Research Article Open Access
Assessment of Toxic Activity of Heat Labile Toxin from Bordetella pertussis Vaccine Strain 134
| Shivanandappa KC1*, Jagannathan S1, Pandiyarajan S2, Vijayakumar R3, Umadevi T4, Jeeva Kalaiselvan1 and B Sekar1 | |
| 1Pasteur Institute of India, Coonoor, The Nilgiris, Tamilnadu, India, PIN 643 103 | |
| 2Department of Microbiology, JJ college of Arts and Science, Pudukkottai, Tamilnadu, India, PIN 622 422 | |
| 3Department of Biochemistry, North Eastern Hill University, Shillong, Meghalaya, India, PIN 793 022 | |
| 4Department of Biochemistry, P.S.G Arts and Science College, Coimbatore, Tamilnadu, India, PIN 641 014 | |
| Corresponding Author : | Shivanandappa KC Pasteur Institute of India, Coonoor The Nilgiris, Tamilnadu, India. PIN 643 103 Tel: +91 9789327696 Fax: +91 423 2231655 E-mail: shivasham_27@yahoo.co.in |
| Received: April 18, 2015; Accepted: June 27, 2015; Published: July 05, 2015 | |
| Citation: Shivanandappa KC, Jagannathan S, Pandiyarajan S, Vijayakumar R, Umadevi T, et al. (2015) Assessment of Toxic Activity of Heat Labile Toxin from Bordetella pertussis Vaccine Strain 134. Biochem Physiol 4:168. doi:10.4172/2168-9652.1000168 | |
| Copyright: © 2015 Shivanandappa KC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Heat Labile toxin (HLT) is a mouse lethal toxin produced by all virulent Bordetella pertussis species. B. pertussis cells were dermo necrotizing and lethal when injected in to animals. Therefore in the present study the biological property of heat labile toxin of B. pertussis strain 134 was assessed before and after heat inactivation. The result found in SDS PAGE analysis of non-heat inactivated sample (NIS) shown apparent molecular mass of 102 kDa whereas it is absent in the Heat inactivated sample (HIS). The Mouse Weight Gain Test (MWGT) results shown that, after the culture cells were heated at 56ºC for 30 min (HIS) detoxification sample shown good weight gain in the mouse .In case of in NIS there was a decreased in weight, death of mice was also observed. The antigenic property was tested by agglutination test were found to be good in both samples proved the presence of factor 1and 3. Further the HIS and NIS samples were evaluated through ischemic and DNT tests, the sample NIS was tested in the guinea pig and mice, induced hemorrhagic and dermonecrosis reactions on the inoculated region of skin. All dermonecrotizing activity and a large part of the lethal toxicity were observed in guinea pig and mice. There was no such reactions were seen in case of HIS sample. If the cells were heated above 56ºC the virulence activities was substantially diminished. These biological effects of organisms are now known to be caused by Heat labile toxin which is also called dermonecrotic toxin. Hence HLT should be detoxified at 56ºC at 30 minutes is most preferred, during the production of Whole Cell Pertussis Vaccine.
| Keywords |
| Whole cell pertussis vaccine; Dermo necrotizing toxin; Ischemic activity; Heat liable toxin; Slide agglutination |
| Introduction |
| Pertussis or whooping cough is a noninvasive illness of the ciliated respiratory epithelium of the lower respiratory tract caused by Bordetella pertussis. Pertussis remains an important disease of infancy and early childhood, particularly in developing countries. For this reason, in recent years, much attention has been paid to Pertussis immunization by WHO in the context of the Expended Programme of Immunization (EPI) [1]. Prior to widespread use of whole-cell Pertussis vaccine (WCPV), There were 2,70,000 cases of Pertussis reported each year in the United States (indeed, the true case count likely approximated the annual birth cohort), with 10,000 deaths, report of the task force on Pertussis and Pertussis immunization [2]. The occurrence of Pertussis declined markedly after the introduction of whole-cell vaccine. B. Pertussis produces multiple antigenic and biologically active products, including Pertussis toxin (PT), Filamentous Haemagglutinin (FH), Agglutinogens (AGG), Adenylate Cylase Toxin (ACT), Pertactin (Pt) and Tracheal Cytotoxin (TCT) and Heat labile toxin (HLT). |
| Heat labile toxin (HLT) is a cytoplasmic protein; present in all the Bordetellae species. Its role in disease is undefined. The toxin was first demonstrated by Bordet and Gengou 1909 [3]. HLT also known as Dermo Necrotizing Toxin (DNT) was the first Bordetella toxin to be described and its role in disease has not yet been defined. It has a potential vaso-constrictive activity and can cause death and loss of normal weight gain in mice. Recent studies state that apart from B. avium, all other strains of Pertussis have an indistinguishable physiochemical, serological and biological properties. Therefore the aim of the present study is analysis of virulence property heat labile toxin of B. Pertussis strain 134 before and after heat inactivation during production of whole cell Pertussis vaccine (WCpV). |
| Materials and Methods |
| Strain of Bordetella pertussis |
| Bordetella pertussis vaccine strain 134 used in this study was obtained from Rijks institute, Bilthovan, Holland. The strain is maintained in lyophilized state at 4ºC. |
| Bordet-Gengou (BG) medium |
| Bordet-Gengou (BG) Medium was prepared as per Cruikshank [4] with the significant modification as per WHO [5]. |
| B2 culture medium |
| The B2 culture medium was prepared with the following compositions. Bacto Casamino acid (BCA) 6g, L glutamic acid 5 g, NaCl 2.5 g, KH2PO4 0.5 g, MgSO4 0.1 g, CaCl2 0.01 g, FeSO4 0.0125 g, CuSO4 0.0005 g, Glutathione 0.01 g, Yeast extract 1.5 g, Soluble starch 1.5 g. Starch solution was prepared by dissolving starch in cold water. The suspension was then added to 100 ml of hot distilled water and steaming in autoclave at 118ºC about 20 minutes separately. The remaining chemicals were dissolved in serial order 100ml of warmed distilled water in separate vessel. L-glutamic acid solution was prepared by dissolving in 50% NaOH still get amorphous solution hot distilled water. BCA was dissolved 100 ml of distilled water and yeast extract was added to this solution. Finally L-glutamic acid solution and other chemicals were added and made up to 1000 ml and mixed properly, transferred to fermenter and sterilized the medium at 121ºC for 30 minutes. |
| Sterility media |
| Nutrient agar medium, Soya bean Casein Digest Medium (SCDM), Alternate Thio Glycolate Medium (ATGM) were used to study the purity and sterility of the culture at appropriate stage to ensure its purity and safety. |
| Preparation of seed culture |
| One ampoule freeze dried working seed stock (134 strain) was opened under sterile environment and resuspended in 2 ml of sterile B2 medium. The suspension was then inoculated BG slope and incubated at 35ºC ± 1ºC for 72 hours, ensured the purity by Gram staining. Furthermore the culture was scraped aseptically and inoculated into a 1 ltr flask containing 400 ml of B2 medium. The flasks were loaded on seed shaker (140 ± 10 RPM) for 24-hours at 35ºC ± 1ºC and sterile filtered air is supplied over the surface of the medium (3 to 5 Lpm). Cultivation is allowed to continue for 30-36 hours till the maximum yield of organism is achieved. Samples are collected and checked for purity, opacity and pH. The pH usually ranges from 7.8 to 8.2. |
| Purity and sterility test |
| The purity, morphology was studied by Gram staining method. The purity of seed samples, fermenter culture was also checked by taking 1 ml of seed sample and inoculated into 3-nutrient agar slopes and other three slopes were kept as control without adding any sample and incubated both control and test samples at 35ºC ± 1ºC for 24 hours and observed for its sterility. Samples (1 ml) of vaccines are inoculated into 4 bottles (100 ml each) of each thioglycolate medium and Soya bean caesin digestive medium. 4 bottles of thioglycolate medium are incubated at 35ºC and other 4 bottles are incubated at 20-22ºC for 14 days. |
| Cell mass determination (opacity test) |
| 0.5 ml of the test vaccine was taken in the opacity tube and diluted the sample with normal saline until the opacity is identical with 5th International reference preparation of opacity when compared by eye under uniform background, taking the dilution factor into account, calculation of opacity [6]. |
| Slide agglutination test |
| Dried mono specific typing sera for factors 1, 2 and 3 were kindly supplied by Dr. M. Zakharova (Gamaleya Institute of Epidemiology and Microbiology, Moscow, USSR). These sera were used in accordance with the instructions provided with them and the test was carried out for slide agglutination [7]. One drop anti Pertussis nonspecific sera 1, 2 and 3 and equal volume of 134 culture (around 50 IOU/ml) and mixed them up and rocked the slide gently mixed and observed for rapid and heavy agglutination, the results are tabulated. |
| Heat inactivation |
| Samples were heated at 56ºC for 30 min in a water bath. The vaccine was shaken frequently during heating. After heating, the bottle was cooled in running water as quickly as possible. |
| Viability test |
| The test for absence of living B. Pertussis is done by inoculating BG agar slope with vaccine and incubated at 35ºC for 7 days. The inoculated BG tubes should be observed for 7 days and there should be no growth of B. Pertussis or of any other organisms. |
| SDS-PAGE analysis |
| The SDS-PAGE was analyzed as per Laemmli with significant modifications in a vertical electrophoresis Unit (Bio-Rad Laboratory USA). Samples were loaded in the wells and the gel was run at 100 V. After the tracking dye reached the bottom the gel was removed gently and silver stained. The gel was submerged in fixative and left overnight (50% methanol, 12% Glacial Acetic Acid, 37 μl formaldehyde). It was them impregnated in Sodium thiosulphate- Na2S2O3.5H2O (0.02 gm %). Finally, it was treated with the developer (6% Na2CO3, 40 μl of 0.02 gm % Sodium Thiosulphate- Na2S2O3. 5H2O, 10 μl of formaldehyde). Once the gel was stained, it was stored in storing solution (50% methanol, 12% Glacial Acetic Acid) [8]. |
| Toxicity/ MWGT test |
| Prior to conduct the animal test the concentration of the material per ml (i.e. Single Human dose 40x109 B. Pertussis organism/ml) and (i.e. Half of the single Human dose 20x109 B. Pertussis organism/ml) as per this criteria all tests was performed. in The toxicity test was performed by mouse weight gain test (MWGT) described by pitman and cox was performed according to the WHO guidelines [5]. The MWGT was executed for each harvested sample of different cultivation hours after heat inactivation at 56ºC / 30 minutes. Two weeks old, 14- 15 g of healthy male Lacca mice are weighed in groups (n = 10) and injected intra peritoneally with 0.5 ml (half of the single Human dose i.e. 20x109 B. Pertussis organism/ml). Control group of 10 mice was injected with an equal volume of 0.9% saline. During the assessment the animals are properly fed, both groups are weighed in the first, third and seventh day after injection. |
| A vaccine is considered non-toxic if, at the end of 72 hours the weight of the group is not less than that at the time of injection, and at the end of 7 days the average weight gained per mouse is no less than 60 percent of that of the control group of mice: In addition, no vaccinerelated deaths should occur. If any mice die the test may be repeated but the aggregated deaths may not exceed 5% [9]. |
| Test for abnormal toxicity (Innocuity) |
| One single human dose (40x109 B. Pertussis organism/ml)but not more than 1.0 ml was injected intraperitoneally into each of five adult three to four weeks old, mice weighing 17–22 gm and one single human dose, but not more than 5.0 (40x109 B. Pertussis organism/ml) ml into each of two guinea pigs, Five to six weeks old, 250–350 gm used for this study [10]. The vaccine inoculated animals were observed for 7 days. |
| Vaccine is acceptable if none of animals shows signs of ill health in the seven days following the inoculation. If one of the animals dies or shows signs of ill health during the time specified, the test is repeated. The substance passes the test if one of the animals in the second group dies or shows signs of ill health in the time interval specified. |
| Dermonecrotizing activity of suckling mice |
| Three days old suckling mice weighting range from 2-3 g was used for this experiment. The 50 μl of sample was taken in a sterile tuberculin syringe (samples NIS and HIS) are injected intracutaneously into 2 different suckling mice. The mice were observed after 3-4 hours injection. |
| Ischemia-inducing activity |
| Samples (100 μl) of an HIS and NIS which had been diluted logarithmically in phosphate-buffered saline, pH 7.2, were injected through intracutaneous into the shaved back of female five to six weeks old Hartley guinea-pigs which weighed 300 to 350 g. At the indicated times, the diameter of the ischemic lesion was measured. The minimum dose of protein producing an ischemic lesion was measured. The minimum dose of protein producing an ischemic lesion 10 mm in diameter is defined as the minimum ischemia-inducing dose (MID). Values are means calculated from the dose response curves obtained from three test animals. |
| Results and Discussion |
| The viability test results was shown in Table 1 for absence of living B. Pertussis (viability test) was done by inoculating with detoxified and non-detoxified vaccine samples in BG agar slants results found that after incubated at 35ºC for 7 days. The results indicates that the growth of B. Pertussis was presence in BG agar slants for sample NIS, and absence contamination was also observed, in case of HIS there was no growth was observed after 7 days incubation, it illustrated that the culture cells were heated at 56ºC for 30 min, all dermonecrotizing activity (HLT) was diminished. The similar study of Endoh et al. [11] shown that after the B. Pertussis organism were heated for 30 min at 56ºC, all dermo necrotizing activity and a large part of the lethal toxicity were destroyed in tested animals. |
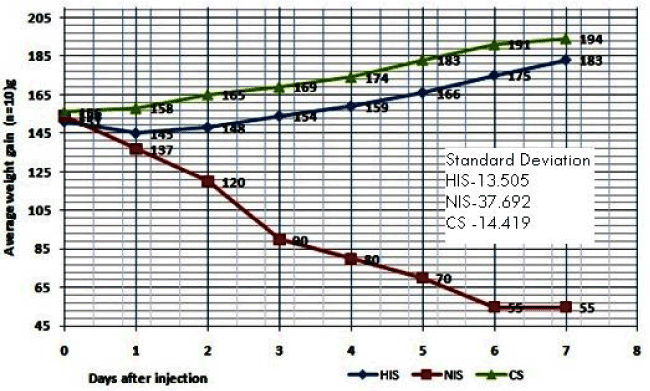
| The Mouse Weight Gain Test (MWGT) was performed according to the guidelines of WHO [5]. The result of HIS vaccine shown the maximum average weight gain for the mice (n = 10) from day 1day to 7, and there is no death of mice also have been observed (Table 2 and Figure 3). Finally after 7th day the total weight gain of the mice was found to be +32 grams from the day one average weight (151 g).Whereas the non-heat inactivated sample (NIS) showed significant decreased weight in the mice after 24 hours inoculation and as well as deaths were also found on the 2, 3 and 5th days it is due to the virulence property of HLT not heat inactivated properly. After the seven the total weight loss of mice (n = 10) found to be -99 g from the day one weight (154 g). Hence 56ºC/ 30 minutes inactivation sample shown passed the mouse weight gain test as per WHO criteria [5,12]. |
| Similarly the synopsis of mouse weight gain test results of both HIS and NIS shown in Table 3 indicated that, the two samples (HIS, NIS) along with control (CS), in the sample HIS, the mice (n = 10) average weight was increased on the day 3rd and 7th (0.3 and 3.3 respectively per mouse) and overall test % control was 86.8 g after 7 days it was passed MWGT. In case of NIS it was observed the mice weight was continual decrease up to the 7th day, on the day 1(-1.7 g), day 3(-6.4 g) and day 7 (-9.9 g) and overall test % control was 26 g after 7 day. Hence the test was failed. It seemed therefore that weight gain expressed as percentage of that the saline control might be more accurate measurement of the toxicity of the vaccine than obsolete weight gain [12]. |
| The mortality rate in the mice, this evidence are higher levels of toxicity encountered by the presence of HLT which was present in the vaccine sample caused the toxic reactions in the mice. For this purpose, Heating at 56ºC for 30 minutes and treatment with 0.012-0.02% Merthiolate is preferred. Thus, Heating ensures that diminish of HLT is achieved and it supports the evidences that increase the stability of vaccine [13]. The MWGT is confirmatory test for the inactivation of HLT [13]. Since the weight decrease in body of mice in NIS inoculated with presence of HLT and it appears early with in one or two days after inoculation of NIS sample, in this aspect the MWGT is mandatory routine method for quality control test of whole cell Pertussis vaccine production [9]. |
| Abnormal toxicity test |
| Further the both HIS and NIS samples abnormal toxicity results tabulated (Table 4). In this test 1.0 ml of NIS and HIS samples was injected into each of five three weeks old adult mice weighing 17–22 gm and 5.0 ml into five to six weeks old, each of two guinea pigs, weighing 250–350 g intraperitoneally [10]. |
| It was observed that two mice were died on 3rd and 6th day after vaccination with NIS sample, but no death of guinea pigs were noticed. There was nonappearance death in mice and guinea pig as seen in the inoculated sample of HIS; it reveals that experiment the NIS sample was toxic to mice due to the presence of virulence factor (HLT). Whereas the NIS samples fall short in MWGT test due to presence of higher toxicity factors, because of increased mortality rate in the mice, this evidence are higher levels of toxicity encountered by the presence of Heat Labile Toxin (HLT) which present in the vaccine sample was caused the toxic reactions. For this purpose, Heating at 56ºC for 30 minutes and treatment with 0.012-0.02% Merthiolate is preferred. Thus, Heating ensures that diminish of HLT is achieved and it supports the evidences that increase the stability of vaccine [13]. |
| The Bacterial Agglutinogen Test (BAT) test was performed for the confirmation of agglutinogen factors (Factor 1, and Factor 3) (Table 5). The antigenic property was tested by agglutination test were found to be good in both sample show the presence of factor 1,and 3 were agglutinated within 3 seconds, which showed in both combination factor were present high purity and normal morphology of B. Pertussis. Many authors believe that serotype–specific Agglutinogens (AGG) are among the surface components of B. Pertussis which are important factors in conferring immunity to the disease, by mediating adhesion to the respiratory mucosa. It is now generally believed that there are 3 major agglutinogens AGG- 1, 2, 3. The strain 134 containing the AGG factor 1 and 3 [14]. Since the presence of AGG- 1, 2, 3 in Whole Cell Pertussis Vaccine (WCPV) is believed) to contribute to its protective efficacy, the WHO requirements for Pertussis vaccine use a test to verify the presence of component of such agglutinogens in vaccine [15]. |
| SDS-PAGE analysis |
| The HIS and NIS samples was structural characterized by SDSPAGE (Figure 1) it shows, in NIS the presence of protein bands at various molecular weights, especially around 102 KDa the presence of small band, the faint polypeptide with molecular mass about 102 KDa [16]. Along with more other bands also presence it reveals the presence of other vital Pertussis proteins. In case of HIS the absence of protein band at around 102 KDa. |
| In the studies of Gentry Weeks et al. [17], the 58,000 MW proteins were also present in E. coli cells, suggesting that 58,000 MW proteins was a common bacterial protein and were unrelated to dermo necrotic toxin. Further the cross reactivity of dermonecrotic toxin antibody with the 58,000 MW protein probably represented nonspecific naturally occurring cross reactive antibody in the rabbit antiserum. B. bronchiseptica and B. Pertussis and B. parapertussis dermo necrotic toxins are reported to be serologically related [16] and molecular weights of 102 KDa and 190 Kda [2]. |
| Ischemic activity |
| The results of Ischemia inducing activity of guinea pig for NIS was shown in Figure 2 after 8 and 24 hours red colored lesions was present on the injected region, this lesion ranging from light reddish (erythema) to purple (Hemorrhagic necrosis) were measured 5 to 10 mm, the similar study of Endoh et al. [18] also reported that, the skin lesions was formed due to injection of crude HLT in guinea pig have been described as hemorrhagic and necrotic it divulge such as whole have contained significant quantities of endotoxin. |
| In other study supernatant of sonicated B. Pertussis which are used in their experiment shown induced hemorrhagic lesions in Guinea pigs and produced ischemic lesion in rabbits, with hemorrhage and accomplished as a result the laboratory animal rabbit is appeared to be less susceptible than guinea pigs to the hemorrhagic action of crude HLT preparations [17]. |
| Dermonecrosis activity in mice |
| For the analysis of HLT activity the HIS and NIS samples was diluted with normal saline and injected to Swiss albino suckling mice. After 24 hrs at the injection site of the suckling skin, induces the dermonecrosis in NIS sample, in case of HIS it was absence (Figure 4). |
| The exact role of the dermonecrotic toxin in the pathogenicities of B. avium and B. Pertussis is unknown. It has been reported that the dermonecrotic toxin of B. Pertussis inhibits a Na+-K+-ATPase in in vitro assays [16] conservation of a functional dermonecrotic toxin in all Bordetella species suggests that this toxin may play a role in the virulence of the organisms [2].The dermonecrotic lesion produced by injection of cell homogenates into turkeys and chickens was much more extensive and comprised an area two to five times larger than the lesions produced in guinea pigs [17]. |
| Preparations of heat-labile toxin from B. parapertussis were shown to interact with endotoxin in producing dermonecrotic lesions [18]. While the possibility for this type of interaction has not been explored in depth with purified HLT the presence of less than 30 ng of endotoxin per mg of pure protein suggests that endotoxin is not essential in purified HLT -mediated dermonecrosis in suckling mice. Similar studies of Weeks et al. [17] and Goldman [19] showed that, Dermonecrotic toxin (HLT) is a virulence factor which may play an important role. |
| The study of Endoh et al. [11] report evident suggested that our study, after the cells were heated for 30 min at 56ºC, all dermo necrotizing activity and a large part of the lethal toxicity were destroyed, and cell homogenates of B. parapertussis, B. bronchiseptica, and B. avium produce similar dermonecrotic lesions [11]. |
| Conclusion |
| A more Potent and Safer Pertussis Vaccine is highly desirable, Whole cell Pertussis vaccine (WCPV) has been used for nearly 30 years in India for the Expendad programme for immunization (EPI) of children to control whooping cough disease in combined with diphtheria and tetanus (DTP vaccine). In the meantime the vaccine preparation has developed considerably owing to increasing knowledge of immunobiologicals property, antigenic pattern and chemical composition of B. Pertussis and extensive immuno epidemiological experience. The basic research during the last five decades has revealed some vital properties of B. Pertussis which are of primary importance in respect of vaccine production. In this seemingly in the present work the importance of HLT was analyzed in heat and non-heat inactivated vaccine samples 134. |
| For the SDS-PAGE analysis of B. Pertussis, in case of NIS showed the faint protein band around 102 KDa along with other important proteins, in case of HIS the HLT protein was absence (102 KDa) due to the heat inactivation at 56ºC at 30 minutes the heat labile protein was ruined. Thus, agglutination test was shown in both (NIS, HIS) the presence of factor 1 and 3 in Pertussis vaccine appears to give an indication of its protective ability. The result of mouse weight gain test to assessed detoxification study results found that, after the culture cells were heated below 56ºC for 30 min all dermonecrotizing activity and a large part of the lethal toxicity were observed in guinea pig and mice. If the cells were heated above 56ºC the activities were destroyed and no such reaction was seen. Hence HLT should be detoxified at 56ºC 30 minutes is most preferred, during Pertussis vaccine (WCPV) preparation. Further the HIS and NIS samples were evaluated through ischemic and DNT tests the sample NIS was tested in the guinea pig and mice, induced hemorrhagic and dermonecrosis reactions. There is no such reaction were seen in HIS sample. However this study concluded that the HLT is a virulence protein of B. Pertussis bacterial cells were dermo necrotizing and lethal when injected in to testing animals, after heat inactivation at 56ºC for 30 minutes the dermonecrotizing and lethal activity of bacterial cells was substantially diminished. The dermonecrotic effect of HLT appears to be due to a specific constructive effect on the vascular smooth muscles therefore the mouse toxicity test for Pertussis vaccine was introduced primarily to ensure that HLT should destroyed during whole cell Pertussis vaccine production. |
| Acknowledgement |
| The authors sincerely thank the Director, PII, Coonoor for providing facility to carry out this study, and also, the technical help and supports received from all technical staffs of Pertussis production division of Pasteur Institute of India Coonoor, Tamilnadu, India is highly acknowledged. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14679
- [From(publication date):
September-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10090
- PDF downloads : 4589
