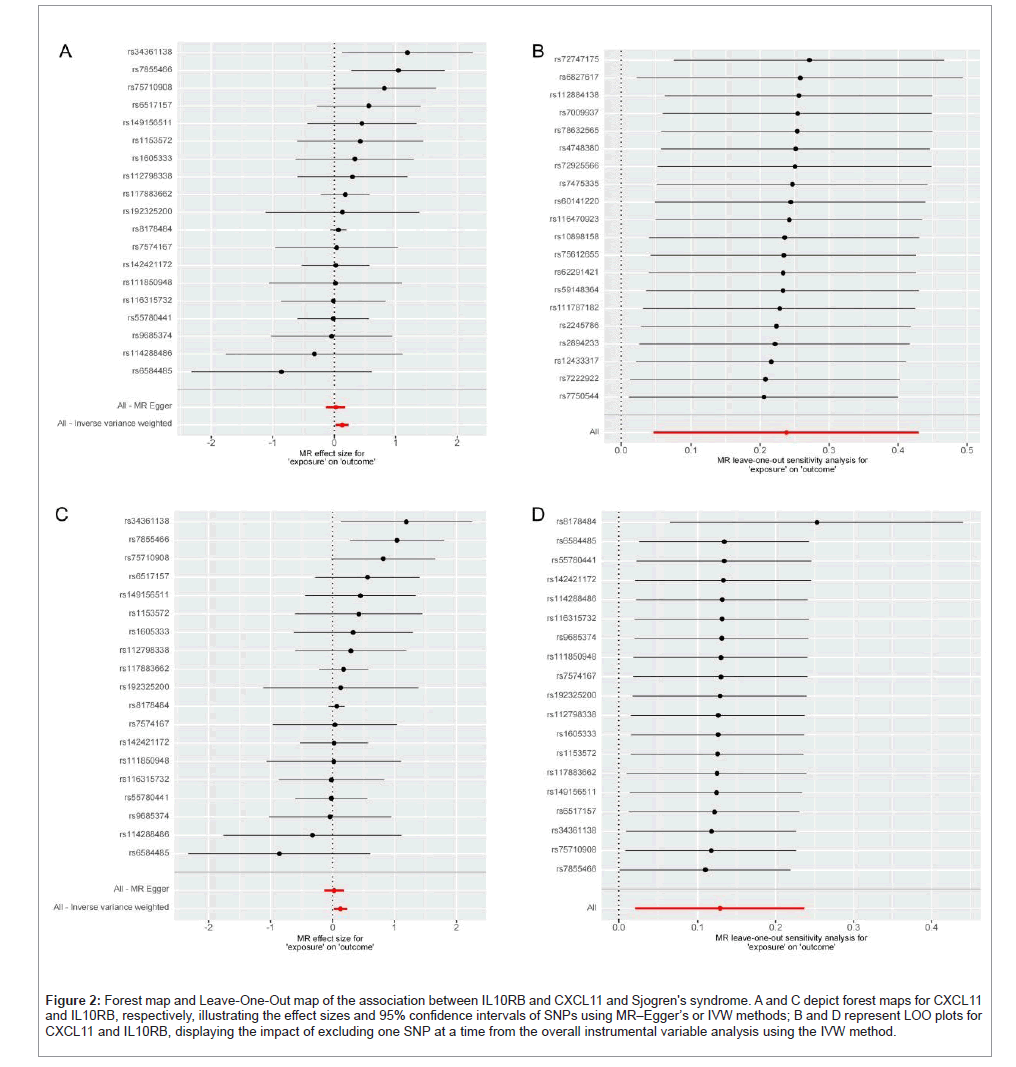
In sensitivity analysis, excluding pleiotropic SNPs, the forest and Leave-One-Out (LOO) maps indicated no significant impact of SNP absence on the outcomes (Figure 2). Heterogeneity testing found no evidence of heterogeneity (IL10RB Q-Pval=0.470, CXCL11 Q=0.604). MR Egger level pleiotropy testing (IL10RB P=0.090, CXCL11 P=0.402) and MRPRESSO testing (CXCL11 P=0.657, IL10RB with no outlier SNP exhibiting level pleiotropy, hence data not shown) did not detect level pleiotropy (Supplementary Table 6). Additionally, the Steiger test confirmed the validity of the research direction (Supplementary Table 7).
Figure 2: Forest map and Leave-One-Out map of the association between IL10RB and CXCL11 and Sjogren's syndrome. A and C depict forest maps for CXCL11 and IL10RB, respectively, illustrating the effect sizes and 95% confidence intervals of SNPs using MR–Egger’s or IVW methods; B and D represent LOO plots for CXCL11 and IL10RB, displaying the impact of excluding one SNP at a time from the overall instrumental variable analysis using the IVW method.
MR analysis of the association between Sjogren's syndrome and cytokines
A reverse MR analysis was executed to ascertain the potential causal relationship between SS and cytokines. Utilizing the Inverse Variance Weighted (IVW) method, preliminary findings suggested a reverse causal association between SS and CXCL9 (P=0.015) and CXCL10. Further investigation for pleiotropy was conducted using the phenoscanner website, leading to the exclusion of three SNPs potentially exhibiting pleiotropy. A subsequent MR analysis, however, revealed no significant association between SS and cytokines (Supplementary Table 8). The outcomes of both heterogeneity and horizontal pleiotropy tests were negative, confirming the absence of heterogeneity or horizontal pleiotropy bias in our MR findings (Supplementary Table 9).
Discussion
This MR study was designed to investigate the potential causal relationship between cytokine cycling levels and SS. Utilizing LDSC analysis, 46 cytokines were identified as genetically correlated with SS. These cytokines served as IVs in the MR analysis, with an additional focus on excluding SNPs indicative of mixed pleiotropy. The analysis provided suggestive evidence that genetically predicted circulating levels of IL-10RB and CXCL11 are associated with SS. Moreover, bidirectional MR analysis indicated that SS does not influence cytokine cycling levels.
Chemokines are a category of small cellular factors with molecular weights ranging from 8 kd-14 kd, characterized by similar basic structures and playing pivotal roles in immune system development, homeostasis, and function [31,32]. CXCL11, a member of the CXC chemokine family, is a low molecular weight cytokine. It induces chemotaxis through γ interferon, binding to CXCR3 receptors on activated T cells, B cells, and NK cells. This interaction influences immune cell migration, promotes inflammation, augments angiogenesis, and facilitates leukocyte exosmosis, there by playing a significant role in various immune-related diseases [33,34].
Clinical studies on CXCL11 and SS are limited. A study examining tear film and ocular surface in 16 SS patients and 15 controls reported increased CXCL11 and CXCR3 expression in SS patients (P<0.05), along with a rise in CD4 (+) cell numbers [35]. Another immunohistochemical analysis of labial salivary glands in 22 SS patients showed high CXCL11 expression and CXCR3+ macrophage migration [36], suggesting CXCL11's involvement in SS inflammatory infiltration. These findings align with our MR analysis, where CXCL11 is positively correlated with SS risk. IFN-γ is known to exacerbate lymphocyte infiltration in SS salivary glands by inducing CXCL11, while inhibiting IFN-γ-induced CXCL11 can reduce lymphocyte infiltration and alleviate inflammation [37]. Animal studies have demonstrated that with reduced inflammation in SS mice's submandibular glands, there is a notable decrease in T and B lymphocytes, including activated CD4 and CD8T cells, along with downregulation of IFN-γ, CXCR3, and CXCL11 expression [38]. This suggests a strong correlation between CXCR3 and CXCL11 expression and SS severity.
These studies collectively underscore a significant association between CXCL11 and SS. Further research, particularly focusing on mechanisms, is essential to elucidate CXCL11's specific role in SS development.
IL-10RB, a surface receptor for IL-10 cytokines, functions in inflammatory and immune regulation through the IL-10 receptor (IL10R) complex [39]. IL-10's anti-inflammatory and pro- inflammatory effects vary based on its binding affinity with different receptors (IL-10RA and IL-10RB) [40]. While numerous studies have focused on IL-10 and SS [41,42], the specific relationship between IL- 10RB and SS has not been extensively reported. Elevated IL-10 levels in SS patients' serum, particularly in those with liver involvement, have been documented [43]. Additionally, a positive correlation between IL-10 levels and autoantibodies has been observed [44], suggesting a significant link between IL-10 and SS.
IL-10RB is crucial for the IL-10 induced signal transduction, acting as a necessary auxiliary chain in the active IL-10 receptor complex. IL- 10 stimulation of B cells and T helper cell 2 (Th2) cells may lead to autoantibody production. IL-10RB interacts with IL-10 through the JAK-STAT pathway, where IL-10 receptor complex activation results in transcription factor signal transduction and phosphorylation of transcription activating factor STAT3. This mediation of IL-10 functions could impact multiple factor interactions and signal transductions, potentially disrupting immune homeostasis and contributing to various immune diseases [45,46].
In studies examining IL-10RB's role in other conditions, its pleiotropic nature has been highlighted. For instance, blocking IL-10RB in allergic asthma was found to affect STAT3 phosphorylation, reducing allergic airway inflammation [47]. In synovial tissue of Rheumatoid Arthritis (RA) rabbits, elevated IL-10RB and abnormal activation of the JAK-STAT pathway were observed, with IL-22R expression in the L-10 family increasing and IL-10RB expression decreasing after treatment, mitigating synovial inflammation [48].
These findings underscore IL-10RB's multifaceted roles and warrant further investigation into its association with SS. Our MR analysis identified a positive correlation between IL-10RB and SS risk. Given the reported close relationship between JAK-STAT and SS development [49,50], the potential role of abnormal IL-10RB expression and mediation of JAK- STAT pathway activation in SS pathogenesis merits in-depth exploration.
To the best of our knowledge, this study is the inaugural one to establish a genetic causal relationship between cytokines and Sjogren's Syndrome (SS) using extensive Genome-Wide Association Study (GWAS) data. The considerable reduction in lifestyle and environmental factor impacts lends enhanced reliability to the results. Additionally, the genetic correlation between cytokines and SS was further investigated through Linkage Disequilibrium Score Regression (LDSC) analysis, thereby strengthening the validity of our findings. The research suggests that variations in cytokine cycling levels might influence SS pathogenesis. Nonetheless, the relationship between less common cytokines such as IL-10RB and SS remains under-researched, presenting a substantial area for further study.
Despite efforts to mitigate various influencing factors, this research is not without limitations. The unavailability or absence of data in many SS GWAS datasets [3], restricted us to a single, albeit large, data source, potentially introducing minor biases. Additionally, the data being derived from a single racial group limits the generalizability of our findings to other populations. Consistent with other studies [51-53], the use of a P-value threshold of <5 × 10^-6 due to a limited number of SNPs may have resulted in false positives. However, the robustness of our results is supported by high F-statistics. Lastly, the precision of LDSC regression analysis could have led to the omission of certain cytokines potentially associated with SS.
Conclusion
In conclusion, this study identified a genetic correlation and causal relationship between cytokine levels (specifically IL10RB and CXCL11) and SS, utilizing MR analysis with LDSC regression and public GWAS data. Further research is necessary to elucidate the biological mechanisms through which cytokine cycling levels influence SS.
Acknowledgements
The author expresses gratitude to every participant in the Sjogren's Syndrome cohort study, as well as to every member involved in cytokine research.
Authors’ Contributions
Conceptualization, Z.J. and X.C.; methodology, Z.J., L.X. and X.C.; software, W.Y., S.Q., J.J and Z.X.; validation, Z.J., L.X. and W.Y.; formal analysis, X.M., Q.W. and X.C.; investigation, F.T. and T.Z.; resources, W.K.; data curation, Q.W., C.M. and J.J; writing—original draft preparation, Z.J. and X.C.; writing—review and editing, F.T.; visualization, X.M. and T.Z.; supervision, W.K.; project administration, F.T.; funding acquisition, W.K. All authors have read and agreed to the published version of the manuscript. ding acquisition, F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guizhou Provincial Clinical Research Center for Rheumatism and Immunology (Qian Kehe Platform Talent No. 2202); Key Laboratory of Integrated Traditional Chinese and Western Medicine in the Prevention and Treatment of Disease Transformation in Higher Education Institutions in Guizhou Province (Qian Jiao Ji [2023] No. 017); Guizhou University of Traditional Chinese Medicine National and Provincial Science and Technology Innovation Talent Team Cultivation Project (Guizhou University of Traditional Chinese Medicine TD NO.[2022]004); Guizhou Provincial Health Commission Science and Technology Fund Project (gzwkj2023-144).
Availability of Data and Materials
All data used in this work are presented in the Additional files that accompany the manuscript and are available in the original publications.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
None.
References
- Bjordal O, Norheim KB, Rodahl E, Jonsson R, Omdal R (2020) Primary Sjogren's syndrome and the eye. Surv Ophthalmol 65:119-32.
[Crossref] [Google Scholar] [PubMed]
- Grange L, Chalayer E, Boutboul D, Paul S, Galicier L, et al. (2022) TAFRO syndrome: A severe manifestation of Sjogren's syndrome? A systematic review. Autoimmun Rev 21:103137.
[Crossref] [Google Scholar] [PubMed]
- Thorlacius GE, Bjork A, Wahren-Herlenius M (2023) Genetics and epigenetics of primary Sjogren syndrome: implications for future therapies. Nat Rev Rheumatol 19: 288-306.
[Crossref] [Google Scholar] [PubMed]
- Yura Y, Hamada M (2023) Outline of salivary gland pathogenesis of sjogren's syndrome and current therapeutic approaches. Int J Mol Sci 24.
[Crossref] [Google Scholar] [PubMed]
- Mavragani CP, Moutsopoulos HM (2009) The geoepidemiology of Sjogren's syndrome. Autoimmun Rev 9:A305-310.
[Crossref] [Google Scholar] [PubMed]
- Goulabchand R, Malafaye N, Jacot W, Witkowski DVP, Morel J, et al. (2021) Cancer incidence in primary Sjogren's syndrome: Data from the French hospitalization database. Autoimmun Rev 20: 102987.
[Crossref] [Google Scholar] [PubMed]
- Yang TH, Cheng YF, Chen CS, Lin HC (2023) Increased prevalences of head and neck cancers in patients with Sjogren's syndrome. Head Neck 45:2874-2881.
[Crossref] [Google Scholar] [PubMed]
- Drosos GC, Vedder D, Houben E, Boekel L, Atzeni F, et al. (2022) EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis 81:768-79.
[Crossref] [Google Scholar] [PubMed]
- Fierro JJ, Prins JR, Verstappen GM, Bootsma H, Westra J, et al. (2023) Preconception clinical factors related to adverse pregnancy outcomes in patients with systemic lupus erythematosus or primary Sjogren's syndrome: A retrospective cohort study. Rmd Open 9.
[Crossref] [Google Scholar] [PubMed]
- Ramos-Casals M, Brito-Zeron P, Bombardieri S, Bootsma H, de Vita S, et al. (2020) EULAR recommendations for the management of Sjogren's syndrome with topical and systemic therapies. Ann Rheum Dis 79:3-18.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, et al. (2021) Cytokines: From clinical significance to quantification. Adv Sci (Weinh) 8: e2004433.
[Crossref] [Google Scholar] [PubMed]
- Aung T, Grubbe WS, Nusbaum RJ, Mendoza JL (2023) Recent and future perspectives on engineering interferons and other cytokines as therapeutics. Trends Biochem Sci 48:259-73.
[Crossref] [Google Scholar] [PubMed]
- Hou X, Hong X, Ou M, Meng S, Wang T, et al. (2022) Analysis of gene expression and tcr/b cell receptor profiling of immune cells in primary sjogren's syndrome by single-cell sequencing. J Immunol 209:238-49.
[Crossref] [Google Scholar] [PubMed]
- Blokland S, Flessa CM, van Roon J, Mavragani CP (2021) Emerging roles for chemokines and cytokines as orchestrators of immunopathology in Sjogren's syndrome. Rheumatology 60:3072-87.
[Crossref] [Google Scholar] [PubMed]
- Dong C (2021) Cytokine regulation and function in t cells. Annu Rev Immunol 39:51-76.
[Crossref] [Google Scholar] [PubMed]
- Yin Z, Dai J, Deng J, Sheikh F, Natalia M, et al. (2012) Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol 189:2735-45.
[Crossref] [Google Scholar] [PubMed]
- Jang DI, Lee AH, Shin HY, Song HR, Park JH, et al. (2021) The role of tumor necrosis factor alpha (TNF-alpha) in autoimmune disease and current TNF-alpha Inhibitors in therapeutics. Int J Mol Sci 22.
[Crossref] [Google Scholar] [PubMed]
- Yoshimoto K, Suzuki K, Takei E, Ikeda Y, Takeuchi T (2020) Elevated expression of BAFF receptor, BR3, on monocytes correlates with B cell activation and clinical features of patients with primary Sjogren's syndrome. Arthritis Res Ther 22:157.
[Crossref] [Google Scholar] [PubMed]
- Tam V, Patel N, Turcotte M, Bosse Y, Pare G, et al. (2019) Benefits and limitations of genome-wide association studies. Nat Rev Genet 20:467-84.
[Crossref] [Google Scholar] [PubMed]
- Lee JJ, McGue M, Iacono WG, Chow CC (2018) The accuracy of LD score regression as an estimator of confounding and genetic correlations in genome-wide association studies. Genet Epidemiol 42:783-95.
[Crossref] [Google Scholar] [PubMed]
- Davies NM, Holmes MV, Davey SG (2018) Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj 362: k601.
[Crossref] [Google Scholar] [PubMed]
- Skrivankova VW, Richmond RC, Woolf B, Davies NM, Swanson SA, et al. (2021) Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. Bmj 375:n2233.
[Crossref] [Google Scholar] [PubMed]
- Smith GD, Ebrahim S (2003) 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32:1-22.
[Crossref] [Google Scholar] [PubMed]
- Ahola-Olli AV, Wurtz P, Havulinna AS, Aalto K, Pitkanen N, et al. (2017) Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am J Hum Genet 100:40-50.
[Crossref] [Google Scholar] [PubMed]
- Zhao JH, Stacey D, Eriksson N, Macdonald-Dunlop E, Hedman AK, et al. (2023) Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol 24:1540-51.
[Crossref] [Google Scholar] [PubMed]
- Kurki MI, Karjalainen J, Palta P, Sipila TP, Kristiansson K, et al. (2023) FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613:508-18.
[Crossref] [Google Scholar] [PubMed]
- Kappelmann N, Arloth J, Georgakis MK, Czamara D, Rost N, et al. (2021) Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-sample mendelian randomization study. Jama Psychiatry 78:161-70.
[Crossref] [Google Scholar] [PubMed]
- Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, et al. (2015) LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 47:291-95.
[Crossref] [Google Scholar] [PubMed]
- Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG (2017) Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology 28:30-42.
[Crossref] [Google Scholar] [PubMed]
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, et al. (2018) The MR-base platform supports systematic causal inference across the human phenome. Elife 7.
[Crossref] [Google Scholar] [PubMed]
- Saxena S, Singh RK (2021) Chemokines orchestrate tumor cells and the microenvironment to achieve metastatic heterogeneity. Cancer Metastasis Rev 40:447-76.
[Crossref] [Google Scholar] [PubMed]
- Cui LY, Chu SF, Chen NH (2020) The role of chemokines and chemokine receptors in multiple sclerosis. Int Immunopharmacol 83:106314.
[Crossref] [Google Scholar] [PubMed]
- Crescioli C, Corinaldesi C, Riccieri V, Raparelli V, Vasile M, et al. (2018) Association of circulating CXCL10 and CXCL11 with systemic sclerosis. Ann Rheum Dis 77:1845-46.
[Crossref] [Google Scholar] [PubMed]
- Yang P, Tan J, Yuan Z, Meng G, Bi L, et al. (2016) Expression profile of cytokines and chemokines in osteoarthritis patients: Proinflammatory roles for CXCL8 and CXCL11 to chondrocytes. Int Immunopharmacol 40:16-23.
[Crossref] [Google Scholar] [PubMed]
- Yoon KC, Park CS, You IC, Choi HJ, Lee KH, et al. (2010) Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci 51:643-50.
[Crossref] [Google Scholar] [PubMed]
- Aota K, Yamanoi T, Kani K, Nakashiro KI, Ishimaru N, et al. (2018) Inverse correlation between the number of CXCR3(+) macrophages and the severity of inflammatory lesions in Sjogren's syndrome salivary glands: A pilot study. J Oral Pathol Med 47:710-18.
[Crossref] [Google Scholar] [PubMed]
- Ogawa N, Shimoyama K, Kawanami T (2005) Molecular mechanisms of salivary gland destruction in patients with Sjogren's syndrome. JPN J Clin Immunol 28:10-20.
[Crossref] [Google Scholar] [PubMed]
- Li D, Onodera S, Deng S, Alnujaydi B, Yu Q, et al. (2022) Alternate-day fasting ameliorates newly established Sjogren's syndrome-like sialadenitis in non-obese diabetic mice. Int J Mol Sci 23.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Wong K, Ouyang W, Rutz S (2019) Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol 11.
[Crossref] [Google Scholar] [PubMed]
- Fernandez-Santamaria R, Satitsuksanoa P (2022) Engineered IL-10: A matter of affinity. Allergy 77:1067-69.
[Crossref] [Google Scholar] [PubMed]
- Colafrancesco S, Ciccacci C, Priori R, Latini A, Picarelli G, et al. (2019) Stat4, Traf3ip2, Il10, and Hcp5 polymorphisms in Sjogren's syndrome: Association with disease susceptibility and clinical aspects. J Immunol Res 2019:7682827.
[Crossref] [Google Scholar] [PubMed]
- Abughanam G, Elkashty OA, Liu Y, Bakkar MO, Tran SD (2019) Mesenchymal stem cells extract (mscse)-based therapy alleviates xerostomia and keratoconjunctivitis sicca in sjogren's syndrome-like disease. Int J Mol Sci 20:4750.
[Crossref] [Google Scholar] [PubMed]
- Garcic-Carrasco M, Font J, Filella X, Cervera R, Ramos-Casals M, et al. (2001) Circulating levels of Th1/Th2 cytokines in patients with primary Sjogren's syndrome: correlation with clinical and immunological features. Clin Exp Rheumatol 19:411-15.
[Google Scholar] [PubMed]
- Vazquez-Villamar M, Palafox-Sanchez CA, Munoz-Valle JF, Valle Y, Orozco-Barocio G, et al. (2015) Analysis of IL10 haplotypes in primary Sjogren's syndrome patients from Western Mexico: Relationship with mRNA expression, IL-10 soluble levels, and autoantibodies. Hum Immunol 76:473-79.
[Crossref] [Google Scholar] [PubMed]
- Grk M, Miskovic R, Jeremic I, Basaric M, Dusanovic PM, et al. (2023) Association of IL10RA, IL10RB, and IL22RA polymorphisms/haplotypes with susceptibility to and clinical manifestations of SLE. Int J Mol Sci 24.
[Crossref] [Google Scholar] [PubMed]
- Saxton RA, Tsutsumi N, Su LL, Abhiraman GC, Mohan K, et al. (2021) Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science 371.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Gao S, Zhang J, Li C, Li H, et al. (2021) Interleukin-22 attenuates allergic airway inflammation in ovalbumin-induced asthma mouse model. Bmc Pulm Med 21:385.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Li JS, Yang SQ, Zhang XX, Zhang TS, et al. (2007) Influence of moxibustion on JAK-STAT signal transduction pathways of synovial cells in rheumatoid arthritis rabbits. Zhen Ci Yan Jiu 32:75-82.
[Google Scholar] [PubMed]
- Hong X, Wang X, Rang X, Yin X, Zhang X, et al. (2022) The shared mechanism and candidate drugs of multiple sclerosis and Sjogren's syndrome analyzed by bioinformatics based on GWAS and transcriptome data. Front Immunol 13:857014.
[Crossref] [Google Scholar] [PubMed]
- Benucci M, Bernardini P, Coccia C, de Luca R, Levani J, et al. (2023) JAK inhibitors and autoimmune rheumatic diseases. Autoimmun Rev 22:103276.
[Crossref] [Google Scholar] [PubMed]
- Liu B, Lyu L, Zhou W, Song J, Ye D, et al. (2023) Associations of the circulating levels of cytokines with risk of amyotrophic lateral sclerosis: a mendelian randomization study. Bmc Med 21:39.
[Crossref] [Google Scholar] [PubMed]
- Yeung C, Schooling CM (2021) Systemic inflammatory regulators and risk of Alzheimer's disease: a bidirectional mendelian-randomization study. Int J Epidemiol 50:829-40.
[Crossref] [Google Scholar] [PubMed]
- Luo J, le Cessie S, Blauw GJ, Franceschi C, Noordam R, et al. (2022) Systemic inflammatory markers in relation to cognitive function and measures of brain atrophy: a Mendelian randomization study. Geroscience 44:2259-70.
[Crossref] [Google Scholar] [PubMed]
Citation: Jiang Z, Cai X, Yao X, Zhang S, Lan W, et al. (2024) Association between Cytokine Cycling Levels and Sjogren's Syndrome: Genetic Correlation and Bidirectional Mendelian Randomization Study. Diagnos Pathol Open 9: 225. DOI: 10.4172/2476-2024.9.1.225
Copyright: © 2024 Jiang Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1006
- [From(publication date): 0-2024 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 774
- PDF downloads: 232