Assessment of Inflammation and Damage in Rheumatoid Arthritis Patients with Methotrexate Inadequate Response Receiving Tocilizumab Using Low Field MRI
Received: 28-Apr-2014 / Accepted Date: 23-Sep-2014 / Published Date: 30-Sep-2014 DOI: 10.4172/2161-1165.1000173
Abstract
Objectives: The aim of the study was to monitor joint inflammation and destruction in rheumatoid arthritis (RA) patients receiving tocilizumab therapy using MRI and compare MRI findings with clinical, biological and radiographic data.
Methods: Inclusion criteria were patients aged between 18 and 65 years, fulfilling American College of Rheumatology 1987 criteria for RA. All patients had methotrexate inadequate response with no prior biologic exposure. All patients were evaluated clinically including disease activity score 28 (DAS28) and by low field dedicated MRI (dominant hand and wrist) at initiation of treatment with anti-IL 6 receptor antibody agents and after 6 months. The MRI images were scored using the Outcome Measures in Rheumatology Clinical Trials RAMRI score (OMERACT RAMRIS).
Results: Among 22 patients with RA included in the study; 19 were female. The mean age was 42years ±13.7. Tree patients were excluded from the study before 24 weeks because of serious side effects. The study population exhibited significant decreases in all measures of disease activity at 24 weeks. At 24 weeks, the median RAMRIS synovitis (p<0.0001) and bone edema (p=0.04) scores were significantly reduced while RAMRIS bone erosion score was unchanged. The baseline RAMRIS synovitis score was strongly correlated with delta RAMRIS edema at 24 weeks (r= - 0.46; p=0.04).
Conclusion: This study suggests a significant reduction in MRI pre-erosive lesions (synovitis and osteitis) using Tocilizumab in patients with RA with inadequate response to DMARDS. Prospective studies with long term follow-up and imaging as an outcome measure are needed.
Keywords: Rheumatoid Arthritis; Tocilizumab (anti-IL6); RAMRIS; low field MRI
162534Introduction
Optimum management of rheumatoid arthritis (RA) requires early diagnosis and timely introduction of agents that reduces inflammation and consequently inhibits structural damage [1]. Indeed, the opportunity to induce remission/low disease activity in Rheumatoid Arthritis (RA) patients has been achieved in recent years by the adoption of more sensitive diagnostic methods; particularly Magnetic Resonance Imaging (MRI), and ultrasonography and early aggressive treatments. Tocilizumab (TCZ), a humanized monoclonal antibody that binds to both forms of the interleukin-6 receptor, showed clinical and structural efficacy in patients with RA [2-5]. The RA Disease Activity Score in 28 Joints (DAS28) is used to evaluate the response to TCZ. Indeed, the DAS28 is an excellent tool for assessing disease activity in RA. However and particularly during treatment with TCZ, CRP and DAS28 decrease very fast and very early and seem to overestimate the remission. Hence, it should be necessary to associate these tools to imaging (US or MRI) [6]. Magnetic resonance imaging (MRI) is more sensitive for the detection of both inflammatory (synovitis) and structural (erosive) joint changes than clinical examination and radiography. While conventional radiographs remain the standard reference methods for assessing destructive skeletal changes in patients with RA, radiographs are inherently limited by the lack of ability to assess pre-erosive changes that precede damage to the osseous component of the joint, a stage of disease that had been thought to be irreversible [7]. In addition to being much more sensitive in detecting joint erosions, MRI can also detect pre-erosive lesions (synovitis and osteitis) [8-11]. The areas of bone that appear as bone oedema or osteitis on MRI have been shown to be heavily infiltrated by inflammatory cells including osteoclasts [12], and MRI-detected synovitis and osteitis have been shown to increase the risk of developing new erosions over time as detected by either MRI or radiograph [13-20]. Detection and treatment of pre-erosive lesion can therefore significantly alter the course of RA.
Thus, MRI offers improved opportunities for investigating the course of joint inflammation and destruction during new therapeutic approaches, as in this case TCZ. Quantitative analysis in MRI has advantages over semi quantitative analysis in measuring the response to therapeutic agents, as can reflect a change in inflammatory activity more sensitively [6,21]. The Outcomes Measures in Rheumatoid Arthritis clinical trials (OMERACT) has developed the RAMRIS (RA MRI scoring system) to measure therapeutic effects in clinical studies in particular with biologic agents. The RAMRIS consists of semi quantitative assessment of bone erosion, bone marrow edema and synovitis and is sensitive to change [19,20].
The aim of the study was to monitor joint inflammation and destruction in rheumatoid arthritis (RA) patients receiving tocilizumab therapy using MRI and compare MRI findings with clinical, biological and radiographic data.
Patients and Methods
Population sample
Twenty two patients with RA (fulfilling American College of Rheumatology 1987 criteria for RA) at the department of Rheumatology Rabat-Sale university hospital enrolled in an open label, 24 weeks follow up study. All patients have had methotrexate inadequate response aged between 18 and 65 years with moderate to severely active disease defined as Disease Activity Score in 28 joints based on erythrocyte sedimentation rate (DAS28-ESR) at baseline and an inadequate response to a stable dose of MTX (=15 mg/week for =6 weeks). TCZ was administered for study purposes only and not for routine treatment. Exclusion criteria for the study, included pregnancy, severe comorbidities, prior use of any biologics and use of csDMARDs other than MTX 1 month (3 months for leflunomide) before the baseline visit. All patients who were eligible agreed to participate in the study. The use of corticoids and analgesic drugs was allowed at baseline and during the follow up. Among 22 patients with RA included in the study; 19 were female. The mean age was 42 years ±13.7; their mean disease duration was 7.9 ±5.2 years. All patients signed a separate informed consent for inclusion in this study. The study was approved by the local independent committee of Rabat and conducted according to the principles of the declaration of Helsinki.
Tocilizumab therapy
Each patient received intravenous injections of tocilizumab monotherapy (8 mg/kg/every 4 weeks). Oral glucocorticoids and non-steroidal anti-inflammatory drugs were permitted.
Standard clinical assessment
Clinical assessment was performed for each patient by one of tree rheumatologists. All of whom were blinded to the imaging findings. Disease activity was assessed by DAS28 ESR. Functional disability was estimated by the Health Assessment questionnaire (HAQ).
Laboratory assessment
Serum markers of inflammation, C reactive protein (CRP) and erythrocyte sedimentation rate were obtained at the same day as MRI. The anti-CCP and rheumatoid factor dosage were performed only at baseline.
Magnetic resonance imaging
Image acquisition
MRI of the dominant wrist and 2nd to 5th metacarpophalangeal (MCP) joints was carried out at baseline and 6 months, using a 0.2 Tesla artoscan system (ESAOTE Biomedica, Genova, Italy) a dedicated extremity MRI unit. Patients ware comfortably seated in an adjustable scanning chair, with the dominant hand positioned in a wrist coil in the magnet in neutral position. The imaging protocol comprised a coronal and axial Short Tau inversion recovery (STIR) sequence followed by coronal and axial T1-weighted Turbo Spin Echo 3D images obtained before and after intravenous injection Gabapentic acid (0.2 mmol/kg body weight). The post contrast coronal and axial images were started 3 min after the injection of contrast agent. The imaging parameters for the STIR sequence were as follows: repetition time 500 ms, Echo time 18 ms, matrix size 256×160, field of view (FOV) 200 mm, slice thickness 3mm. For the T1 weighted Spin Echo 3D sequences, the imaging parameters were as follows: repetition time 500 ms, Echo time 18 ms, matrix size 256×192, field of view (FOV) 200 mm, slice thickness 1 mm.
Image evaluation
All the MRI were scored for synovitis, bone edema and bone erosion as defined in the OMERACT MRI RA recommendations (Table 1). Synovitis: is assessed in three wrist regions (the distal radioulnar joint; the radiocarpal joint; the intercarpal and carpometacarpal joints) and in each MCP joint. The first carpometacarpal joint and the first MCP joint are not scored. The scale is 0-3. Score 0 is normal, and 1-3 (mild, moderate, severe) are by thirds of the presumed maximum volume of enhancing tissue in the synovial compartment. Bone erosions: each bone (wrists: carpal bones, distal radius, distal ulna, metacarpal bases; MCP joints: metacarpal heads, phalangeal bases) is scored separately. The scale is 0-10, based on the proportion of eroded bone compared to the ‘‘assessed bone volume’’, judged on all available images—0: no erosion; 1: 1-10% of bone eroded; 2; 11-20%, etc. Bone edema: each bone is scored separately (as for erosions). The scale is 0-3 based on the proportion of bone with oedema, as follows—0: no oedema; 1: 1-33% of bone edematous; 2: 34-66% of bone edematous; 3: 67-100% (Table 1). Sum scores of synovitis, erosion, and oedema can be calculated by summation of individual joint scores, as a total sum or separately in the evaluated wrist and second to fifth MCP joints, respectively. For synovitis, the possible range of sum scores of unilateral second to fifth MCP joints, wrist joint, and both are 0-12, 0-9, and 0-21, respectively. The corresponding values for bone erosion are 0-80, 0-150, and 0-230 and for bone edema 0-24, 0-45, and 0-69, respectively.
| Synovitis | Bone edema | Bone erosion | ||||
|---|---|---|---|---|---|---|
| Selection | MCP joint | Wrist | MCP joint | Wrist | MCP joint | Wrist |
| Areas | 2-5 proximal and distal | Distal radio-ulnar joint Radio-capral joint Inter-carpal -CMCJ | 2-5 proximal and distal | Trapezium, trapezoid, capitates, hamate, scaphoid, lunatr, triquetrum, pisiform Distal radius Distal ulna | 2-5 proximal and distal | Trapezium, trapezoid, capitates, hamate, scaphoid, lunate, triquetrum, pisiform Distal radius Distal ulna |
| Grades | All (0-3) | All (0-3) | All (0-10) | |||
Table 1: OMERACT MRI in RA group recommendations of MRI definitions of important RA joint, and an RA RAMRIS scoring. MCP: metacarpo-phalangeal joint, CMC J: carpo-metacarpal joint. Synovitis scale is 0-3. Score 0 is normal, and 1-3 (mild, moderate, severe). Bone erosions: each bone is scored separately. The scale is 0-10, 0: no erosion; 1: 1-10% of bone eroded; 2; 11-20%, etc. Bone edema: each bone is scored separately. The scale is 0-3; 0: no oedema; 1: 1-33% of bone edematous; 2: 34-66% of bone edematous; 3: 67-100%.
OMERACT RAMRIS was assessed by two rheumatologists with documented experience and high inter reader agreement. The assessors were blinded to clinical and radiographic findings.
X rays
Conventional x rays of both wrists and hands in anteroposterior projection were obtained at inclusion and 6 months. Radiological assessment were examined according to modified Sharp score of Van Der Heidje
Statistical analysis
Non parametric statistical methods were applied and description statistics (numbers, medians, ranges, and interquartile ranges were calculated. The Mann Whitney U test was used for pairwise group comparisons. The Wilcoxon matched pairs rank signed test was used to compare baseline values with data at 24 weeks. Correlations were calculated by means of the Spearman’s rank correlation test. P values <0.05 were considered significant. The statistical analyses were carried out with SPSS.
Results
Clinical measures of disease activity at baseline and at follow up
Table 2 present baseline and follow up characteristics of the 22 patients. Three patients were excluded from the study before 24 weeks because of serious side effects (pulmonary embolism in one 1 case, acute hepatitis in one case, and heart failure in one case). The study population exhibited significant decreases in all measures of disease activity at 24 weeks. The dose of corticosteroids decreased progressively through the period of the study (p=0.005). Physical activity expressed by the HAQ score increased through the observation period (p<0.001).
| Baseline | 24 weeks | p value | |
|---|---|---|---|
| N= 22 | N=19 | ||
| Age (years)1 | 42±13.7 | 40±12.7 | - |
| Female Sex2 | 19 (86.4) | 16 (84.2) | - |
| VASpain (0-100)3 | 55 (50-60) | 5 (0-10) | <0.0001 |
| VAS fatigue (0-100)3 | 55 (50-60) | (0-12.5) | <0.0001 |
| Tenderness joints (0-28)3 | 10 (9-17) | 0 (0-4) | <0.0001 |
| Swollen joints (0-28)3 | 8.5 (6 -11) | 0 (0-1) | <0.0001 |
| DAS281 | 5.78±0.87 | 1.9±1.4 | <0.0001 |
| Number of MRI synovitis1(dominant hand) | 5.8±1.2 | 3.6±2.3 | 0.05 |
| HAQ3 | 1.06 (0.34-1.53) | 0.12 (0-0.43) | <0.0001 |
| ESR (mm/h)3 | 28.5 (18-46.7) | 4(2-17) | 0.001 |
| CRP (mg /l)3 | 16 (6.7-36.2) | 2 (2-4.5) | <0.0001 |
| Anti-CCP (UI/l)3 | 98 (5-145) | - | - |
| Rheumatoid factor (UI/ml)3 | 40 (0-96) | - | - |
| corticoïdes dose (mg/day)3 | 7.5 (0-10) | 5 (0-5) | 0.005 |
Table 2: Demographic and clinical characteristics of population sample and evolution at 24 weeks follow up;
1mean and standard deviation,
2number and percentage,
3median and quartiles; VAS: visual analogic scale, DAS 28: disease activity score in 28 joints, HAQ: Health assessment questionnaire, ESR: erythrocyte sedimentation rate, CRP: C reactive protein.
Bone erosions on X rays and MRI at baseline and at follow up
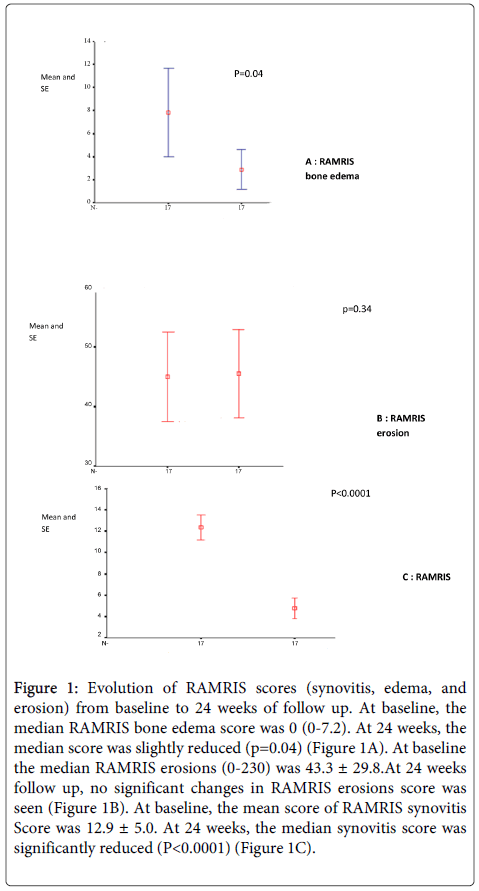
No change of the modified Sharp score was observed at 24 weeks follow up. At baseline the median RAMRIS erosions (0-230) was 43.3 ± 29.8.At 24 weeks follow up, no significant changes in RAMRIS erosions score was seen (Figure 1B). However 2 new erosions in the wrist (1 patient) were seen and 3 erosions had enlarged (2 patients).
MRI synovitis, bone edema and tenosynovitis at baseline and follow up
At baseline the mean score of RAMRIS synovitis Score was 12.9 ± 5.0. At 24 weeks, the median synovitis score was significantly reduced (P<0.0001) (Figure 1C). This coincided with a reduction in tender and swollen joints counts and considerable decreases in laboratory markers of inflammation (CRP and ESR) (Table 2). At baseline, the median RAMRIS bone edema score was 0 (0-7.2); at 24 weeks, the median score was slightly reduced (p=0.04) (Figure 1A).
Figure 1: Evolution of RAMRIS scores (synovitis, edema, and erosion) from baseline to 24 weeks of follow up. At baseline, the median RAMRIS bone edema score was 0 (0-7.2). At 24 weeks, the median score was slightly reduced (p=0.04) (Figure 1A). At baseline the median RAMRIS erosions (0-230) was 43.3 ± 29.8.At 24 weeks follow up, no significant changes in RAMRIS erosions score was seen (Figure 1B). At baseline, the mean score of RAMRIS synovitis Score was 12.9 ± 5.0. At 24 weeks, the median synovitis score was significantly reduced (P<0.0001) (Figure 1C).
Relation between MRI synovitis scores, clinical and biochemical assessments
Table 3 resumes the correlations between RAMRIS score of erosion, edema, and synovitis and disease parameters relative to RA at baseline.
| Baseline | 24 weeks | p value | |
|---|---|---|---|
| N= 22 | N=19 | ||
| Age (years)1 | 42±13.7 | 40±12.7 | - |
| Female Sex2 | 19 (86.4) | 16 (84.2) | - |
| VASpain (0-100)3 | 55 (50-60) | 5 (0-10) | <0.0001 |
| VAS fatigue (0-100)3 | 55 (50-60) | (0-12.5) | <0.0001 |
| Tenderness joints (0-28)3 | 10 (9-17) | 0 (0-4) | <0.0001 |
| Swollen joints (0-28)3 | 8.5 (6 -11) | 0 (0-1) | <0.0001 |
| DAS281 | 5.78±0.87 | 1.9±1.4 | <0.0001 |
| Number of MRI synovitis1(dominant hand) | 5.8±1.2 | 3.6±2.3 | 0.05 |
| HAQ3 | 1.06 (0.34-1.53) | 0.12 (0-0.43) | <0.0001 |
| ESR (mm/h)3 | 28.5 (18-46.7) | 4(2-17) | 0.001 |
| CRP (mg /l)3 | 16 (6.7-36.2) | 2 (2-4.5) | <0.0001 |
| Anti-CCP (UI/l)3 | 98 (5-145) | - | - |
| Rheumatoid factor (UI/ml)3 | 40 (0-96) | - | - |
| corticoïdes dose (mg/day)3 | 7.5 (0-10) | 5 (0-5) | 0.005 |
Table 3: Correlations between RAMRIS score of erosion, edema, and synovitis and disease parameters relative to RA at baseline.
At baseline; there was a significant correlation between RAMRIS synovitis and swollen joints counts (p=0.02), however there was no correlation between RAMRIS synovitis and VAS pain, HAQ, DAS 28, VS, and CRP.
There was a significant correlation between RAMRIS edema, age, CRP, anti-CCP and bone erosion at X-ray (for all p<0.05). However there was no correlation between RAMRIS edema and disease parameters (Table 3).
Baseline MRI synovitis changes as predictors of delta RAMRIS bone edema
Table 4 resumes the correlation between clinical and imaging variables at baseline and bone damage progression as assessed by delta RAMRIS bone edema. The delta RAMRIS edema at 24 weeks was substantially correlated with the baseline RAMRIS synovitis score (r= - 0.46; p=0.04). No correlation was found between delta RAMRIS edema, the DAS28, CRP, ESR, anti-CCP, and rheumatoid factor level at baseline (Table 3).
| RAMRIS erosion r | RAMRIS erosion r | RAMRIS synovitisr | |
|---|---|---|---|
| Age (years) | -0.50* | 0.18 | -0.18 |
| Disease duration(years) | 0.20 | -0.39 | 0.27 |
| Swollen joints (0-28) | 0.06 | 0.19 | 0.51* |
| DAS28 | 0.04 | 0.32 | 0.32 |
| HAQ | 0.13 | -0.01 | -0.01 |
| ESR (mm/h) | -0.05 | 0.03 | 0.03 |
| CRP (mg/l) | -0.52* | -0.12 | -0.12 |
| Anti-CCP | 0.56* | 0.17 | 0.23 |
| Rheumatoid factor | 0.14 | 0.17 | 0 .26 |
| Modified Sharp of van Der hedje | 0.73** | 0.32 | 0.32 |
| r: coefficient of correlation of Spearman,*p<0,01, **p < 0,001. DAS 28: disease activity score in 28 joints. HAQ: Health assessment questionnaire, ESR: erythrocyte sedimentation rate. CRP: C reactive protein, anti-CCP: anti-cyclic citrullinated peptide | |||
Table 4: correlation between clinical and imaging variables at baseline and bone damage progression as assessed by delta RAMRIS bone edema
Discussion
The present 6 months follow-up study of a cohort of patients with RA treated with TCZ suggested a significant reduction in pre-erosive lesions (synovitis and osteitis). MRI allows an objective evaluation of the effect of TCZ on disease activity, while biological and clinical parameters of disease activity remain inappropriate in this case. Indeed, DAS28 is inappropriate marker because TCZ normalizes C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) in the early stage of treatment. The aim of the treatment in rheumatoid arthritis (RA) is to prevent articular damage and functional loss by decreasing the activity of the disease. The overall goal is the full suppression of the activity of the disease, also called clinical remission or low disease activity. Evaluation of disease activity in RA is not easy, and no single marker can reflect all these aspects [18]. Remission is often selected as the 'treat to target'. Imaging remission can apply to structural damage and/or inflammation. The reason for arguing that imaging remission should be included for inflammation is that inflammation may still be present in patients who are in clinical remission. This concept has been reported in several studies [21]. Haavardsholm et al. [22] reported that in RA patients treated with anti-TNFa, the MRI inflammation score displayed superior responsiveness to conventional measures of disease activity and may be a promising outcome measure in clinical studies and for clinical practice. Lagana [23] reported in preliminary study an excellent clinical and imaging response in early RA patients treated with Etanercept with total remission in 40 % of them after a 1-year therapy period. However, imaging progression occurred despite clinical remission in early rheumatoid arthritis patients after etanercept interruption. Others studies [6,18] have observed the same finding in patients with RA treated with anti-IL 6 medication with a more pronounced discrepancy. This is explained by the fact that anti-IL 6 medication directly inhibits the production of acute phase proteins, such as CRP and fibrinogen from hepatocytes, by directly inhibiting the action of IL-6. Consequently, the CRP level and ESR rapidly and intensively decrease with the initiation of anti-IL 6 treatment before any improvement in swollen or tender joint counts is observed [24], possibly resulting in a discrepancy between an improvement in inflammatory markers and an improvement in actual RA disease activity [25]. Our findings raise the question as to whether a clinically nontender, nonswollen joint is in fact inflamed when the MRI indicates the presence of inflammation. In the present study, we have found a significant improvement in clinical and imaging outcome trough follow up; however in 6 months while 46% of patients were in clinical remission, there was persistence of MRI inflammation. Hence, synovial inflammatory activity estimated by the mean MRI may offer a more precise measurement of disease activity and will allow for easier monitoring of changes in the degree of inflammation [26]. Indeed, modern imaging techniques are becoming increasingly important in assessing the course of arthritis and as part of patient follow-up in evaluating response to treatment. The more effective schedule plan administration of traditional disease modifying drugs, as well as the highly effective new drugs, has changed the outcome of rheumatoid arthritis (RA), not only in the individual patient but also at the population level [27-28]. These therapeutic strategies can achieve low course of arthritis. Sensitivity to change is the key feature of a quantifying method. A reliable measure should show good responsiveness and allow prediction of future structural damage. Several studies have shown that, in patients with RA, clinical evaluation, even after careful training and standardization, is significantly less sensitive than either US [29] or MRI [30]. As a result, imaging is a useful alternative to achieve proper assessment of disease activity and predict structural damage [27,31]. In the cohort of patients receiving the anti-IL 6 receptor antibody tocilizumab, Kamishima T et al. [18] have shown that conventional measures are responsive but less reflective of future bone destruction than image analysis. In the evaluation of disease activity in RA patients within 1 year of beginning treatment with the anti-IL 6 receptor antibody tocilizumab, MR bone erosion is both responsive and predictive of structural damage progression at 1 year. The second point that deserves discussion: that is the low field MRI is also reliable and useful than the high field Low field dedicated E-MRI units have previously, been shown to allow detection of inflammatory and destructive joint damage in RA, moreover, E-MRI have shown the ability to detect the development of structural joint damage [6,18, 32,33]. E-MRI is favored by low costs and also because patient positioning is more comfortable and claustrophobia is avoided and can be easily used for follow-up of treatment. The disadvantages of E MRI include a smaller field of view meaning that fewer joints can be examined at the same time and a longer imaging time. OMERACT has developed a score to evaluate RA [34]: this Rheumatoid Arthritis MRI Score (RAMRIS) includes assessment of synovitis, BME, and erosions [35]. The resulting total score offers a comprehensive evaluation of the global burden of RA, including disease activity and extent of damage. As a result, novel drugs for rheumatoid have been evaluated with MRI after only three [36] or six [37] months from treatment start. In our study, we have found that pre erosive lesions (RAMRIS synovitis and oedema) were improved significantly at 24 weeks follow up. The same results were found in the study of P. G Conaghan et al. [7] in patients with RA treated with Golimumab plus MTX significantly improved MRI-detected synovitis and osteitis at weeks 12 and 24, and in the study of Suzuki et al. [6] in patients with RA treated with tocilizumab at 44 weeks but not at 20 weeks. Recently, Conaghan PG et al. [38] had found a rapid suppression of synovitis and osteitis with reduction in structural joint damage progression occurred with TCZ, as monotherapy or in combination with MTX, through week 52. However, in 2 others studies, there was an improvement of the RAMRIS erosions score, while the modification of RAMRIS synovitis and RAMRIS oedema scores remain trivial. Hence, it is expected that the beneficial effects of new drugs on synovitis and bone oedema (prognosticators of future structural damage) may prevent further progression of structural damage. The use of imaging as an outcome measure will move away from the concept of scoring to that of measuring and consistent with this there will be a move from plain radiography to MRI [39]. However, even if several authors found the MRI to be more sensitive in detecting subclinical local inflammation, it is unclear whether that is of clinical significance, Moreover, the literature so far does not necessarily support a worse functional outcome in these patients. A cutoff point for determining an MRI inflammatory activity acceptable state score needs to be established. Hence, a definition of remission by imaging needs to be established. A choice has to be made about the level of inflammation that can be tolerated and how this needs to be assessed (which imaging method, which feature, which joints, which cut-off point). Many unanswered questions remain to recommend including imaging remission of inflammation in a definition of remission [21]. This study has some strengths and weaknesses. Weaknesses include the small population sample. Another limitation of this study was a lack a control group. Hence, it may not be representative; and limits the ability to describe causal relationships to the associations detected.
Conclusion
This study suggests a significant reduction in MRI pre-erosive lesions (synovitis and osteitis) using Tocilizumab in patients with RA with inadequate response to DMARDS, however in 6 months, while 46% of patients were in clinical remission, there was persistence of MRI inflammation. Indeed, modern imaging techniques are becoming increasingly important in assessing the course of arthritis and in permitting measurement of response to treatment as part of the follow up of patients. Estimation of synovial inflammatory activity by the MRI possibly with a significant role for low field imaging appears to be a promising method of detecting and monitoring inflammatory activity in patients with RA. Recently, EULAR had produced ten recommendations encompassing the role of imaging in making a diagnosis of RA, detecting inflammation and damage, predicting outcome and response to treatment, monitoring disease activity, progression and remission [40]. Prospective studies with long term follow-up and imaging as an outcome measure are needed.
Acknowledgments
The study was entirely funded by the University Med V-Souissi and Avicenne University Hospital of Rabat.
References
- Terslev L, Torp-Pedersen S, Savnik A, von der Recke P, Qvistgaard E, et al. (2003) Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study.Arthritis Rheum 48: 2434-2441.
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Josef R, et al. (2008) For the OPTION Investigators.Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371
- Schoels MM, van der Heijde D, Breedveld FC, Burmester GR, Dougados M, et al. (2012) Blocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta-analysis informing a consensus statement. Ann Rheum Dis 10.
- Smolen JS, Schoels MM, Nishimoto N, Breedveld FC, Burmester GR, et al (2012) Consensus statement on blocking the effects of interleukin-6 and in particular by interleukin-6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis 21.
- Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, et al. (2013) Adding tocilizumab or switching to tocilizumabmonotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY).Ann Rheum Dis 72: 43-50.
- Suzuki T, Horikoshi M, Sugihara M, Hirota T, Ogishima H, et al (2012) Therapeutic efficacy of tocilizumab in patients with rheumatoid arthritis refractory to anti-tumor-necrosis-factor inhibitors: 1 year follow-up with low-field extremity MRI. Mod Rheumatol14.
- Conaghan PG, Emery P, Østergaard M, Keystone EC, Genovese MC, et al (2011) Assessment by MRI of inflammation and damage in rheumatoid arthritis patients with methotrexate inadequate response receiving golimumab: results of the GO-FORWARD trial. Ann Rheum Dis 70:1968-1974.
- McQueen F, Lassere M, Edmonds J, Conaghan P, Peterfy C, et al. (2003) OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Summary of OMERACT 6 MR Imaging Module.J Rheumatol 30: 1387-1392.
- Hoving JL, Buchbinder R, Hall S, Lawler G, Coombs P, et al. (2004) A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis.J Rheumatol 31: 663-675.
- Østergaard M, Hansen M, Stoltenberg M, Jensen KE, Szkudlarek M, et al. (2003) New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier.Arthritis Rheum 48: 2128-2131.
- Ejbjerg BJ, Vestergaard A, Jacobsen S (2005) The smallest detectable difference and sensitivity to change of magnetic resonance imaging and radiographic scoring of structural joint damage in rheumatoid arthritis finger, wrist, and toe joints: a comparison of the OMERACT rheumatoid arthritis magnetic resonance imaging score applied to different joint combinations and the Sharp/van der Heijde radiographic score. Arthritis Rheum52:2300-2306.
- Dalbeth N, Smith T, Gray S, Doyle A, Antill P, et al. (2009) Cellular characterisation of magnetic resonance imaging bone oedema in rheumatoid arthritis; implications for pathogenesis of erosive disease.Ann Rheum Dis 68: 279-282.
- Conaghan PG, O'Connor P, McGonagle D (2003) Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum 48:64-71.
- Huang J, Stewart N, Crabbe J (2000) A 1-year follow-up study of dynamic magnetic resonance imaging in early rheumatoid arthritis reveals synovitis to be increased in shared epitope-positive patients and predictive of erosions at 1 year. Rheumatology (Oxford)39:407-416.
- McQueen FM, Benton N, Perry D (2003) Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum 48:1814-1827.
- Haavardsholm EA, Bøyesen P, Østergaard M, Schildvold A, Kvien TK (2008) Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression.Ann Rheum Dis 67: 794-800.
- Bøyesen P, Haavardsholm EA, van der Heijde D, Østergaard M, Hammer HB, et al. (2011) Prediction of MRI erosive progression: a comparison of modern imaging modalities in early rheumatoid arthritis patients.Ann Rheum Dis 70: 176-179.
- Kamishima T, Tanimura K, Shimizu M, Matsuhashi M, Fukae J, et al. (2011) Monitoring anti-interleukin 6 receptor antibody treatment for rheumatoid arthritis by quantitative magnetic resonance imaging of the hand and power Doppler ultrasonography of the finger. Skeletal Radiol 40:745-755
- Ostergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, et al. (2005) An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 64:3-7.
- Lisbon, Maymo J, Perich J, Almirall M, Perez-Garcia C, Carbonell J. Etanercept reduces synovitis as measured by magnetic resonance imaging in patients with active rheumatoid arthritis after only 6 weeks. J Rheumatol. 2008;35(3):394-7.a MP
- van der Heijde D (2012) Remission by imaging in rheumatoid arthritis: should this be the ultimate goal?Ann Rheum Dis 71 Suppl 2: i89-92.
- Laganà B, Diamanti PA,Ferlito C, Germano V, Migliore A, et al. (2009) Imaging progression despite clinical remission in early rheumatoid arthritis patients after etanercept interruption. Int J ImmunopatholPharmacol22: 447-454.
- Nishimoto N, Yoshizaki K, Maeda K, Kuritani T, Deguchi H, et al. (2003) Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol 30: 1426-1435.
- Nishimoto N, Takagi N (2010) Assessment of the validity of the 28-joint disease activity score using erythrocyte sedimentation rate (DAS28-ESR) as a disease activity index of rheumatoid arthritis in the efficacy evaluation of 24-week treatment with tocilizumab: subanalysis of the SATORI study. Mod Rheumatol
- Cimmino MA, Barbieri F, Zampogna G, Camellino D, Paparo F, et al. (2012) Imaging in arthritis: quantifying effects of therapeutic intervention using MRI and molecular imaging.Swiss Med Wkly 141: w13326.
- Cimmino MA, Masocco M, Torre M (2011) Hospital admission for rheumatoid arthritis dwindled in Italy between 2001 and 2008.Rheumatology (Oxford) 50: 2140-2141.
- Szkudlarek M, Klarlund M, Narvestad E, Court-Payen M, Strandberg C, et al. (2006) Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther 8: R52.
- Tamai M, Kawakami A, Iwamoto N, Kawashiri SY, Fujikawa K, et al. (2011) Comparative study of the detection of joint injury in early-stage rheumatoid arthritis by magnetic resonance imaging of the wrists and finger joints and physical examination. Arthritis Care Res 63:436-439.
- Gandjbakhch F, Foltz V, Mallet A, Bourgeois P, Fautrel B (2011) Bone marrow oedema predicts structural progression in a 1-year follow-up of 85 patients with RA in remission or with low disease activity with low-field MRI.Ann Rheum Dis 70: 2159-2162.
- Suzuki T, Ito S, Handa S, Kose K, Okamoto Y, et al. (2009) A new low-field extremity magnetic resonance imaging and proposed compact MRI score: evaluation of anti-tumor necrosis factor biologics on rheumatoid arthritis. Mod Rheumatol 19: 358-365.
- Østergaard M, Duer A, Nielsen H, Johansen JS, Narvestad E, et al (2005) Magnetic resonance imaging for accelerated assessment of drug effet and prediction of subsequent radiographic progression in rheumatoid arthritis: a study of patients receiving combined anakinra and méthotrexate treatment. Ann Rheum Dis 64:1503-1506.
- Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, et al. (2003) OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system.J Rheumatol 30: 1385-1386.
- Bird P, Conaghan P, Ejbjerg B, McQueen F, Lassere M, et al. (2005) The development of the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 64: 8-10.
- Genovese MC, Kavanaugh A, Weinblatt ME, Peterfy C, DiCarlo J, et al. (2011) An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents.Arthritis Rheum 63: 337-345.
- Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, et al. (2011) Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial.Arthritis Rheum 63: 939-948.
- Conaghan PG, Peterfy C, Olech E, Kaine J, Ridley D, et al. (2014) The effects of tocilizumab on osteitis, synovitis and erosion progression in rheumatoid arthritis: results from the ACT-RAY MRI substudy.Ann Rheum Dis 73: 810-816.
- Freeston J, Emery P (2007) The role of MRI and ultrasound as surrogate markers of structural efficacy of treatments in rheumatoid arthritis.Joint Bone Spine 74: 227-229.
- Colebatch AN, Edwards CJ, Østergaard M, van der Heijde D, Balint PV, et al. (2013) EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. European League Against Rheumatism Ann Rheum Dis 72: 804-814.
Citation: Rostom S, Amine B, Bahiri R, Allali F, Abouqa R, et al. (2014) Assessment of Inflammation and Damage in Rheumatoid Arthritis Patients with Methotrexate Inadequate Response Receiving Tocilizumab Using Low Field MRI. Epidemiology (Sunnyvale) 4:173. DOI: 10.4172/2161-1165.1000173
Copyright: © 2014 Rostom S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.