Assessment of the Prevalence of Zidovudine Induced Anemia among Adult HIV/AIDS Patients on HAART in an Ethiopian Hospital
Received: 15-Jan-2018 / Accepted Date: 29-Jan-2018 / Published Date: 09-Feb-2018 DOI: 10.4172/2329-6879.1000271
Abstract
Background: Zidovudine is one of a nucleoside reverse transcriptase inhibitor in the first line ART regimen in Ethiopia. It is, however, known to be associated with life threatening toxicity like anemia. Unfortunately, zidovudine related anemia at Mizan-Aman General Hospital has not yet been known before. Therefore, the aim of this study is to determine the prevalence of zidovudine induced anemia among adult HIV/AIDS patients on highly active antiretroviral therapy.
Methods: Retrospective cross-sectional study design was conducted in ART pharmacy in Mizan-Aman General Hospital by observing patient charts. Simple random sampling technique was used to select the charts. Data was collected by randomly extracting documented information in the patients’ medical chart who initiated AZT containing ART regimen. Result: Around on third of the participants (27.9%) were in the age range of 32 – 38 years. Of the assessed patient charts, charts with female patients constitute 51.9%. More than two-third of participants (73.7%) were urban residents while about forty percent of the total patients included in the study (39.1%) were secondary school achievers but 11.9% of participants were illiterate. About one fifth of the patients (22.6%) developed anemia. The occurrence of zidovudine induced anemia was higher among illiterates (29.4%). The number of participants who developed AZT induced anemia while using social drugs was relatively lower 14.1%. Majority of patients who developed anemia had a baseline hemoglobin level of ≤ 12 g/dl (75.3%).
Conclusion: The prevalence of AZT induced anemia was found to be highest on patients whose baseline Hgb level ≤ 12 g/dl. Frequent monitoring of Hgb concentration in patients on AZT based regimen will be an essential strategy to overcome anemia itself and anemia related costs like blood transfusion.
Keywords: Anemia; Ethiopia; Mizan aman; Zidovudine
Background
Without a cure now it is a decade, acquired immunodeficiency syndrome (AIDS) is a cause of serious public health concern in the world [1]. HIV is a retrovirus of lentivirinae subfamily having the ability to infect its host chronically and progressively rain its host immune system [2]. Two major viral types have identified in human as HIV-1 and 2. HIV-1 is the predominant throughout the world; while HIV-2 is primarily found in West Africa. Infection with HIV and subsequent AIDS is rampant problem worldwide with its broad community implications; making AIDS the most devastating emerging disease that human kind has ever faced [3-6].
The highly active antiretroviral therapy (HAART) is the only treatment option for treating the HIV-positive virus patients for improving the immune system by increasing the number of CD4 cells essential to protect body from infections and cancers [7,8]. Approximately, 7 million people who live with AIDS can now access HAART. The increasing number of patients receiving HAART has led to a reduction in AIDS-related morbidity and mortality. The primary goal of ART is to prevent HIV associated morbidity and mortality. This goal is best accomplished by using effective HHART to maximally inhibit HIV replication so that the viral load remains below levels detectable by commercially available assays [9].
Anemia has been found to be a significant reason for progression to AIDS and moderate to severe anemia is associated with an increased risk of death. Anemia may impair physical, socio-emotional, neurophysiological functioning negatively affects the quality of life [10]. Zidovudine (AZT) is unique among antiretroviral drugs because of its hematological toxicity. Zidovudine-induced anemia may be mild, moderate, or severe depending on patient's conditions. The long term toxicity of AZT remains an important obstacle for sustained therapy with this drug. Although the initial nausea associated with the commencement of zidovudine-containing therapy usually settles within a matter of weeks, anemia, neutropenia, dyslipidemia and peripheral lipoatrophy are all well-documented adverse events associated with long-term use of zidovudine [11,12].
This study will determine the prevalence of AZT induced anemia. Accordingly, this study helps in preventing this type of anemia by identifying and stratifying individuals at higher risk of developing anemia. Moreover the result of this study will help the stake holders and the patients to save significant costs associated with the hospitalization resulting from severe anemia and its complications. Finally the result of the study will help other researchers to conduct further research on the issue.
Methodology
Study area and period
The study was conducted in Mizan aman general hospital (MAGH) located 561 km South West of Addis Ababa. The hospital has different units including hospital pharmacy, internal medicine, pediatrics, gynecology, surgery. The study was conducted at ART pharmacy; MAGH from March 28 April, 2016.
Study design
A retrospective cross-sectional design was conducted among adult HIV/AIDS patients taking AZT based ART regimen in MAGH.
Sample size determination
Using the formula for estimation of a single proportion, n=(Ƶ ɑ/2)2 p(1-p) ̸ d2. Where the z value is taken as 1.96; p, proportion of new onset anemia patients, was assumed to be 50%; and d, the margin of error of estimation was assumed to be 5% or 0.05. Based on these assumptions, the final sample size determination was calculated to be 385.
ni; =(Ƶ ɑ/2)2 p(1-p) ̸ d2, ni=(1.96)2 0.5(1-0.5)/(0.05)2=385 But the total HIV patients is less than 10,000. So that we use correction formula as:
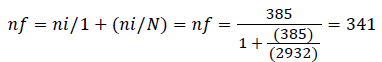
So, after adding the 10% of contingency 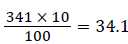 was considered for non-response rates.
was considered for non-response rates.
The final total sample size was =341 +34.1=376
Sampled documents were then randomly selected to extract relevant information from the patient chart. Simple random sampling technique was used to select the charts.
Data collection procedure and analysis
Data was collected by randomly extracting documented information in the patients’ medical chart who initiated AZT containing ART regimen using check list paper. The collected data was checked for completeness, processed and analyzed manually. The results of were presented using tables.
Results
Study population characteristics
Around on third of the participants (27.9%) were in the age range of 32–38 years. Of the assessed patient charts, charts with female patients constitute 51.9%. More than two-third of participants (73.7%) were urban residents while about forty percent of the total patients included in the study (39.1%) were secondary school achievers but 11.9% of participants were illiterate. Approximately, one-third of the participants’ are social drug users such as cigarette, alcohol or khat along with their ART medications (Table 1).
| Variables | Frequency | Percent (%) | |
|---|---|---|---|
| Sex | Male | 181 | 48.10% |
| Female | 195 | 51.90% | |
| Age (years) | 18-24 | 11 | 2.90% |
| 25-31 | 68 | 18.10% | |
| 32-38 | 105 | 27.90% | |
| 39-45 | 79 | 21.10% | |
| 46-52 | 93 | 24.70% | |
| >52 | 20 | 5.30% | |
| Residence | Urban | 277 | 73.70% |
| Rural | 99 | 26.30% | |
| Educational status | Primary school | 108 | 28.70% |
| Secondary school | 147 | 39.20% | |
| Higher education | 36 | 9.60% | |
| Illiterate | 45 | 11.90% | |
| Read and write | 40 | 10.60% | |
| Social drug use | Cigarette | 11 | 2.90% |
| Alcohol | 40 | 10.60% | |
| Khat | 14 | 3.70% | |
| Tobacco | 23 | 6.20% | |
| None | 288 | 76.60% |
Table 1: Socio demographic characteristics of participants on ART in MAGH.
Among all participants, 65.4% of them started ART with their baseline CD4 less than and equal to 500 cells/mm3. Majority of patients were in WHO clinical stages stage III 130[34.6%] at the time of ART initiation. Forty seven percent of them had OIs at the time of HAART initiation and 73.4% patients had a baseline Hgb level of >12 g/dl (Table 2).
| Variables | Frequency | Percentage (%) | |
|---|---|---|---|
| Initial HAART regimen | AZT based | 376 | 10000.00% |
| Baseline CD4 count | ≤ 500 | 246 | 6540.00% |
| >500 | 130 | 3460.00% | |
| Current CD4 count | ≤ 500 | 214 | 5690.00% |
| >500 | 162 | 4310.00% | |
| WHO stage | I | 68 | 1800.00% |
| II | 113 | 3000.00% | |
| III | 130 | 3460.00% | |
| IV | 65 | 1740.00% | |
| OIs | TB | 48 | 1280.00% |
| Cryptococcus infection | 28 | 740.00% | |
| Candidiasis | 40 | 1060.00% | |
| PCP | 51 | 1360.00% | |
| Others | 6 | 160.00% | |
| None | 203 | 5400.00% | |
| Baseline Hgb level | ≤ 12 g/dl | 100 | 2660.00% |
| >12 g/dl | 276 | 7340.00% |
Table 2: Initial HAART regimen, CD4 count, WHO stage, OIs and Hgb levels of participants.
According to the finding of this study, 22.6% patients developed anemia. Among these most [75.3%] of them developed grade I anemia. Out of the total 376 patients there is only one patient who have grade IV anemia and grade III were not observed (Table 3).
| Variables | Frequency | Percentage (%) | |
|---|---|---|---|
| Prevalence of anemia (Total=85) | AZT | 85 | 22.60% |
| Grade of anemia (N=85) | Grade I | 64 | 75.30% |
| Grade II | 20 | 23.50% | |
| Grade III | 0 | 0% | |
| Grade IV | 1 | 1.20% | |
Table 3: Prevalence and grades of anemia.
The occurrence of Zidovudine induced anemia was higher among illiterates [29.4%]. The number of participants who developed AZT induced anemia while using social drugs was relatively lower 14.1%. Majority of patients who developed anemia had a baseline hemoglobin level of ≤ 12 g/dl. 64 [75.3%] (Table 4).
| Variables | Presence of anemia | |||
| Yes | % | No | % | |
| Education status | ||||
| Illiterate | 25 | 29.40% | 20 | 6.90% |
| Secondary | 32 | 37.60% | 115 | 39.50% |
| Higher education | 11 | 12.90% | 25 | 8.60% |
| Social drug use | ||||
| Yes | 12 | 14.10% | 76 | 26.10% |
| No | 73 | 85.90% | 215 | 73.90% |
| Baseline Hgb Level (g/dl) | ||||
| ≤ 12 | 64 | 75.30% | 36 | 12.40% |
| >12 g/d | 21 | 24.70% | 239 | 82.10% |
Table 4: Occurrence of anemia among selected independent variables.
Discussion
The In this study, 22.6% patients developed anemia from. This was higher than observed in other studies while a bit lower from some others [13]. These differences might be due to different factors such as differences in socio-economic level of the study populations, use of different methods such as inclusion and exclusion criteria for other causes of anemia. Furthermore, the lower nutritional status of our society, as compared to the relatively developed parts of the world, can also have an effect to the development of AZT induced anemia.
In contrast, lower level of anemia in this study compared to some other findings could be described to many confounding factors that may vary geographically. For instance, malaria and other comorbidities were known to overestimate anemia prevalence in different part of the world. Other published reports showed BMI to be a risk factor for early severe anaemia, which may be a surrogate marker for patients who are more severely immunocompromised [14]. However, I didn’t consider this factor for the development of AZT induced anaemia due to incompleteness of medical charts. The prevalence of anaemia in illiterate patients was higher than those with advanced education; this may be explained by illiterate segment of those populations who were not fully aware of their anaemia and come to ART clinics quite late with high anaemic grades [15,16].
As mentioned in previous findings low baseline Hgb level came to strongest risk factor for developing anemia, the present studies also demonstrated similar result. The fact that anemia is a reduced level of Hgb, it is not surprising to see patients with lower value of baseline Hgb level developed anemia. Low level of Hgb supported by the additive effect of AZT for anemia due to suppression of bone marrow precursor cells.
In one study conducted in India in 2010, the prevalence of grade IV anemia was observed to be highest (42.1%) of total anemic patients while grade three anemia was to the lowest [17]. This study indicated that the prevalence of grade one anemia was highest (75.3%) of the total AZT based ART regimen users while grade three anemias were the lowest. This difference could be attributed to types of sampled participants and time points after commencing ART since anemia greatly varied as time goes on.
Conclusion
In this study the prevalence of AZT induced anemia in MAGH is high and it needs an attention. The prevalence of AZT induced anemia was found to be highest on patients whose baseline Hgb level ≤ 12 g/dl. Frequent monitoring of Hgb concentration in patients on AZT based regimen will be an essential strategy to overcome anemia itself and anemia related costs. If the patient come with low Hgb level, it is better to initiate HAART regimens other than zidovudine. But if the patients have to be on AZT based regimen regular monitoring of Hemoglobin concentration is mandatory. There should be guidelines regarding the initiation of ART for anemic patients.
Limitation of the study
Because this study was retrospective, we were unable to access patients’ real health condition as well as the role and care status of the nursing professionals.
Competing interest
The authors declare that they have no competing interests.
Authors' contributions
Both of the authors contributed in the design, data collection and analysis of data as well as in the manuscript preparation.
References
- Gashaw A, Afework K, Feleke M Yigzaw K, Molla G, et al. (2007) Low prevalence of HIV infection, and knowledge, attitude and practice on HIV/AIDS among high school students in Gondar, Northwest Ethiopia. J Health Dev 21: 1-4.
- Guidelines for the implementation of ART in Addis Ababa, Ethiopia (2007).
- Guidelines for the use of ARV drugs in adolescent and adults in Ethiopia, Addis Ababa, Ethiopia (2008).
- The population and housing census of Ethiopia : Results at country level (1994).
- Srikanth BA, Babu SC, Yadav HN, Jain SK (2012) Incidence of adverse drug reactions in human immune deficiency virus-positive patients using highly active antiretroviral therapy. J Adv Pharm Technol Res 3: 62–67.
- Kuwalairat P, Winit-Watjana W (2014) Determinants for zidovudine-induced anemia in HIV adult patients: A Thai multicenter study. APP 5: 6.
- Renner LA, Dicko F, Malateste K, Gueye RD, Aka E, et al. (2013) Anaemia and zidovudineâ€containing antiretroviral therapy in paediatric antiretroviral programmes in the IeDEA Paediatric West African database to evaluate AIDS. J Int AIDS Soc 16: 18024.
- Agarwal D, Chakravarty J, Chaube L, Rai M, Agrawal NR, et al. (2010) High incidence of zidovudine induced anaemia in HIV infected patients in eastern India. Indian J Med 132: 386-389.
- Spaulding A, Rutherford GW, Siegfried N (2010) Tenofovir or zidovudine in threeâ€drug combination therapy with one nucleoside reverse transcriptase inhibitor and one nonâ€nucleoside reverse transcriptase inhibitor for initial treatment of HIV infection in antiretroviralâ€naïve individuals. Cochrane Database Syst Rev. 10:CD008740.
- Kiragga AN, Castelnuovo B, Nakanjako D, Manabe YC (2010) Baseline severe anaemia should not preclude use of zidovudine in antiretroviral-eligible patients in resource-limited settings. JIAS 13: 42.
- Ssali F, Stohr W, Munderi P, Reid A, Walker AS, et al. (2006) Prevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trial. Antivir Ther 11: 741-749.
- Moh R, Danel C, Sorho S, Sauvageot D, Anzian A, et al. (2005) Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with co-trimoxazole in Côte d’Ivoire. Antivir Ther 10: 615-624.
- Kumarasamy N, Venkatesh KK, Cecelia AJ, Devaleenal B, Lair AR, et al. (2008) Adverse event of HAART in southern Indian HIV infected patients. AIDS patient care STDs 22: 337-344
- Rajesh R, Vidyasagar S, Varma DM, Mohiuddin S (2011) Evaluation of incidence of zidovudine induced anemia in Indian human immunodeficiency virus positive patients in comparison with stavudine based highly active antiretroviral therapy. Int J Risk Saf Med 23: 171-80.
Citation: Muluken W, Epherem M (2018) Assessment of the Prevalence of Zidovudine Induced Anemia among Adult HIV/AIDS Patients on HAART in an Ethiopian Hospital. Occup Med Health Aff 6: 271. DOI: 10.4172/2329-6879.1000271
Copyright: © 2018 Wubetu M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7850
- [From(publication date): 0-2018 - Dec 21, 2024]
- Breakdown by view type
- HTML page views: 7115
- PDF downloads: 735
