Research Article Open Access
Assessment of Triacylglycerol Content in Chlorella vulgaris Cultivated in a Two-Stage Process
Raquel R dos Santos1*, Claudete N Kunigami2, Donato AG Aranda3 and Cláudia MLL Teixeira11Divisão de Energia, Instituto Nacional de Tecnologia, 20081-312 Rio de Janeiro - RJ, Brazil
2Divisão de Química Analítica, Instituto Nacional de Tecnologia, 20081-312 Rio de Janeiro - RJ, Brazil
3Departamento de Engenharia Química, Escola de Química, Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro - RJ, Brazil
- Corresponding Author:
- Raquel R dos Santos
Divisão de Energia
Instituto Nacional de Tecnologia
Rio de Janeiro, Rio de Janeiro 20081-312, Brazil
Tel: 55 21 996574980
E-mail: raquel.c.rezende@gmail.com
Received date: October 15, 2015; Accepted date: November 30, 2015; Published date: December 07, 2015
Citation: dos Santos RR, Kunigami CN, Aranda DAG, Teixeira CMLL (2015) Assessment of Triacylglycerol Content in Chlorella vulgaris Cultivated in a Two- Stage Process. J Biotechnol Biomater 5:212. doi:10.4172/2155-952X.1000212
Copyright: © 2015 dos Santos RR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Chlorella vulgaris cultivation in two-stage process was applied to increase the lipid productivity without compromising the biomass productivity. At the first stage the microalgae was cultivated under nutrient-sufficient conditions to obtain a maximized cell density, at the second stage the nitrate conditions are changed to trigger the accumulation of TAG. During the first stage, the maximum biomass productivity (0.032 g.L-1.d-1) was observed after 13 days under nutrient-sufficient conditions with 1.21 g.L-1 NaNO3 and 0.00449 g.L-1 K2HPO4. The maximum lipid content (25.4%), lipid productivity (7.5 mg.L-1.d-1) and TAGs content in total lipids (41.3%) were favored by the nitrogen-starvation conditions for more 4 days, at the second stage. The oil extracted at the second stage contained lower percentage of PUFAs being more suitable for the production of biodiesel when compared with the oil extracted at the first stage. This two-stage phototrophic process is promising to provide a more efficient way for large-scale production of algal biomass and biodiesel production.
Keywords
Microalgae; Biomass productivity; Nitrogen starvation; Triacylglycerol (TAG) content; Two-stage process
Introduction
Microalgae are prokaryotic or eukaryotic photosynthetic microorganisms that grow at an exceptional fast rate in open oceans, rocky shores and fresh water habitats including rivers, lakes, ditches and ponds [1]. The biodiversity of microalgae is enormous and represents an almost untapped resource [2]. This biodiversity permits the use of microalgae in biotechnology applications as pharmaceutical and nutraceutical products as well as raw material for biofuels production [1].
Microalgae lipids have attracted attention as future raw materials for biodiesel synthesis, among other factors, due to the microalgae potential of attaining higher lipid productivity in relation to the oilseed crops and the modulation of the biochemical composition of the microalgae biomass by varying growth conditions [3]. Accordingly, the choice of the most appropriate condition for the cultivation of microalgae is of paramount importance to the viability of the process.
Usually, the biochemical composition of microalgae in exponential phase and growth in batch respects to the following order: protein > carbohydrate = lipid > nucleic acid. However, under conditions of nutritional stress and aging culture, this ratio can change with the increase of lipids and decrease of the protein concentration [4].
Being the nitrogen essential for the synthesis of amino acids, limiting conditions of this nutrient makes it impossible to the synthesis of other proteins involved in the cell division. When the cells are no longer able to divide, the lipids are synthesized and stored within cells as an energy source [5]. For this reason, it is easy to realize that the amount of nitrogen is an important intracellular factor involved in lipid biosynthesis. On one side nitrogen starvation favors biosynthesis of intracellular lipids in cells already developed, on the other, it can affect the cell growth of new cells. In this sense, increasing the lipid content and getting biomass simultaneously become a challenge for researchers.
A two-stage culture strategy can be an option for enhancing lipid productivity [6]. In order to increase the biomass productivity, a high-density culture of microalgae can be obtained under favorable conditions of temperature [7], light intensity [8], CO2 concentration [9] and other nutrients with high mass transfer rates. In relation to the increase of the lipid content in cells, other cultivation conditions such as nitrogen deprivation [8-10], phosphate limitation [11] and iron supplementation [11] have been tested. In some works, the two-stage culture strategy involves the application of heterotrophic cultivation [12-14] at the second stage, being used glycerol, glucose, sucrose or wastewater derived from industrial and agricultural as organic carbon.
Microalgae produce a large variety of lipid-like compounds, such as waxes, sterols, hydrocarbons, and glycerolipids. Glycerolipids are characterized when the carboxylate end of the fatty acid molecule is bonded to an uncharged head group (e.g. glycerol) and can be divided into two large subclasses based on their specific function (e.g. energy storage or cell membrane lipids). Cell membrane lipids consists of fatty acids ester-bonded to a glycerol backbone and is categorized according to its number of fatty acids. Lipids with three fatty acyl groups attached to glycerol backbone are known as triacylglycerols (TAG) [15].
TAG is the major compound of the oils used for biodiesel production and therefore the main raw material for its production. Other lipid molecules, which have fatty acid in its structure, can also be a raw material for biodiesel production in principle. Lipids as hydrocarbons and sterols cannot be used for biodiesel (as fatty acid methyl esters) production. In this case of hydrocarbon it is possible to produce a biooil with fuel properties but it is not appropriately a biodiesel. Thus, it is very important to quantify this compound in total lipids to predict the potential of microalgae biodiesel. In fact, when we have a total lipid value and make comparisons among different results from different cultivation conditions we might sub or super estimate the potential for biodiesel production since in these total lipids we can have different percentages of TAG [16]. Several investigations focus on the total lipids determination to indicate biodiesel production potential in studies where two-stage cultivation was carried out but in many cases, the TAG content in the total lipids has not been considered.
In the present study, two-stage cultivation strategy was applied in order to enhance lipid productivity. At the first stage, the microalgae were cultivated under nutrient-sufficient conditions to obtain a maximized cell density. At the second stage, the nitrate conditions are changed to trigger the accumulation of TAG. Keeping in mind the importance of TAG determination for a real potential for biodiesel production, evaluation of this compound was quantified in total lipids extracted from algal biomass.
Methods
Strain and maintenance
The microalgae Chlorella vulgaris was kindly donated by Dr. Armando Augusto Henriques from Federal University of São Carlos, Brazil. The strain was preserved in tubes containing 8 mL of sterile WC medium [17]. The tubes were kept in a germination chamber under a photon flux density of 20 μmol of photons.m-2s-1 provided by fluorescent lamps and 21±1°C manually shaken every 48 hours.
The WC medium was composed of TRIS buffer (0.5 gL-1), NaNO3 (0.085 gL-1), NaHCO3 (0.0126 g.L-1), CaCl2.2H2O (0.03676 g.L-1), MgSO4.7H2O (0.03697 g.L-1), K2HPO4 (0.00871 g.L-1), 1% H3BO3 (0.1 mL.L-1), vitamin solution (1 mL.L-1) and trace metals solution (1 mL.L- 1). The initial pH was adjusted to 8.5 with HCl 1M. The vitamin solution was composed of thiamine (0.1 g.L-1), cyanocobalamin (0.0005 g.L-1) and biotin (0.0005 g.L-1) being filtered through 0.22 μm membrane. The trace metals solution was composed of CuSO4.5H2O (0.0098 g), ZnSO4.7H2O (0.022 g), CoCl2.6H2O (0.01 g), MnCl2.4H2O (0.18 g), NaMoO4.2H2O (0.0063 g) and chelated iron (1 L). The chelated iron was composed of Na2EDTA (4.36 g.L-1) and FeCl3.6H2O (3.5 g.L-1).
Microalgae cultivation process
First stage cultivation: nutrient-sufficient conditions: To obtain the inoculum, the cells were grown in 500-mL Erlenmeyer flasks containing 300 mL of WC medium. The flasks were kept under constant agitation of 180 rpm, photon flux density of 100 μmol of photons.m-2s-1 provided by fluorescent lamps and 25±2°C. The culture was cultivated until it achieved an optical density at 730 nm (OD730nm) of approximately 0.8 (exponential phase).
The inoculum obtained was transferred to clear 6-L bottles containing 5 L of WC medium. At this stage, the WC medium was modified in relation to K2HPO4 (0.00449 g.L-1) and NaNO3 (1.21 g.L- 1) concentrations. This change was made to obtain a maximized cell density, what was observed in previous experiments. Before the transfer, the cells were centrifuged and washed with the medium modified. The final OD730nm of the cellular suspension in the bottles with 5 L of medium was 0.2.
The cultivation was carried out in batch and kept under pneumatic stirring, a photon flux density of 100 μmol of photons.m-2s-1 and a room temperature of 25±2°C until they achieved an OD730nm around 1.0 (early stationary phase). The biomass obtained was harvested by centrifugation at 2607g for 10 minutes, being a part lyophilized and stored at 4°C until lipid (total lipids and TAG) content analyses and the other part was resuspended in bottles containing WC medium without nitrate.
Second stage cultivation: nitrate-starvation conditions: The cultivations were carried out in 6-L bottles containing 5 L of WC medium without nitrate. The WC medium used at this stage contained K2HPO4 (0.00449 g.L-1) and other nutrients (as described in item 2.1) except NaNO3. This medium was named stress medium. This nitratedeficient condition was carried out to trigger the accumulation of TAG in biomass obtained at the first stage. Before the transfer, the cells were washed with the medium used in the cultivation. The conditions were the same and the cultivation lasted more four days (late stationary phase). The stressed biomass was harvested by centrifugation at 2607g for 10 minutes, being lyophilized and stored at 4°C until lipid content analyses.
Monitoring of biomass in culture medium: The microalga grown in culture medium was monitored by measuring the optical density at 730 nm. The optical density was correlated with dry weight X (gL-1) by the following equations:
X = (OD730nm)/3.5275 (R2= 0.9925) Exponential phase
X = (OD730nm)/2.331 (R2= 0.8572) Stationary phase
The dry weight (DW) was obtained by vacuum filtering in glass fiber membrane (0.45 μm) previously weighed. After filtering, the membranes were dried in an oven at 105°C overnight. The dry membranes were cooled in a desiccator and weighed again. The weight of the dry biomass was calculated from the subtraction between the final weight and the initial weight of membrane.
The cellular concentration was calculated as g.L-1 in terms of DW. The biomass productivity at the end of each stage was calculated as g.L-1.d-1 by dividing the increment of biomass concentration by the cultivation time.
The specific growth rate (μ) of the culture was determined in the exponential phase exponential (during the first stage) and it was calculated according to the following formula:
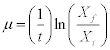 ,being
,being
t the total time of cell growth, Xf the final concentration of the culture and Xi the initial concentration of the culture. The result was expressed in d-1.
Monitoring of nitrate concentration in culture medium: Nitrate concentration in the culture medium was determined by a spectrophotometric method [18]. Culture samples were diluted with WC medium without nitrate and vacuum filtered in glass fiber membrane (0.45 μm). The nitrate concentration in filtrate was determined by UV spectrophotometer (GENESYS 10 UV-Vis spectrophotometer - Thermo Scientific) at 220 nm. The calibration curve between the absorbance and nitrate concentration (g.L-1) was established by using sodium nitrate as the standard.
Lipid content analyses
Lipid extraction: The lipids extraction was carried out using chloroform: methanol (2:1) [19]. All solvents used in the extraction were of chromatographic grade and were obtained from commercial source (Tedia Brazil). It was used a mass of 0.5 g of dry microalga for each 36 mL of the extracting mixture chloroform: methanol (2:1) at room temperature. This mixture was submitted to ultrasonic bath working at 40 kHz and producing an ultrasonic intensity of 34.74 W/L (Unique model 1800 USC – Indaiatuba, Brazil, 3.8 L, internal dimensions: 30 x 15.1 x 10 cm) for 20 min. The flask containing the mixture was put in the ultrasonic bath with the help of a metal support in order to be centralized and not touch in the bottom tank. Later the mixture was centrifuged at 2607g for 8 min at 4 ºC. The organic phase was carefully collected and transferred to other tube where 9 mL of 0.88% KCl was added. At this moment, there were two phases and the upper phase was discarded with a pipette. Then 4.5 mL of chloroform:methanol:water (3:48:47) was added to the lower phase, so that two phases formed again. Again, the upper phase was discarded with pipette. The washing with chloroform:methanol:water was repeated twice. The organic phase was carefully collected and the solvent evaporated with a rotary evaporator at 40 ºC. The lipid fraction was dried to constant weight in an oven with air circulation at 30 ºC. The lipid mass was determined by gravimetric analysis.
The lipid productivity at the end of each stage was calculated as mg.L-1.d-1 by dividing the lipid content obtained by volume culture by the cultivation time.
Quantification of triacylglycerol (TAG): The mass of TAG in the total lipids solubilized in isopropyl alcohol was determined by the glycerol-3-phosphate oxidase-p-chlorophenol method [20] (monoreagent triglycerides K117; Bioclin, Brazil) using triolein as the standard.
Fatty acid profiles: The transesterification reaction in the total lipids fraction was carried out according to ASTM D 3457 standard method. The analysis was performed by gas chromatography and detection by mass spectrometry (Agilent, 6890N model to gas chromatography and 5975 model to mass spectrometry, USA). It was used an HP-5MS column (30 m length x 0.25 mm i.d. x 0.25 μm stationary phase), split injection of 10:1 and injection volume of 1μL. The initial temperature of the oven was 40°C, which was increased until 300 ºC at a temperature gradient of 10°C.min-1. Helium was used as the carrier gas with flow of 1 mL.min-1. The fatty acids were identified by consulting the Wiley 7 Nist 05 digital library’s mass spectral database.
Statistical analysis
The results obtained were evaluated using a one-way ANOVA and Turkey test. The level of significance adopted was p < 0.05.
Results and Discussion
Assessment of biomass, total lipid and TAG
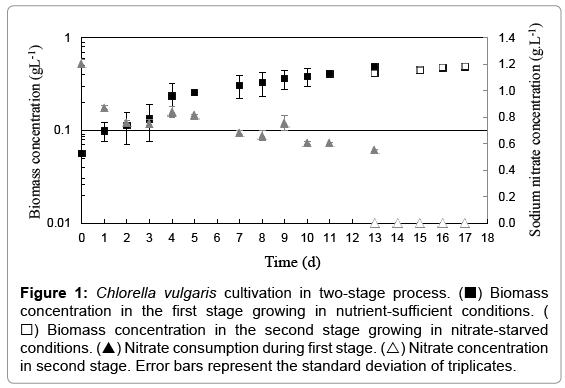
Microalga growth and nitrate concentration throughout the twostage of cultivation are presented in Figure 1. Cultivation in stage 1 (nutrient sufficient) was carried out with initial cell concentration and nitrate concentration of 0.056 g.L-1 and 1.21 gL-1, respectively. The cultivation proceeded until the cell concentration achieve 0.485 gL-1 (OD730nm around 1.0 - early stationary phase), following the biomass obtained by centrifugation that was resuspended in stress medium (without nitrate). The harvest efficient was around 97% (determined through OD730nm of the cellular suspension before centrifugation and OD730nm of the final supernatant). In this new cell suspension, the initial cell concentration was 0.412 gL-1 (OD730nm around 0.961) and the cells were kept under illumination for four more days (late stationary phase). In the phase 1 it was observed a decreasing of around 50% of nitrate concentration in the cell suspensions (Figure 1).
Figure 1: Chlorella vulgaris cultivation in two-stage process. (■) Biomass concentration in the first stage growing in nutrient-sufficient conditions. (□) Biomass concentration in the second stage growing in nitrate-starved conditions. (▲) Nitrate consumption during first stage. (△) Nitrate concentration in second stage. Error bars represent the standard deviation of triplicates.
Table 1 shows results of biomass concentration (g.L-1), biomass productivity (g.L-1.d-1), specific growth rate (d-1), lipid content (%), lipid productivity (mg.L-1.d-1) and TAG content in total lipids (%) for the two-stage of cultivation. The cell cultivation in nutrient-sufficient conditions (stage 1) for 13 days resulted in 0.032 g.L-1.d-1 of biomass productivity with 0.151 d-1 of specific growth rate, 19.6% of total lipids with 6.7 mg.L-1.d-1of productivity and 27.7% of TAG in total lipids.
| Parameters | First Stage (early stationary phase) |
Second Stage (late stationary phase) |
|---|---|---|
| Total time (d) Biomass concentration (g.L-1) µ (d-1) (exponential phase) Biomass productivity (g.L-1.d-1) Lipid content (%) Lipid productivity (mg.L-1.d-1) TAG content (% in total lipids) |
13 0.485 0.151 0.032 ± 0.001 19.6 ± 0.6 6.7 ± 0.2 27.7 ± 1.1 |
17 0.488 - 0.027 ± 0.004 25.4 ± 0.4 7.5 ± 0.1 41.3 ± 0.1 |
Table 1: Comparative analysis between the first and second stage cultivation. The productivity data are reported as mean ± standard deviation of triplicates.
Liang et al. [21] compared the biomass productivity in C. vulgaris when maintained under various culture conditions. After 23 days cell growth under autotrophic conditions (starting inoculum around of 0.1 g.L-1), it was obtained 10 mg.L-1.d-1 of biomass productivity, 38% of lipid content and 4 mg.L-1.d-1 of lipid productivity. A greater biomass productivity in relation to the quoted publication attained in this investigation was provided by the nitrate-sufficient conditions, which made possible the development of various metabolic reactions necessary for cell growth. In addition, a shorter cultivation time was a result of this satisfactory condition for growing. The reduction of the cultivation time reduces process costs such as the energy necessary for the functioning of the cultivation system. The cost of increasing the nitrate concentration is often more advantageous than the high-energy expenditure required for maintenance culture system in a pilot plant [22].
From stage 1 to stage 2, as shown in Table 1, in spite of the fact that the biomass productivity has decreased to 0.027 g.L-1.d-1 since the lipid content increased (25.4 %) and the lipid productivity increased (7.5 mg.L-1.d-1 ). Another very interesting and important result was TAG content in total lipids that increased from 27.7% to 41.3%.
These results indicate that the proposed two-stage culture strategy is effective to increase TAG production from microalgae. This may be due to two factors: (1) Lack of nitrate limited the protein biosynthesis necessary for biomass production in photosynthesis thus increasing the content lipid (store fatty acids as TAG). (2) Photosynthesis and the electron transport produce ATP and NADPH as energy “storage” and electron carrier metabolites, respectively. These metabolites are required during carbon fixation by key enzymes resulting in ADP and NADP+. Under favorable growth conditions, this cycle is regenerated via photosystems; however, under adverse growth conditions (e.g. lack of nitrate), the pool of NADP+ and ADP can become depleted. This can lead to a potentially dangerous situation for the cell because photosynthesis is mainly controlled by light availability, and can not be shut off completely. TAG synthesis requires NADPH and ATP; therefore, increased TAG synthesis replenishes the pool of required electron acceptors in the form of NADP+ [16].
Determination of the fatty acid profile
The total lipids obtained at the two phases were assessed for fatty acid profile. Table 2 summarizes the fatty acid composition of the total lipids of C. vulgaris at the first stage and the second stage cultivation. Unsaturated oils produce a biodiesel that is more susceptible to oxidation due to their instability when stored for long periods. Therefore, the fatty acid profile is mainly evaluated to detect fatty acids containing two or more double bonds (PUFAs) [23]. The oil extracted at the second stage contains lower percentage of PUFAs being more suitable for the production of biodiesel when compared with the oil extracted at the first stage (Table 2).
| Acid name | Composition | Content in first stage (%) | Content in second stage (%) |
|---|---|---|---|
| Myristic Palmitic Palmitoleic Stearic Linoleic γ Linolenic Arachidonic |
C14:0 C16:0 C16:1 C18:0 C18:2 C18:3 C20:4 |
1.96 17.57 13.13 2.78 28.53 1.20 1.38 |
1.37 23.65 12.91 1.77 12.86 0.69 0.68 |
Table 2: Principal acids obtained after the first and second stage cultivation.
The two-stage operation strategy is currently the most widely studied strategy to increase the productivity of important biomolecules as astaxanthin [24], protein [25], carbohydrate [26,27] and lipid. In terms of C. vulgaris, although there are many reports about its lipid productivity of in nitrate-starvation conditions and more recently some reports in two-stage process, it often becomes difficult to compare the data obtained in these studies because cultivation and stress conditions as light intensity, aeration and temperature, as well as composition of the stress medium are different. Besides this, analytical methods employed in the evaluations of total lipids differ among the works. Another example is the monitoring of cell density by measuring optical density in diverse wavelengths; depending on the used wavelength, cell density can be overestimated or underestimated because the absorption of cell pigments in the some ranges of wavelength used.
Mujtaba et al. [6] evaluated the lipid production by C. vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. At the first stage, the microalgae was cultivated photoautotrophically with air at a rate of 0.15 vvm supplied with 4% CO2, continuous illumination (200 μmol.m-2s-1 ), temperature of 25°C, with and without N-feeding. At the second stage, different phosphate concentrations, light intensities, extent of aeration, concentrations and sources of inorganic carbon were applied to induce quick lipid accumulation. The final content lipid was 53% in dry cell weight. Since lipids were extracted by chloroform: methanol and this mixture extracts both neutral lipids (i.e., TAG) by chloroform and polar lipids (i.e., phospholipids, glycolipids, sterols, carotenoids) by methanol, it is necessary to quantity TAG for a real potential for biodiesel production evaluation.
Yen and Chang [28] evaluated the two-stage cultivation process of C. vulgaris also. At the first stage, the cells were cultivated under autotrophy conditions with air at a rate of 1 vvm supplied with 2% CO2, continuous illumination (1300 μmol.m-2s-1), temperature of 25°C and agitation of 100 rpm. At the second stage, the cells were cultivated under mixotrophic conditions. Carbon sources (glucose or glycogen) were added once a day to the medium after the biomass stopped growing. At the end of the, the maximum content lipid obtained was less than 20% in dry cell weight. In this situation, the fatty acid profile obtained was 13.5% of C16:0, 2.2% of C18:0, 13.9% of C18:1, 57.1% of C18:2 and 13.2% of C18:3. Unlike the results obtained in this present study, the cultivation process would not significantly affect the fatty acid profile in order to generate a fuel more suitable for the production of biodiesel when compared with the oil extracted at the first stage.
According to the Brazilian crop of grains 2013/14, soybean cultivation (main raw material used to produce biodiesel) in Brazil has occupied an area of 30.000 hectares, with agricultural productivity of 2.841 kg.ha-1 per year (or 0.78 g.m-2.d-1). Imagining a microalgae cultivation tank with 30 cm of height where 1 L culture occupies an area of 0.0033 m2, the biomass productivity after 17 days in a culture with density of 0.5 gL-1 would be approximately 8.9 g.m-2.d-1. In this condition, the microalgae productivity can be 11 times higher than the soybean productivity. In addition to increased biomass productivity, the lipid content of microalgae (25.4% being) can be higher than the lipid content of soybean (between 18 and 21%) [29]. Although the soybean can have higher TAG content (approximately 95% in total lipids) when compared with microalgae (about 41.3% in total lipids in the present study), the lipid productivity is still superior and besides this the microalgae can have the fatty acid profile more suitable for the production of biodiesel. In the case of microalgae the lipid profile can change depending on the cultivation conditions whereas that the soybean has in average the following profile: 0.1% of C14:0, 11% of C16:0, 0.1% of C16:1, 4.0% of C18:0, 23% of C18:1, 53% of C18:2, 8% of C18:3 and 0.3% of C20:4 [29].
Conclusion
The maximum biomass productivity was observed in the cultivation with 1.21 g.L-1 NaNO3 and 0.00449 g.L-1 K2HPO4. The increment in the concentration of nitrate favored the development of metabolic reactions associated with cell growth. Except the biomass productivity, content and lipid productivity as well as TAGs content were favored by the starvation condition of nitrate in the extracellular medium. In the present study, it was possible to increase the TAG content without significant compromising of the final biomass productivity by microalgae cultivation in two-stage. In addition, based on these results, the total cultivation time was reduced. This two-stage phototrophic process is promising to provide a more efficient way for large-scale production of algal biomass and biodiesel production.
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP) for their financial support of this work.
References
- Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renew SustEnerg Rev 14: 217-232.
- Stengel DB, Connan S, Popper ZA (2011) Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. BiotechnolAdv 29: 483-501.
- Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, et al. (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res 1: 20-43.
- Tokusoglu Ö, Ünal MK (2003) Biomass nutrient profiles of three microalgae: Spirulinaplatensis, Chlorella vulgaris and Isochrisisgalbana. J Food Sci 68: 1144-1148.
- Singh A, Nigam PS, Murphy JD (2011) Mechanism and challenges in commercialisation of algal biofuels. BioresourTechnol 102: 26-34.
- Mujtaba G, Choi W, Lee C, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions.BioresourTechnol 123: 279-283.
- Converti A, Casazza AA, Ortiz EY, Perego P, Borghi MD (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsisoculata and Chlorella vulgaris for biodiesel production. ChemEng Process 48: 1146-1151.
- Lv J, Cheng L, Xu X, Zhang L, Chen H (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. BioresourTechnol 101: 6797-6804.
- Ördög V, Stirk WA, Bálint P, Staden JV, Lovász C (2012) Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J ApplPhycol 24: 907-914.
- Li Z, Yuan H, Yang J, Li B (2011) Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341. BioresourTechnol 102: 9128-9134.
- Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. BioresourTechnol 99: 4717-4722.
- Farooq W, Lee YC, Ryu BG, Kim BH, Kim HS, et al. (2013) Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. BioresourTechnol 132: 230-238.
- Zheng Y, Chi Z, Lucker B, Chen S (2012) Two-stage heterotrophic and phototrophic culture strategy for algal biomass and lipid production. BioresourTechnol 103: 484-488.
- Zhou W, Min M, Li Y, Hu B, Ma X, et al. (2012) A hetero-photoautotrophic two-stage cultivation process to improve wastewater nutrient removal and enhance algal lipid accumulation. BioresourTechnol 110: 448-455.
- Klok AJ, Lamers PP2, Martens DE2, Draaisma RB3, Wijffels RH2 (2014) Edible oils from microalgae: insights in TAG accumulation. Trends Biotechnol 32: 521-528.
- Fields MW, Hise A, Lohman EJ, Bell T, Gardner RD, et al. (2014) Sources and resources: importance of nutrients, resource allocation, and ecology in microalgal cultivation for lipid accumulation. ApplMicrobiolBiotechnol 98: 4805-4816.
- Guillard RRL, Lorenzen CJ (1979) Yellow-green algae with chlorophyllide. J Phycol 8: 10-14.
- Collos Y, Mornet F, Sciandra A, Waser N, Larson A, et al. (1999) An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. Journal of Applied Phycology 11: 179-184.
- dos Santos RR, Moreira DM2, Kunigami CN3, Aranda DA4, Teixeira CM5 (2015) Comparison between several methods of total lipid extraction from Chlorella vulgaris biomass. UltrasonSonochem 22: 95-99.
- Takagi M, Karseno, Yoshida T (2006) Effect of Salt Concentration on Intracellular Accumulation of Lipids and Triacylglyceride in Marine Microalgae Dunaliella Cells. J BiosciBioeng 101: 223-226.
- Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. BiotechnolLett 31: 1043-1049.
- Duerr EO, Edralin MR, Price NM (1997) Facilities requirements and procedures for the laboratory and outdoor raceway culture of Spirulina spp. J Mar Biotechnol 5: 1-11.
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, et al. (2008) Microalgaltriacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54: 621-639.
- Choi Y, Yun Y, Park JM, Yang J (2011) Determination of the time transferring cells for astaxanthin production considering two-stage process of Haematococcuspluvialis cultivation. Bioresour Techno 102: 11249-11253.
- Li Y, Mu J, Chen D, Han F, Xu H, et al. (2013) Production of biomass and lipid by the microalgae Chlorella protothecoides with heterotrophic-Cu(II) stressed (HCuS) coupling cultivation. BioresourTechnol 148: 283-292.
- Zhu S, Wang Y, Huang W, Xu J, Wang Z, et al. (2014) Enhanced accumulation of carbohydrate and starch in Chlorella zofingiensis induced by nitrogen starvation. ApplBiochemBiotechnol 174: 2435-2445.
- Sun X, Cao Y, Xu H, Liu Y, Sun J, et al. (2014) Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochlorisoleoabundans HK-129 by a two-stage process. BioresourTechnol 155: 204-212.
- Yen HW, Chang JT (2013) A two-stage cultivation process for the growth enhancement of Chlorella vulgaris. Bioprocess BiosystEng 36: 1797-1801.
- Gunstone F.D (2002) Vegetable oils in food technology: composition, properties and uses. CRC Press LLC, Unites States America and Canada.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 11291
- [From(publication date):
December-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10368
- PDF downloads : 923