Assessment of Parasitological Behaviour, Clinical Changes and Serology during Experimental Infection of a Calf with a Venezuelan Isolated of Trypanosoma evansi: A Preliminary Study
Received: 18-Mar-2017 / Accepted Date: 22-Mar-2017 / Published Date: 30-Apr-2017 DOI: 10.4172/2476-2024.1000125
Abstract
Background: Trypanosoma evansi is the agent of trypanosomosis that affect domestic and wild animals causing anaemia, degeneration, necrosis and inflammatory processes. This disease is of great concern because it produces growth retardation, loss of body weight, low production of animal proteins and diminished fertility and traction power. On the other hand could be become in an emergent zoonosis affecting human beings as it has been recently described in India and Vietnam. Due in Venezuela experimental infections of cattle with T. evansi have been referred as "benign", the aim of this study was to assess the effect of T. evansi on parasitological, clinical and serological parameters during experimental infection of a bovine.
Methods: The evolution of the experimental infection of one bovine with T. evansi EcF1991 was assessed at parasitological, serological and clinical level for 30 days by measuring levels of parasitemia, IgG anti-T. evansi bovine antibodies by ELISA, body temperature, packed cell volume and levels of haemoglobin.
Results: Infected bovine developed a fluctuating low-level and often cryptic parasitaemia without fluctuations in body temperature. Parasitaemia was represented by four main peaks at days 1, 10 and 17. Although the two first parasitaemia peaks occurred with only a slight decreasing of haematocrit and increasing of IgG anti-T. evansi levels, beginning of third parasitaemia peak was accompanied by a marked decrease on haematocrit and haemoglobin values reaching levels 60% and 64% below of preinfection values respectively. The most marked diminution of haematocrit was observed after third parasitaemia peak (day 17). This decreasing trend of haematocrit was accompanied by a pronounced increasing on IgG levels against T. evansi during infection.
Conclusion: Results herein presented reports for the very first time the evolution of some clinical, parasitological and serological parameters during T. evansi infection of a bovine and confirm molecular studies about role of Venezuelan bovines as susceptible and natural hosts of T. evansi as it has been reported in Africa, Asia and South America. In addition presents an indirect ELISA technique for detection of bovine IgG antibodies to T. evansi suggesting that more research is needed to define the clinical, pathological and immunological profiles of these infections in bovines from Venezuela.
Keywords: Trypanosoma evansi; Bovine; Experimental infection; PCV; Haemoglobin; IgG-antibodies; Indirect ELISA
7624Abbreviations
ELISA: Enzyme Linked Immunosorbent Assay; EcF1991: Equus caballus Frío 1991; IgG: Immunoglobulin G; PCV: Packed Cell Volume; IFAT: Immunofluorescence Antibody Test; PCR: Polymerase Chain Reaction; PSG: Phosphate Saline Glucose Buffer; EDTA: Ethlylene-Diamine-Tetra-Acetic Acid; Hb: Haemoglobin; PBS: Phosphate Buffered Saline; ABTS-H2O2: 2,2'-azino-bis(3- ethylbenzothiazoline-6-sulphonic acid)-Hydrogen Peroxide; OD: Optical Density; nm: Nanometers.
Introduction
Trypanosoma evansi has the widest geographical distribution of all the pathogenic trypanosome species and infects a wide range of mammals in many countries of Africa, Asia and South America [1-3]. In this regard, T. evansi infections have been demonstrated in domestic animals such as horses [4], donkeys, camels, water buffaloes [5], cattle [6,7] and dogs [8,9], as well as in wild animals such as capybaras (Hydrochoerus hidrochaeris) [10-12], coati (Nasua nasua) [13], haematophagous bats (Desmondus rotundus) [14] and nectar-feeding bats [15].
In Venezuela animal trypanosomosis is mainly caused by T. evansi and Trypanosoma vivax, the former is the aetiological agent of Surra (Derrengadera) in horses, and was first described for Venezuela by Rangel [16], while the second one was first described by Tejera as the causative agent of trypanosomosis that affects bovines, ovines and caprines known as Secadera, Peste Boba or Peste Bonita [17], as well as trypanosomosis of wild ungulates such as the white tailed deer or caricou (Odoicoileus gymnotis ) [18-20].
Both trypanosome species are mechanically transmitted by blood-sucking arthropods as horseflies Tabanus importunus and Tabanus nebulosus for South American T. vivax [21,22] and Stomoxys calcitrans for T. evansi [23]; and were brought in America during XVI century by the Spaniards conquerors from infected horses of North Africa and infected bovines from West Africa [14].
Venezuelan savannahs are important zones to breed cattle under an extensive livestock ranching. According to this production system, horses are used for different agricultural activities. Additionally, cattle, dogs, blood-sucking arthropods, capybaras, hemathophagous and nectar-feeding bats, also belonging to Venezuelan savannah fauna, live together with infected horses promoting the transmission and infection maintenance. In these regions, trypanosomosis caused by T. evansi (75, 8% seroprevalence by IFAT) is an important factor that wastes health and productivity of horses [24].
Concerning to infections of bovines with T. evansi in Venezuela, there are few works about natural and experimental infections with this trypanosomatid. According to the first report of Tejera [17], inoculation of a heifer with T. evansi results in the appearing trypanosomes in blood few days after inoculation but parasitemia was transient and parasites disappeared quickly from the bloodstream. Despite this parasitaemia pattern was accompanied by not visible clinical alterations, inoculation of guinea pigs with blood from infected heifer suggested the occurrence of an active but cryptic infection. Similar results were obtained by Kubes [17] in a work which demonstrates the development of resistance against T. evansi when self-cured animals were reinoculated with the same strain of trypanosome.
More recently, a PCR based on established primer pairs (21-mer/22- mer and ILO1264/ILO1265) indicated that 68 (19.9%) of 342 blood samples investigated including 316 from water buffalo herds, contained T. vivax and that none contained T. evansi or any other member of the subgenus Trypanozoon [5]. On the other hand a PCR using specific ITS1 primers that differentiate T. vivax and T. evansi infections allowed to demonstrate that from 47 cattle evaluated in the ‘‘Laguneta de la Montaña’’ sector (Miranda State), 9 animals resulted positive for T. vivax, 3 for T. evansi and 2 with double infections. Whilst in the ‘‘San Casimiro’’ sector (Aragua State), out of the 38 cattle evaluated 19 animals were diagnosed as positive by PCR, determining only the presence of T. evansi in this locality [7].
At the time this communication was prepared, there were no studies related to the parasitological behaviour of the infection caused by Venezuelan T. evansi isolates in cattle, as well as the clinical changes that occur and the follow-up of seroconversion by ELISA. On the other hand, due we have used crude antigens of T. evansi for detection of bovine IgG antibodies against T. vivax in seroepidemiological studies of bovine trypanosomosis [25], [26], the aim of this work was to describe the behaviour of bovine infection caused by T. evansi as well as the capability of this trypanosome specie to produce changes in clinical parameters and the applicability of an indirect-ELISA originally standardized for detection of bovine antibodies to T. vivax, in analysis of seroconversion of bovine infected with T. evansi.
Materials and Methods
Experimental infection
For reasons of economical scarcity only one male crossbred (Holstein-Zebu) calf was used. It was kept in an animal-isolation unit and fed on hay, concentrate and water ad libitum. Calf was dewormed before use in the experimental infection. Inoculum was derived from a stabilate originally collected in 1991 from an infected horse with Derrengadera, from the Hato El Frío (7°56' North latitude and 68°57' West longitude) in the Apure State of Venezuela.
Typanosomes were characterized as monomorphic trypomastigote forms, and were classified as T. evansi, according to clinical, morphological (T. brucei -like) and biological (susceptibility for experimentation animals) criteria. Furthermore trypanosomes were also recently classified as T. evansi according to its ultrastructure feature [27] and low number of coated vesicles in the flagellar pocket in relation to T. equiperdum a co-endemic specie of trypanosome [3]. A T. evansi EcF1991 stabilate was defrosted and then 0.5 ml injected intraperitoneally into Spragüe-Dawley rats that were daily examined for presence of parasites by making wet smears from the tail blood. When number of parasites was scored as a +4 ( 20 trypanosomes at 40 X) a tail blood aliquot was diluted in phosphate saline glucose buffer pH 8.0 (PSG) [28] and number of viable trypanosomes was counted into a Neubauer haemocytometer. Rats were then anesthetized by chlorophorm inhalation and blood was taken directly by heart puncture with heparin as anticoagulant. Infected blood was diluted in PSG (2 ml final volume) and the inoculum (106 trypanosomes/calf) was made intravenously by venipuncture of jugular vein. The infection of calf was followed up for 30 days and after the period of experimentation was treated with diminacene aceturate (Berenil®) at a dose of 3.5 mg of diminacene/Kg of body weight. Calf was maintained under veterinary supervision until the recovery of normal PCV values and no trypanosomes were observed in the buffy coat of microhematocrit tubes.
Clinical and parasitological observations
Clinical observations were daily made at 9:00 a.m., and included loss of appetite, depression, weakness, refusal to walk, body temperature and haematocrit. Body temperature was determined using a rectal thermometer. The percentage of Packed Cell Volume (% PCV) or haematocrit, was estimated with blood samples collected from jugular vein into ethylene-diamine-tetra-acetic acid (EDTA) tubes. For the % PCV estimation, 3 heparinized capillary tubes (75 x 1.5 mm) were filled with blood and spun in a microhaematocrit centrifuge for 5 minutes at 12,000 rpm. The % PCV was measured with a microhematocrit reader. Haemoglobin (Hb) level was estimated from % PCV values using corrected equation of Flores-Torres et al. [29] as follow:
Estimated Hb (g/dL) = 0.257 + (%) PCV/ 3.135
Calf parasitemia was daily estimated by the dark ground buffy coat method and the intensity of infection was graded from 0 to 6 as described by Paris et al. [30]. For this purpose filled micro capillaries used for PCV estimation were microscopically analysed and bloodstream trypomastigotes counted in the upper layer of buffy coat using a 40X objective.
Kinetic of bovine IgG anti-T. evansi antibodies
Levels of bovine IgG anti-T. evansi during infection were determined by an indirect-ELISA previously standardized for detection of bovine IgG antibodies against T. vivax [25,26]. For this purpose a T. evansi crude antigen was prepared from DEAE-cellulose purificated trypanosomes by dilution of 6 x 108 trypanosomes in 1 ml of phosphate buffered saline (PBS) pH 7.4 and ultrasonic disruption of trypanosomes for 60 seconds at 4°C with an ultrasonic cell disruptor at 50% of maximun allowable amplitude. After centrifugation at 12,000 × g for 5 minutes, supernatant was aliquoted and stored under liquid nitrogen as stock antigen. Protein concentration of antigen stock was 5.00 g/l as determined by Folin Phenol Protein assay method [31]. ELISA used to detect serum antibodies to T. evansi was performed according to method described by Luckins [32] and Reyna-Bello et al. [33]. Optimal dilutions of antigen and sera were determined by checkerboard titration using positive and negative control sera. Assays were performed on microplates Immulon Nº 1, Dynatech. Table 1 shows the optimal conditions for indirect-ELISA applied for detection of bovine IgG antibodies against T. evansi.
| ELISA steps | Assay conditions |
|---|---|
| 1. Microplates sensitization with T. evansi crude antigens | Antigen dilution: 1/480 Buffer: Carbonate-Bicarbonate 0.1 M pH 9.6 Time: Overnight Temperature: 4°C in humid chamber |
| 2. Blocking of non-antigen sites | Blocking solution: PBS-0.1% Tween 20 pH 7.2, 2% gelatin. Time: 2 hours Temperature: 37 ºC in humid chamber |
| 3.Microplates washing | Washing buffer: PBS-0.1% Tween 20 pH 7.2 Temperature: Ambient Time: 5 minutes x 3 |
| 4. Incubation with bovine sera | Dilution: 1/100 Dilution buffer: PBS-0.1% Tween 20 pH 7.2, 2% gelatin Time: 1 h Temperature: 37°C in humid chamber |
| 5. Microplates washing | Washing buffer: PBS-0.1% Tween 20 pH 7.2 Temperature: Ambient Time: 5 minutes × 3 |
| 6. Incubation with secondary antibody couple to horseradish peroxidase | Secondary antibody: Rabbit IgG-Peroxidase anti-bovine IgG whole molecule (SIGMA) Dilution: 1/1000 Dilution buffer: PBS-0.1% Tween 20 pH 7.2, 2% gelatin Time: 1 h Temperature: 37°C in humid chamber |
| 7. Detection of antigen-antibodies reactions | Chromogenic substrate: ABTS-H2O2 Reaction buffer: Citrate-Citric Acid 0,1 M pH 4.6 Time: 1 h Temperature: Ambient Stop solution: H2SO4 |
| 8. Microplate reading | Wavelength (λ) for O.D reading: 405 nm |
Table 1: ELISA optimal conditions for detection of IgG antibodies anti- T. evansi in bovine sera according to Rossi et al. [26].
Results
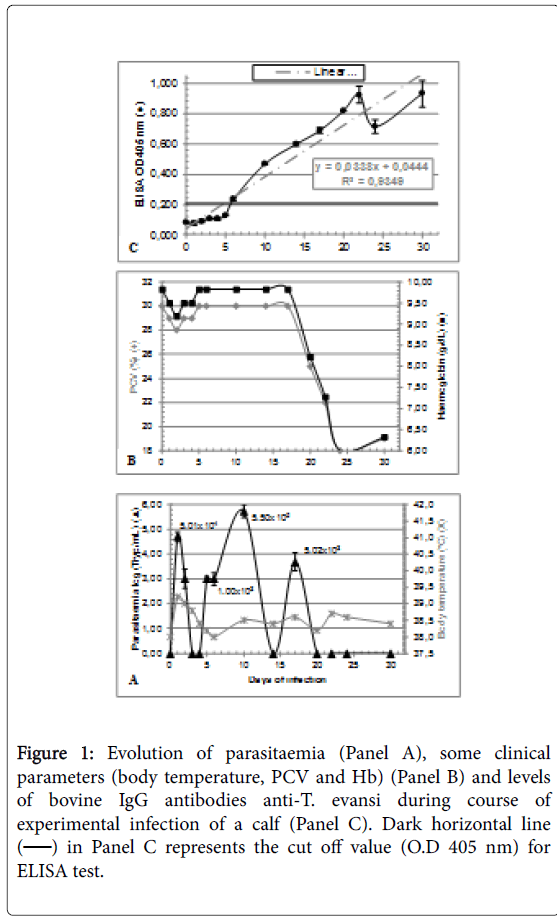
Table 2 shows the results of the analysis for hematological, biochemical and serological parameters of the calf used in the trial, in the 30 days prior to infection. As it can be seen results of IgG anti-T. evansi by ELISA were negative in concordance with the absence of bloodstream trypanosomes. At the beginning of the trial the calf had a healthy state as evidenced by the values of PCV and Hb. Results from parasitological, clinical and serological parameters assessed during infection are shown in Panels A, B and C of (Figure 1). Prepatent period of T. evansi infection in calf was 1 day. During experimental infection of a calf with T. evansi, parasitaemia showed an undulating behaviour in which three evident parasitaemia peaks were observed at days 1 (5.01 × 104 Tryp/mL), 10 (5.00 × 105 Tryp/mL) and 17 (5.02 × 103 Tryp/mL), with an increasing trend from day 0 to 10 and a decreasing trend between day 10 and 30. Maximal parasitaemia level was reached at day 10 (5.50 × 105 tryp/mL) while minimal detectable level of 1 × 103 Tryp/mL was reached on days 3, 5 and 6. Bloodstream trypomastigotes were undetectable on days 3, 4, and 14, and from day 20 to day 30 post infection (Panel A).
Figure 1: Evolution of parasitaemia (Panel A), some clinical parameters (body temperature, PCV and Hb) (Panel B) and levels of bovine IgG antibodies anti-T. evansi during course of experimental infection of a calf (Panel C). Dark horizontal line (??) in Panel C represents the cut off value (O.D 405 nm) for ELISA test.
| Days | % PCV | Haemoglobin (g/dL) | O.D 405 nm | Tryp/mL |
|---|---|---|---|---|
| 0 | 32 | 10.46 | 0.160 | 0.00 |
| 5 | 32 | 10.50 | 0.148 | 0.00 |
| 10 | 32 | 10.55 | 0.150 | 0.00 |
| 15 | 34 | 10.10 | 0.164 | 0.00 |
| 20 | 32 | 10.20 | 0.170 | 0.00 |
| 25 | 33 | 10.78 | 0.175 | 0.00 |
| 30 | 32 | 10.50 | 0.172 | 0.00 |
Table 2: Hematological, biochemical and serological parameters of calf in the 30 days prior to infection with T. evansi.
As it can be seen in Panel A, first peak of parasitaemia (day 1) was accompanied by an increase in body temperature (39°C) and a simultaneous transient-decreasing on values of PCV and Hb from day 1 to 4, moment at which they begin to increase, reaching preinfection values (day 0). From day 1 to 6 body temperature experienced a marked decreasing to preinfection value followed by an evolving with slight fluctuations near 38°C during rest of infection. No fluctuations on PCV and Hb values were recorded from day 5 to 15 (Panel B).
Panel C shows that seroconversion on bovine was achieved on day 6, moment at which O.D 405 nm developed by serum during ELISA test overcame the cut off value for assay. From these moment calf experienced a sustained linear increase (y = 0,0338x + 0,0444) on levels of IgG antibodies anti-T. evansi during infection, as it can be inferred by value of Pearson's coefficient calculated for association of variables Days of infection and ELISA O.D 405 nm (R2 = 0,9349).
Marked increasing in antibody levels, reaching values of O.D 405 nm=0.713 was detected on day 24, allowing to infer that concentration of bovine IgG reached in serum was of 200 g/L, as it can be inferred by O.D 405 nm=0.769, developed by the positive control of indirect ELISA in which 20 ng of bovine IgG were sensitized to microplate by adding 100 μL of bovine IgG from a stock solution of 0.2 μg/mL.
Maximal decreasing on PCV and Hb levels begin on day 17, moment in which ELISA O.D 405 nm reached a value of 0.688. This pronounced decreasing trend occurred from day 17 to 24 determining minimal value of 18% for PCV and 6 g/dL for Hb when an ELISA O.D 405 nm value of 0.713 was reached. This diminuition on PCV and Hb values in relation to preinfection values was of 60% (18/30) and 64% (6.32/9.83) respectively.
Discussion
This study shows values of clinical, parasitological and serological parameters obtained during 30 days from experimental infection of one calf with Venezuelan isolated EcF1991 of T. evansi. The main limitation of our study is due limited number of experimental subjects used as a consequence of economical resources scarcity. For this reason only a descriptive approach is presented and the work herein presented should be considered as a preliminary study. Despite it was impossible to present a statistical analysis, results about establishment of a bovine experimental infection with Venezuelan isolated used, not only confirm results about molecular detection of this African trypanosome in cattle [7], but also describe for the very first time behaviour of experimental infection and application of an indirect-ELISA for detection of IgG antibodies against T. evansi.
Despite increasing of body temperature in infected calf was only described for first three days of infection, results herein presented are in agreement with those obtained by Payne et al. [34,35] in their works, which describe a marked reduction in the rate of weight gain and fall in PCV values of Friesian Holstein calves and heifers experimentally infected with an Indonesian isolate of T. evansi. In this study body temperature of calf was normal throughout 30 days, with the exception of the first three days of infection (parasitemia peak). This result was not in agreement with the fact that the marked increasing trend in level of antibodies recorded by ELISA, should be followed by fever as a consequence of the effects of antigen-antibody immune complexes formed during infections, that acts as a potent stimulus for pyrogen release.
This finding also differs with results obtained by Damayanti et al. [36] in which fluctuating pyrexia of buffaloes experimentally infected with Indonesian isolate of T. evansi commenced 5-8 days after infection and was preceded by a parasitemia wave. On the other hand, differs with results obtained during experimental infections of sheeps [37] and goats [38] with T. evansi, in which no significant changes of body temperature were recorded.
Parasitological behaviour of T. evansi EcF1991 infection was quite similar to those described for Tejera in 1920 [17]. In this regard trypanosomes were observed in blood few days after infection (from day 1 to 17) but parasitemia was transient and parasites disappeared of blood for the next 13 days of infection. This behaviour of T. evansi infection is characteristic of chronic infections and agrees with observations made in rabbits, sheep and goats following experimental and natural infections [37-41], as well as in Indonesian buffaloes (Bubalus bubalis) [36].
Disappearing of T. evansi EcF1991 from bloodstream during infection of calf, should be attributable to antibodies-mediated destruction, but recently published evidences by Rossi et al. [42] about description of intracellular stages of T. evansi in cells of adrenal cortex, hepatocytes, plasma cells and lymphocytes of mice experimentally infected, suggest the possibility that cryptic parasitemias and relapses could be related with T. evansi EcF1991 tissue tropism and its capability of hiding intracellularly.
Despite disease caused by T. evansi in horses is manifested by increased temperature, anaemia and weakness, that may develop over a few months or years, leading to death [43-45], the clinical pattern of calves and heifers infections with T. evansi are characterized by marked reduction in the rate of weight gain increase in body temperatures and fall of PCV values [34,35], and seems to be less severe than described for horses and buffaloes [36].
Intermitent fever and relapsing parasitemia are characteristic of African trypanosomosis and T. evansi infections of cattle, other domestic animals [43]. Periodical fluctuations of parasitemia seems to be attributable to antigenic variation, the main mechanism of immune evasion in African trypanosomes [46] that plays an important role in causing the relapsing course of disease as it has been described by Perrone and colaborators for the same isolated used in this work.
Despite the low level of parasitemia recorded, there were significant changes in PCV and Hb values reaching levels of 60% and 64% below preinfected values. Due this marked reduction on PCV and Hb values are interpreted as signs of intense infection and anaemia, infection of calf with T. evansi EcF1991 should be interpreted as an infection with serious pathological consequences instead a benign infection of bovines as it has been inferred from Tejera and Kubes works of 1920 and 1939 [17]. In this regard, although reduction of PCV and erythrocyte counts in sheep infected with T. evansi TRUE 2143 cannot be regarded as indicative of severe haematological changes [37], reduction of the PCV value in calf infected with T. evansi EcF1991 should be considered as severe anemia, as it has been described for buffaloes and cattle in which PCV values fall between 24-46% below non-infected controls and normal range for cattle [36].
Several enzyme linked Immunosorbent assays (ELISA) using crude antigens of T. evansi has been developed and applied for serological surveys in dairy cattle [47], cattle, buffaloes and horses from Eastern Region of India [48] and buffaloes from Thailand [49], in order to detect antibodies anti-T. evansi with sensitivities and specificities of 92.5% and 94.2%, respectively.
In this study, we have applied an indirect-ELISA test for the detection of bovine IgG anti-T. evansi that allowed the discrimination between seropositive and seronegative bovines to T. vivax with a sensitivity of 94.87% and specificity of 92.86% [25,26]. Serological results herein presented demonstrated that indirect-ELISA applied, was capable to obtain the kinetics of bovine IgG antibodies anti-T. evansi during infection and that calf was capable to develop an IgG antibody response against T. evansi. In this regard, the maximal level of bovine IgG anti-T. evansi reached on day 24 was 200 g/L, a value that was 7 to 12 times higher than normal level of bovine IgG (17-27 g/L) described by Tizard [50] in healthy animals.
These levels of IgG anti-T. evansi during infection were surely responsible for parasitemia control and should be related with PCV reduction as it has been reported in infections with other African trypanosomes [43,51,52]. In fact, maximal decrease on PCV and Hb values was registered at the same time that the maximal increase in OD 405nm was achieved. This result was consistent with an immunologically mediated anemia as it has been described in mice experimentally infected with T. brucei, mice infected with T. evansi [53] and calves infected with T. congolense [54].
During this process, calf erythrocytes surface could experience a biochemical modification by different mechanisms, such as peroxidation of erythrocyte´s lipids [55], remotion of sialic acid residues by proteases or phospholipases extracellularly secreted by live or dead trypanosomes [56,57] or by changes in the surface oligosaccharide patterns during adhesion of T. evansi to mice erythrocytes [53]. All these pathogenic mecanisms have the common effect of modify erythrocyte antigenicity, rendering them more prone to phagocytosis by mononuclear phagocitic system (macrophages and Küpffer cells), as it has been described for periferic- and deep-circulating erythrocytes of adrenal gland cortex [58] and liver [59].
Conclusion
This work not only reports for the very first time the kinetics of bovine IgG antibodies anti-T. evansi by indirect ELISA during infection of a calf with the Venezuelan isolated EcF1991, but also changes in some clinical parameters that suggest its pathogenic effect for Venezuelan bovines instead of "benign" appreciation of this infection. On the other hand, our work report that ELISA assay conditions used for detection of bovine IgG antibodies to T. vivax in cattle, can be also used for screening of T. evansi seroprevalence in bovines.
The transient and cryptic pattern of T. evansi parasitemia in bovines, confirmed by molecular studies of Ramírez-Iglesias et al. [7] together with the possibility of the developing of intracellular stages by T. evansi, as it has been described in mice [42], suggest the need to assess tissue tropism of T. evansi in bovines, because this peculiarity could explain not only parasitemia pattern herein presented, but also the capability of bovines to act as auto chronic hosts (reservoirs), as well as the effects of cryptic parasitemia on the prevalence of T. evansi in horses and other domestic and wild animals.
Conflicts of Interest
The authors (Marcello Salvatore Rossi Spadafora and Pedro María Aso) declare that there are no competing interests regarding the publication of this paper.
Funding
This research was supported by Project BTS-53 del Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICIT) of Venezuela Republic and own funds of the Laboratory of Biochemistry and Immunology of Hemoparasites, Cell Biology Department, Universidad Simón Bolívar, Caracas, Venezuela.
Acknowledgement
The authors would like to thank to the animal facility of Cell Biology Department (Universidad Simón Bolívar) for the care and maintenance of experimental bovine used in this work, to Laboratory Preparers by their invaluable assistance during sampling and obtention of sera and to DVM Alfredo Noda for his clinical assistance of sick animal during infection period.
This work is dedicated to the memory of Dr. Trina Perrone, Dr. Francisco Garcia and Dr. María Isabel Camejo tireless colleagues, friends and researchers of the animal trypanosomosis in Venezuela.
References
- Luckins AG (1988) Trypanosoma evansi in Asia. Parasitol Today 4: 137-142.
- Franke RC, Greiner M, Mehlitz D (1994) Investigations on naturally occurring Trypanosoma evansi infections in horses, cattle, dogs and capybaras (Hydrochoerus hydrochaeris) in Pantanal de Poconé (MatoGrosso, Brazil). Acta Trop 58: 159-169.
- Brun R, Hecker H, Lun ZR (1998) Trypanosoma evansi and Trypanosoma equiperdum: distribution, biology, treatment and phylogenetic relationship. Vet Parasitol 79: 95-107.
- Aquino LP, Machado RZ, Lemos KR, Marques LC, Garcia MV, et al. (2010) Antigenic characterization of Trypanosoma evansi using sera from experimentally and naturally infected bovines, equines, dogs, and coatis. Rev Bras Parasitol Vet 19: 112-118.
- Garcia H, Garcia ME, Perez H, Mendoza-Leon A (2005) The detection and PCR-based characterization of the parasites causing trypanosomiasis in water-buffalo herds in Venezuela. Ann Trop Med Parasitol 99: 359-370.
- Jaimes-Dueñez J, Triana-Chávez O, MejÃa-Jaramillo AM (2017) Parasitological and molecular surveys reveal high rates of infection with vector-borne pathogens and clinical anemia signs associated with infection in cattle from two important livestock areas in Colombia. Ticks Tick Borne Dis 8: 290-299.
- RamÃrez-Iglesias JR, Eleizalde MC, Reyna-Bello A, Mendoza M (2016) Molecular diagnosis of cattle trypanosomes in Venezuela: evidences of Trypanosoma evansi and Trypanosoma vivax infections. J Parasit Dis pp: 1-9.
- GarcÃa FA, Rivera M, Bermúdez V, Alvárez L (1983) Ocular lesions in a dog with natural infection by Trypanosoma venezuelense. Acta Cient Venez 34: 105.
- Correa-Salgado AM, Pacheco De Araujo FA, Cañón-Franco WA (2010)Infección Natural por Trypanosoma evansi en Canino, Manizales - Colombia: CasoclÃnico. Rev Ibero-Latinoam Parasitol 69: 99-100.
- Toro-Benitez M, López R, León E, RuÃz A, GarcÃa A, et al.(1982)ProteÃnasSéricas y presencia de anticuerpos de tripanosomas en chigüires (Hydrochoerushidrochaeris) de Guasdalito, Estado Apure. Rev FacCiens Vets Universidad Central de Venezuela 11: 47-53.
- Arias JF, Garcia F, Rivera M, Lopez R (1997) Trypanosoma evansi in capybara from Venezuela. J Wildl Dis 33: 359-361.
- Eberhardt AT, Monje LD, Zurvera DA, Beldomenico PM (2014) Detection of Trypanosoma evansi infection in wild capybaras from Argentina using smear microscopy and real-time PCR assays. Vet Parasitol 28: 226-233.
- Rjeibi MR, Ben Hamida T, Dalgatova Z, Mahjoub T, Rejeb A, et al. 2015) First report of surra (Trypanosoma evansi infection) in a Tunisian dog. Parasite 22: 3.
- Hoare C (1972) The Trypanosomes of Mammals. A Zoological Monograph Blackwell Scientific Publications. Oxford pp: 555-593.
- Silva-Iturriza A, Nassar JM, GarcÃa-Rawlins AM, Rosales R, Mijares A, et al. (2013) Trypanosoma evansi kDNAminicircle found in the Venezuelan nectar-feeding bat Leptonycteriscurasoae (Glossophaginae), supports the hypothesis of multiple origins of that parasite in South America. Parasitol Int 62: 95-99.
- Rangel R (1905) Nota preliminarsobre la PesteBoba y la Derrengadera de los EquÃdeos de los Llanos de Venezuela. (Tripanosomiasis). GacMéd Caracas 12: 105-113.
- DÃaz-UngrÃa C (1960) ParasitologÃa Venezolana Volumen I. Sociedad de Ciencias Naturales La Salle, Fundación La Salle de Ciencias Naturales. Editorial Sucre CA. Caracas, Venezuela.
- Fernández AJ (1931) Tripanosomiasis de los bovÃdeos de Venezuela. GacMéd Caracas 38: 17-21.
- Fiasson R, Mayer M, Pifano F (1948) Le cariacou (Odoicoileusgymnotis) porteur de Trypanosoma vivax en Venezuela. Bull Soc Path Exot 41: 206-208.
- Gonzatti MI, González-Baradat B, Aso P, Reyna-Bello A (2013) Trypanosoma (Duttonella) vivax and Trypanosomosis in Latin America: Secadera/Huequera/Cacho hueco. Trypanosomes and Trypanosomiasis". edits: Stefan Magez and Magdalena Radwanska. Springer pp: 261-285.
- Raymond HL (1990) Tabanusimportunus, experimental mechanical vector of Trypanosoma vivax in French Guiana. Ann Parasitol Hum Comp 65:44-46.
- Otte MJ, Abuabara JY (1991) Transmition of South American Trypanosoma vivax by the neotropical horsefly Tabanusnebulosus. Acta Trop 49: 73-76.
- Coronado A, Suarez C, Román D (2006) Survival of Trypanosoma evansi in the intestinal tract of experimentally infected Stomoxyscalcitrans. Gac Cien Vet 12: 72-76.
- GarcÃa FA, Rivera M, Ortega M (1992) Natural Infection and Seroprevalence of Trypanosoma evansi in Horses of Apure State, Venezuela. 1th eminaire International Sur Les Trypanosomoses Animales Non Transmises Par Les Glossines, Annecy, France. (Sup): 182.
- Rossi M (1994) Enzyme-Linked Immunosorbent Assay (ELISA) for the diagnosis of bovine trypanosomiasis caused by Trypanosoma vivax. Licentiate in Biology Thesis, Central University of Venezuela, Caracas, Venezuela. pp: 113.
- Rossi S MS, Reyna-Bello A, Perrone T, León T, GarcÃa F, et al. (2017) Development and Application of an Standardized Enzyme-Linked Immunosorbent Assay (ELISA) using Trypanosoma evansi crude antigens for the detection of bovine IgG antibodies against Trypanosoma sp in Venezuela. Submitted to Veterinary World: Open Access.
- Rossi S MS (2009) Trypanosoma evansi: Parasitological, Ultrastructural and Chemotherapeutical Studies in the Experimental Infection of Mice, Doctor Scientarum (DSc) in Biology Thesis, Central University of Venezuela, Caracas, Venezuela. pp: 397.
- Lanham SM, Godfrey DG (1970) Isolation of salivarian Trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol 28: 521-534.
- Flores-Torres J, Echeverria-Ortega AM, Arria-Bohorquez M, Hidalgo G, Albano-Ramos C, et al. (2011) Diferencias entre la hemoglobinaobservada y estimadaporhematocrito y suimportancia en el diagnóstico de anemia en poblacióncosteravenezolana:análisis del segundoestudionacional de crecimiento y desarrollohumano (SENACREDH). Rev Peru Med Exp Salud Pública 28: 47-53.
- Paris J, Murray M, McOdimba F (1982) A comparative Evaluation of Parasitological Techniques Currently Available for the Diagnosis of African Trypanosomiasis. Acta Trop 39: 307-316.
- Lowry OH, Rosebrough NJ, Farr LA, Randall RJ (1951) Protein Measurement with the Folin Phenol Reagent. J Biol Chem193: 265-272.
- Luckins AG (1977) Detection of antibodies in trypanosome infected cattle by means of microplate enzyme-linked immunosorbent assay. Trop AnimHlth Prod 9: 53-62.
- Reyna-Bello A, GarcÃaFA, Rivera M, Sansó B, Aso PM, et al. (1998) Enzyme-linked immunosorbent assay (ELISA) for the detection of anti-Trypanosoma evansi equine antibodies. Vet Parasitol 80: 149-157.
- Payne RC, Sukanto IP, Partoutomo S, Polytedi F (1992) Experimental infection of Friesian Holstein calves with an Indonesian isolate of Trypanosoma evansi. Trop Med Parasitol 43: 115-117.
- Payne RC, Sukanto IP, Bazeley K, Jones TW (1993) The effect of Trypanosoma evansi infection on the oestrous cycle of Friesian Holstein heifers. Vet Parasitol 51: 1-11.
- Damayanti R, Graydon RJ, Ladds PW (1994) The Pathology of Experimental Trypanosoma evansi Infection in the Indonesian Buffalo (Bubalusbubalis). J Comp Pathol 110: 237-252.
- Onah DN, Hopkins J, Luckins AG (1996)Haematological changes in sheep experimentally infected with Trypanosoma evansi. Parasitol Res 82: 659-663.
- González B, Mota M, Espinoza E, Rossi M, Bello A, et al. (1995) Experimental Infection of goats with a Venezuelan isolated of Trypanosoma evansi. Acta Cient Venez 46 Suppl 1: 89.
- Luckins AG, Gray AR, Rae P (1978) Comparison of the diagnostic value of serum immunoglobulin levels, an enzyme-linked immunosorbent assay and a fluorescent antibody test in experimental infections with Trypanosoma evansi in rabbits. Ann Trop Med Parasitol 72: 429-441.
- Boid R, Amin EA, Mahmoud MM, Luckins AG (1981) Trypanosoma evansi infections and antibodies in goats, sheep and camels in Sudan. Trop Anim Hlth Prod 13: 141-146.
- Ngeranwa JJN, Mutiga ER, Agumbah GJO, Gatumbi PK, Munyua WK, et al. (1991) The effects of experimental Trypanosoma (Trypanozoon) (brucei) evansi infection on the fertility of male goats. Vet Res Comm 15: 301-308.
- Rossi S MS, Boada-Sucre AA, Marquez ML, RodrÃguez P, Hernández G, Payares G, et al. (2017) Description of an intracellular stage in the experimental infections of albino mice with a Venezuelan isolated of Trypanosoma evansi using scanning and transmission electron microscopy techniques. Submitted to VeterinariaItaliana (In press).
- Losos GJ (1980) Diseases caused by Trypanosoma evansi. A review. Vet Res Comm 4: 165-180.
- Mahmoud MM, Gray AR (1980) Trypanosomiasis due to Trypanosoma evansi (Steel, 1885) Balbiani, 1888. A review of recent research. Trop Anim Hlth Prod 12: 35-47.
- Hörchner F, Schönefeld A, Wüst B (1983) Experimental infection of horses with Trypanosoma evansi, Parasitological and clinical results. Ann Soc Belge Méd Trop 63: 127-135.
- Stijlemans B, Caljon G, Van Den Abbeele J, Van Ginderachter J.A, Magez S, et al. (2016) Immune Evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity. Front Immunol 7: 233.
- Desquesnes M, Kamyingkird K, Pruvot M, Kengradomkij C, Bossard G, Sarataphan N, et al. (2009) Antibody-ELISA for Trypanosoma evansi: application in a serological survey of dairy cattle, Thailand, and validation of a locally produced antigen. Prev Vet Med 1: 233-241.
- Laha R, Sasmal NK (2009) Detection of Trypanosoma evansi infection in clinically ill cattle, buffaloes and horses using various diagnostic tests. Epidemiol Infect, 137: 1583-1585.
- Kocher A, Desquesnes M, Kamyingkird K, Yangtara S, Leboucher E, et al. (2015) Evaluation of an Indirect-ELISA Test for Trypanosoma evansi Infection (Surra) in Buffaloes and Its Application to a Serological Survey in Thailand. Biomed Res Int.
- Tizard IR (2009) InmunologiaVeterinaria. Editorial Elsevier, 8va edición. Madrid, España.
- Van Den Ingh TS, Zwart D, Schootman AJ, Van Miert AS, Veenendaal GH (1976) The pathology and pathogenesis of Trypanosoma vivax infection in goat. Res Vet Sci 1: 264-270.
- Poltera AA (1985) Pathology of human African trypanosomiasis with reference to experimental African trypanosomiasis and infections of the central nervous system. Br Med Bull 41:Â 169-174.
- Rossi S, Boada-Sucre AA, Simoes MT, Boher, Rodriguez P, et al. (2017) Adhesion of Trypanosoma evansi to Red Blood Cells (RBCs): Implications in the Pathogenesis of Anaemia and Evasion of Immune System. Diagnostic Pathology: Open Access 2: 122.
- Esievo KAN, Saror DI (1991) Immunochemistry and Immunopathology of Animal Trypanosomiasis. Vet Bull 61: 765-777.
- Mijares A, Vivas J, Abad C, Betancourt C, Pinero S, et al. (2010) Trypanosoma evansi: Effect of experimental infection on the osmotic fragility, lipid peroxidation and calcium-ATPase activity of rat red blood cells. Exp Parasitol 124: 301–305.
- Igbokwe IO (1994) Mechanisms of cellular injury in African trypanosomiasis. Vet Bull, 64: 611- 620.
- Rossi M, Bremo A, Gonzatti MI, Giardina S (1995) Trypanosoma evansi secretes proteins extracellularly. Acta Cient Venez 1: 88.
- Rossi M, Boada-Sucre A, Finol HJ, Tejero F, Bello B, et al. (1999) Ultrastructural alterations in the adrenal gland cortex of mice experimentally infected with a Venezuelan isolate of Trypanosoma evansi. J Submicrosc Cytol Pathol 31: 509-513.
- Rossi S MS, Boada-Sucre AA, Hernández G, Bello B, Finol HJ, et al. (2008) Análisis Ultra estructural del HÃgado en RatonesInfectadosExperimentalmente con un Aislado Venezolano de Trypanosoma evansi (KINETOPLASTIDA: TRYPANOSOMATIDAE). Acta Micros 17: 5-12.
Citation: Rossi S MS, Aso PM, García F (2017) Assessment of Parasitological Behaviour, Clinical Changes and Serology during Experimental Infection of a Calf with a Venezuelan Isolated of Trypanosoma evansi: A Preliminary Study. Diagn Pathol Open 2: 125. DOI: 10.4172/2476-2024.1000125
Copyright: © 2017 Rossi MSS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3727
- [From(publication date): 0-2017 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 2870
- PDF downloads: 857