Assessment of In vitro X-Ray Radiation Overexposure by Cytokinesis-Block Micronucleus Assay in Human Peripheral Blood Lymphocytes (HPBLs) in Saudi Population
Received: 17-Apr-2018 / Accepted Date: 28-Jun-2018 / Published Date: 30-Jun-2018
Keywords: Micronucleus assay; Biodosimetry; Radiation accidents; Ionizing radiation and human blood lymphocytes
Introduction
Radiation exposure due to radiotherapy or following a large-scale radiological accident in a populated area can damage the human tissues considerably [1-3]. The ability to achieve an accurate estimate of the absorbed radiation dose is critical for people exposed to significant levels of ionizing radiation, as this information can predict the health risks. Typically, rare cases of radiation exposure and limited number of potential casualty’s scenario. The main focus for such isolated cases, the primary focus is on providing the most accurate dose estimate, considering details of the exposure, such as the type and quality of the radiation and the uniformity, duration, and timing of the exposure [4,5]. The ionizing radiation transferred in the living cells by the genetic material in nucleus and mitochondrial [6]. Chromosomal aberration is widely known as cytogenetic indicators used to assess radiation damage by ionizing radiation exposure for supporting the treatment of radiation casualties [7] using a biodosimetry assays as cytokinesisblock micronucleus (CBMN) and dicentric chromosome assay (DCA) [8]. The CBMN assay is a very reliable documented method to quantify chromosome breakage and loss in nucleated cells [9,10]. And in vitro, to assess cancer susceptibility or radiosensitivity in individuals exposed to occupational, medical, and accidentally radiation [11-13]. To study chromosomal damage in vitro induced by IR or chemicals using a quality dependence dosimeter, MN assay of HPBLs for radiation protection purposes according to the International Atomic Energy Agency recommendation [14-16].
In human and mammalian cells The CBMN assay used for genetic toxicology testing by assessing micronuclei (small nuclei) arise during exposure to various clastogenic agents due to mis-repaired DNA double strand breaks, during cell division small acentric chromosome fragments are not transfer to nuclei of daughter cells. MN scoring very restricted preventing confounding effects during the cell division result in a cell recognized by their binucleate (BN) appearance [9,17,18]. The in vitro micronucleus test is simple, useful, and applicable standard cytogenetic test for genotoxicity in different cell types [19-21]. The most useful micronucleus assay performed by adding the cytochalasin B in cell culture for produce binucleated cells (BNCs) [22]. In addition, scoring of micronuclei in the CBMN assay is easy and quick, making it much less labor-intensive than the DCA method and an attractive option for genetic damage assessment in population a high dose of radiation in the case of large-scale radiation accidents [17]. The main aim of this study was to assess the effect of overexposure to radiation on the peripheral blood lymphocytes cells isolated from the blood of Saudi volunteers as a reliable biomarker for measuring the emerging DNA damage, this assessment was carried out by visual scoring the micronuclei frequency, micronucleated cells and The nuclear division index in 1000 peripheral blood lymphocytes cultures of non-irradiated (control) and irradiated (0.5-5 Gy) lymphocytes samples.
Material and Methods
Blood sampling
This study is a prospective study performed in six-month period between May - Oct. 2016. a total of 20 Peripheral blood samples were collected from nonsmoking and apparently healthy human volunteers had no history of exposure to radiation and did not complain from acute or chronic illness at king sultan hospital. The age range was between 25 and 35 years that consist of 10 males and 10 females. Draw aliquots of 2 mL of whole blood in heparinized vials using a vacutainer system. The study Subjects gave informed consent and the approval of a local ethics committee.
Exposure of HPBLs to X-ray radiation
In vitro, irradiated the Heparinized blood samples immediately after venipuncture using single doses of X-ray with a mean photon energy of 320 keV (filtered with 1 mm) using X-RAD 320 System (Precision X-Ray, United States) at 37°C. The dose rate was approximately 0.913 Gy/min and to obtain a calibration curve, blood samples were exposed at the dose levels of 0, 0.5, 1, 2, 3, 4, and 5 Gy. The irradiation was performed according to IAEA procedure [16], then, kept the blood samples at 37°C to allow for any chromosomal repair to take place.
Isolation and culture of lymphocyte
Blood culture and harvest according to the IAEA protocol [16,23] with slightly modified. Briefly, 0.5 ml of non-irradiated and irradiated peripheral blood samples was added to RPMI 1640 cul ture medium (4.5 ml) enriched with L-glutamine containing 10-15% fetal calf serum, 2% penicillin, 3% strep tomycin and stimulated with 3% phytohaemagglutinin (Sig ma, USA) at 37 °C. After 48 hours of culture, add 45 μl Cytochalasin B (Sigma Aldrich). After 72 hours of incubation period, centrifuging the blood sample for cell collection at 800 rpm for 5 minutes. The collected cells were suspended in 6 ml of cold hypotonic solution (0.075 M cold KCl), centrifuged at 800 rpm for 5 min. the cells were fixed in methanol: Glacial acetic acid (6:1) for 3 times.
Slide preparation and micronuclei scoring
The cytokinesis-block micronucleus test was utilized to evaluate chromosomal damages [23]. Lymphocytes fixed cells were dropped on the glass slides, air-dried and stained with 5% aqueous solution of Giemsa dye Sigma Aldrich) for 15 min. As sessment of slides was carried out using Nikon microscope with ×100 magnification to assess the MN frequency in CBMN according to the criteria proposed by (9, 16). A total of 1000 lymphocytes were examined for the Number of BN and the Number of MN cells.
Statistical analysis
The obtained data of this study was subjected to calculate the frequency, statistical description (Mean, SE) and using statistical analysis of variance (ANOVA) test and least significantly difference (LDS) was set to P<0.05 using SPSS statistical software V. 19.
Results
In the preliminary study, lymphocytes cultures of non-irradiated (control) and irradiated peripheral blood samples were subjected to the cytokinesis-block micronucleus assay according to standard protocol as described by Varga D [24] as a potential method to assess radiation overexposure, Visual scoring the frequencies of binucleated cells (BNCs), micronuclei (BNi) and Nuclear division index (NDI) on 1000 peripheral blood lymphocytes samples exposed to X-rays from 0.5 to 5 Gy as compared with control samples (Table 1). The (NDI) was significantly higher in non-irradiated male control lymphocytes cultures (0.918 ± 0.03, 0.89 ± 0.03) and gradually increased by increasing x-ray dose, while minim average numbers of NDI (120 ± 0.03) was in irradiated male lymphocytes with 5 Gy dose.
| Irradiated Dose (Gy) |
Gender | BNCs (Mean ± SE) |
MNi (Mean ± SE) |
NDI (Mean ± SE) |
|---|---|---|---|---|
| Dose 0 Gy | M | 920.40 ± 30.038 | 1.800 ± 14.343 | 0.918 ± 0.03 |
| F | 893.1 ± 30.038 | 0.700 ± 14.343 | 0.89 ± 0.03 | |
| Dose 0.5 Gy | M | 816.7 ± 30.038 | 82.500 ± 14.343 | 0.816 ± 0.03 |
| F | 816.8 ± 30.038 | 88.100 ± 14.343 | 0.817 ± 0.03 | |
| Dose 1 Gy | M | 613.40 ± 30.038 | 164.600 ± 14.343 | 0.613 ± 0.03 |
| F | 679.5 ± 30.038 | 166.900 ± 14.343 | 0.679 ± 0.03 | |
| Dose 2 Gy | M | 555.4 ± 30.038 | 242.500 ± 14.343 | 0.555 ± 0.03 |
| F | 604.0 ± 30.038 | 256.900 ± 14.343 | 0.604 ± 0.03 | |
| Dose 3 Gy | M | 374.4 ± 30.038 | 333.800 ± 14.343 | 0.374 ± 0.03 |
| F | 410.80 ± 30.038 | 321.100 ± 14.343 | 0.411 ± 0.03 | |
| Dose 4 Gy | M | 188.299 ± 30.038 | 291.800 ± 14.343 | 0.188 ± 0.03 |
| F | 205.60 ± 30.038 | 399.700 ± 14.343 | 0.206 ± 0.03 | |
| Dose 5 Gy | M | 120.40 ± 30.038 | 224.700 ± 14.343 | 0.120 ± 0.03 |
| F | 136.60 ± 30.038 | 400.700 ± 14.343 | 0.137 ± 0.03 |
Table 1: The average values of binucleated cells, micronuclei and division index scored in 1000 lymphocyte cells of male and female after X-ray exposure.
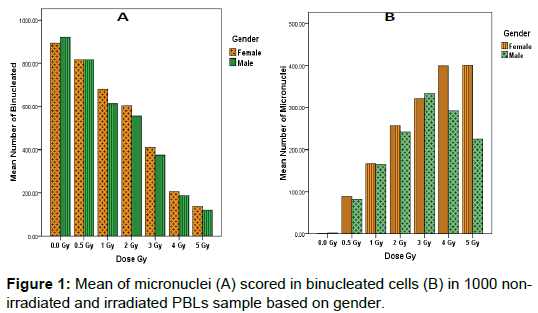
Study results showed that the highest average numbers of BNCs scored in 1000 non-irradiated and irradiated lymphocytes cultures were found in non-irradiated male samples (920.40 ± 30.038) and relatively decreased in accordance with increase radiation dose in both sex, while minim average numbers of BNCs (120.40 ± 30.038) was in irradiated male lymphocytes at 5Gy dose (Figure 1a). We observed that the number of MNi scored in BNCs increased proportionally with radiation dose rate (R2=0.89; Figure 1b). The highest average number of MNi (400.700 ± 14.343) was found in irradiated female lymphocytes at 5Gy dose; while minimum average numbers of MNi (0.700 ± 14.343) was in non-irradiated female lymphocytes samples (Figure 2).
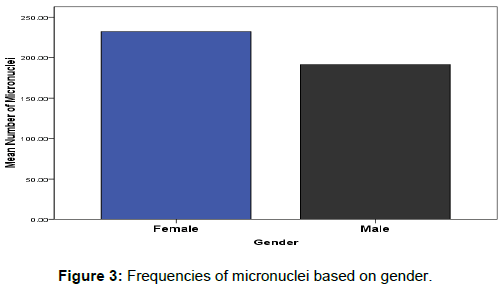
The effects of gender on DNA damage measured using micronuclei frequencies in peripheral blood lymphocytes were firstly reported by Fenech, M [25]. Our observations in this study indicated the influence of gender on the average of micronuclei scores in binucleated lymphocyte cells for healthy tended to be greater in females relative to males (Mean; 191.671 ± 5.421 and 233.443 ± 5.421) respectively as represented in (Figure 3). However, this was only true at lower doses of radiation (0.5– 2 Gy), but at high doses (3–5 Gy), there was no statistically significant difference in MNi percentage between groups 4 Gy4: female (399.7) and male (291.8) and Gy5: female (400.7) and male (224.7) (Figure 1b).
Discussion
The present study investigated the effects of X-ray irradiation on human peripheral blood lymphocytes (HPBLs) by evaluating DNA damage using CBMN assay, as potential tool for assessing cytogenetic damage induced by radiation [26,27]. In general, Parameters of BNCs, MNi and NDI were significantly, and dose dependently changed after irradiation by 1 – 5 Gy as compared with control subjects. On the other hand, cellular proliferation decreased with increasing radiation dose, in agreement with previous work on HPBL irradiated with X-rays [28]. We also investigated differences in DNA damage induced by X-rays by measuring MN in binucleated cells. The mean baseline frequency of MN in non-irradiated lymphocytes was similar to that of previous reports and was within the expected range [8]. A decrease in cellular proliferation and increased cytogenetic damage were showed at higher doses, thereby increasing cell death [29]. The biological responses of irradiated cells also depend on the dose rate [25]. Specifically, a decreased dose rate can result in decreased micronuclei formation in HPBL, possibly due to efficient repair [30,31].
NDI is a measure of cytostasis and cell death (cytotoxicity) as a marker of cell proliferation. The rationale behind NDI is cells with greater chromosomal damage are less likely to enter cell division or cell death occurs before cell division [32]. Our findings showed decreased BNCS and NDI in the x-ray irradiated samples in comparison to the controls subjects. If all viable cells complete one division during the cytokinesis-block phase the binucleated cells will be formed and they will contain more than two nuclei If some viable cells complete more than one nuclear division [32,33]. Analysis of nuclear division index revealed significant differences in lymphocyte proliferation rate upon irradiation; this agrees with an earlier study reporting that DNA damage response was altered by irradiation [18].
A reduction in the dose rate may favor the arrest of cells with DNA damage in the G1 and G2 phases over cell, and the transducer protein kinase ataxia-telangiectasia mutated (ATM) preventing the cells from replicating damaged DNA and the DNA repaired with time, in contrast to the induction of apoptosis of damage cells that occurs at a high dose rate [30]. Micronuclei are formed because of chromosome either breaks or fail to engage with the mitotic spindle fibers.
In 1985, Fenech established the CBMN assay in HPBL (9) this cytogenetic dosimetry assay is no requirement for metaphase cells, speed and ease of analysis, identification the cells completed one nuclear division [34,35]. In an emergency, radiation dose estimates should be provided as soon as possible with sufficient accuracy to support clinical decision-making. Cell-cycle progression delayed in cells that exposed to the radiation and produces micronuclei and quickly died this can result in fewer cells reaching mitosis. The CMBN assay requires 72 hours of culture time, after which cell processing can occur followed by slide preparation. MN scoring is easier with this assay than the DCA method [36]. The biodosimetry recommended that scoring 1,000 binuclear cells [37] in approximately 2 hours, recently Lindholm and colleagues demonstrated that scoring of 200 binuclear cells in about 15 minutes was sufficient to identify radiation doses of >1 Gy [38].
There are some drawbacks to the CBMN assay; however, new improvements have made the assay more adapted to radiation exposure [39] as automated and high-throughput scoring analysis [40,41]. A limited dose range of CBMN assay (0.3–5 Gy), although it has been suggested that the modifications to the method may be attained a range of 0.1–15 Gy [8,36,39,41-45]. Experiments proven that CBMN assay is reliable for up to 6 months after exposure (46), but with a correction factor the time can be extended to approximately 1 year [46-48].
Previous study examines the micronuclei frequency in HPBL, the results showed a significant increase in the number of micronuclei in irradiated blood samples. Other studies have proved that the yield of dicentric chromosomes and micronuclei is increased by the prolonged culture of irradiated lymphocytes cells with PHA at different time intervals [49,50]. Comparative outcomes have been reported that levels chromosomal aberrations result from delay of lymphocytes stimulation cells due to radiation exposure that were elevated compared to lymphocytes stimulated immediately [51]. Furthermore, in stimulated lymphocytes A critical decrease in micronuclei frequency by checkpoint activation result in cell-cycle arrest or by repair of DNA damage induced by the radiation exposure at twenty hours postirradiation, compared to two or six hours post-irradiation [52].
Conclusion
As a result of this study we can conclude, accumulation of genetic damage is detectable in peripheral lymphocytes of healthy individuals exposed to x-ray radiation. The MN frequency increases and affected by gender while the BNCs and NDI decreases with increase the dose rate. Our results suggest that Establishing a laboratory which competent enough to perform cytogenetic analysis for biodosimetry is very important in Saudi Arabia for measuring micronuclei in BNCs cells as best biomarker to assess radiation damage evaluated by CBMN assay.
Acknowledgments
Also, deep thanks for my research group and co-authors for kindly supporting in this study, and king sultan hospital stuff at Riyadh, Saudi Arabia on their roles and responsibilities in perform the samples collection and using laboratories and providing all clinical approval and data about the study cases.
Conflict of Interest
Between me and co-authors, there is no any indirect or direct financial interest we have in the subject matter of a submitted manuscript, or any other potential conflict of interest. Also, thank you for receiving our manuscript and considering it for review. We appreciate your time and look forward to your response.
References
- Iyer R, & Lehnert BE. Effects of ionizing radiation in targetÂed and nontargeted cells. Arch Biochem Biophys, 2000. 376: 14-25.
- Singh VK, Ducey EJ, Brown DS & Whitnall MH. A review of radiation countermeasure works ongoing at the armed forces radiobiology research Institute. Int J Radiation Biol. 2012. 88: 296-310.
- Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, Bertucci A, Xu Y, Wang H &Simaan N, et al. The RABIT: A rapid automated biodosimetry tool for radiological triage. Health physics, 2010. 98: 209.
- Ainsbury EA, Bakhanova E, Barquinero JF, Brai M, Chumak V, Correcher V, Darroudi F, Fattibene P, Gruel G & Guclu I, et al. Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiation Protection Dosimetry, 2010. 147: 573-592.
- Rothkamm K, Beinke C, Romm H, Badie C, Balagurunathan Y, Barnard S, N. Bernard N, Boulay-Greene H, Â Brengues M & De Amicis A, et al. Comparison of established and emerging biodosimetry assays. Radiation Research, 2013. 180: 111-119.
- Steenken S. Purine bases, nucleosides, and nucleotides: aqueous solution redox chemistry and transformation reÂactions of their radical cations and e– and OH adducts. Chem Rev, 1989. 89: 503-520.
- Thierens H, Vral A, Barbé M, Meijlaers M, Baeyens A, Ridder LD. Chromosomal radiosensitivity study of temporary nuclear workers and the support of the adaptive response induced by occupational exposure. Int J Radiat Biol, 2002. 78:  1117‑1126.
- World Health Organization (WHO) Cytogenetic dosimetry: applications in preparedness for and response to radiation emergencies (Spanish Edition).International Atomic Energy Agency. 2014.
- Fenech M & Morley AA. Measurement of micronuclei in lymphocytes. Mutation Research/Environmental Mutagenesis and Related Subjects, 1985. 147: 29-36.
- Tucker JD, Vadapalli M, Joiner MC, Ceppi M, Fenech M, Bonassi S. Estimating the lowest detectable dose of ionizing radiation by the cytokinesis-block micronucleus assay. Radiation res, 2013. 180: 284-291.
- Thierens H, Vral A, de Ridder L, Touil N, Kirsch-Volders M, Lambert V, et al. Inter-laboratory comparison of cytogenetic endpoints for the biomonitoring of radiological workers.Int J Radiat Biol, 1999. 75: 23-24.
- Sari‑Minodier I, Orsière T, Auquier P, Martin F & Botta A. Cytogenetic monitoring by use of the micronucleus assay among hospital workers exposed to low doses of ionizing radiation. Mutat Res, 2007. 629: 111‑121.
- Fucic A, Brunborg G, Lasan R, Jezek D, Knudsen LE & Merlo DF. Genomic damage in children accidentally exposed to ionizing radiation: A review of the literature. Mutat Res, 2008. 658: 111‑123.
- Mill AJ, Wells J, Hall SC & Butler A. Micronucleus induction in human lymphocytes: Comparative effects of X-rays, alpha particles, beta particles and neutrons and implications for biological dosimetry. Radiat Res, 1996.145: 575-585.
- Hall EJ, Giaccia AJ. Radiobiology for the Radiobiologist. 7th edn. Philadelphia: JB Lippincott Company, 2012.
- IAEA Cytogenetic dosimetry: Applications in preparedness for and response to radiation emergencies. Vienna: IAEA‑EPR. 2011.
- Deng B, Hou J, Quan Y, Dong L & Tan Z. Cytogenetic effects of low-dose tritiated water in human peripheral blood lymphocytes—experimental studies on the relative biological effectiveness and chromosome aberration rate and cbmn in human blood lymphocyte irradiated by tritium low dose tritium â-rays and 60Co ã-rays. Open J Diagnostics, 2015. 5: 125.
- Shahane SA, Nishihara K & Xia M. High-throughput and high-content micronucleus assay in CHO-K1 Cells. High-Throughput Screening Assays in Toxicology, 2016. 2: 77-85.
- Kirklanda D, Reeve L & Gatehousec D, et al. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2011. 2: 721: 27–37.
- Lindberga HK, Falcka GCM, Suhonena S, et al. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicology Letters, 2009. 186: 166–173.
- Meschini R, Berni A & Filippi S, et al. The micronucleus assay in mammalian cells in vitro to assess health benefits of various phytochemicals. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2015. 793: 2: 79–85.
- Battal D, C¸ Elik A and Guler G, et al. SiO2 nanoparticleinduced size-dependent genotoxicity- an in vitro study using sister chromatid exchange, micronucleus and comet assay. Drug and Chemical Toxicology, 2015. 38: 196–204.
- Serpil Ko¨nen-Adıgu¨ zel and Serap Ergene In vitro evaluation of the genotoxicity of CeO2 nanoparticles in human peripheral blood lymphocytes using cytokinesis-block micronucleus test, comet assay, and gamma H2AX. Toxicology and Industrial Health, 2018. 34:  293–300.
- Varga D, Johannes T, Jainta S, Schuster S, Schwarz-Boeger U& Kiechle M, et al. An automated scoring procedure for the micronucleus test by image analysis. Mutagenesis 2004. 19: 391–397.
- Fenech, M., Neville, S. & Rinaldi, J. Sex is an important variable affecting spontaneous micronucleus frequency in cytokinesis-blocked lymphocytes. Mutat Res, 1994. 313: 203–207.
- Sram RJ, Svecova V & Rossnerova A. Systematic review of the use of the lymphocyte cytokinesis-block micronucleus assay to measure DNA damage induced by exposure to polycyclic aromatic hydrocarbons. Mutation Research/Reviews in Mutation Research, 2016. 770: 162-169.
- Bolognesi C, Bruzzone M, Ceppi M & Kirsch-Volders M. The lymphocyte cytokinesis block micronucleus test in human populations occupationally exposed to vinyl chloride: A systematic review and meta-analysis. Mutation Research/Reviews in Mutation Research, 2017. 774: 1-1.
- Heimers A, Brede HJ, Giesen U & Hoffmann W. Influence of mitotic delay on the results of biological dosimetry for high doses of ionizing radiation. Radiation and Environmental Biophysics, 2005. 44(3): 211-218.
- Müller WU & Rode A. The micronucleus assay in human lymphocytes after high radiation doses (5–15 Gy). Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2002. 502: 47-51.
- Konopacka M & Rogoliński J. Clastogenic effects in human lymphocytes exposed to low and high dose rate X-ray irradiation and vitamin C. Nukleonika, 2011. 56: 253-257.
- Vral A, Thierens H & De Ridder L. Study of dose-rate and split-dose effects on the in vitro micronucleus yield in human lymphocytes exposed to X-rays. International journal of radiation biology, 1992. 61: 777-784.
- Ionescu ME, Ciocirlan M, Becheanu G, Nicolaie T, Ditescu C & Teiusanu AG, et al. Nuclear division index may predict neoplastic colorectal lesions. Maedica (Buchar), 2011. 6: 173-178.
- Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc, 2007. 2: 1084-1104.
- [IAEA] International Atomic Energy Agency. Cytogenetic analysis for radiation dose assessment. IAEA technical reports series no. 405. Vienna. International Atomic Energy Agency, 2001. 2: 1–127.
- Yang H, Feng R, Liu J, Wang H & Wang Y. Increased frequency of micronuclei in binucleated lymphocytes among occupationally pesticide-exposed populations: a meta-analysis. Asian Pac J Cancer Prev, 2014. 15: 6955-6960.
- [AFRRI] Armed Forces Radiobiology Research Institute (USA) AFRRI TRIGA Reactor Facility to satisfy the requirements of U.S. 2010 1-31 Dec. Nuclear Regulatory Commission License No. R-84 (Docket No. 50-170), Technical Specification 6.6. b.
- Voisin P, Benderitter M, Claraz M, Chambrette V, Sorokine-Durm I, Delbos M, Durand V , Leroy A & Paillole N. The cytogenetic dosimetry of recent accidental overexposure. Cellular and Molecular Biology (Noisy-le-Grand, France), 2001. 47: 557-564.
- Lindholm C, Stricklin D, Jaworska A, Koivistoinen A, Paile W, Arvidsson E, Deperas-Standylo J & Wojcik A. Premature chromosome condensation (PCC) assay for dose assessment in mass casualty accidents. Radiation Research, 2010. 173: 71-78.
- Fenech M. The lymphocyte cytokinesis-block micronucleus cytome assay and its application in radiation biodosimetry. Health Physics, 2010. 98: 234-243.
- Decordier I, Papine A, Plas G, Roesems S, Vande Loock K, Moreno-Palomo J, Cemeli E, Anderson D, Fucic A, Marcos R. Automated image analysis of cytokinesis-blocked micronuclei: an adapted protocol and a validated scoring procedure for biomonitoring. Mutagenesis, 2008. 24: 85-93.
- Willems P, August L, Slabbert J, Romm H, Oestreicher U, Thierens H, Vral A. Automated micronucleus (MN) scoring for population triage in case of large scale radiation events. Int J Radiation Biol, 2010. 86: 2-11.
- Darroudi F, Fomina J, Meijers M, Natarajan AT. Kinetics of the formation of chromosome aberrations in X-irradiated human lymphocytes, using PCC and FISH. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 1998. 404: 55-56.
- Darroudi F. Detection of total-and partial-body irradiation in a monkey model: a comparative study of chromosomal aberration, micronucleus and premature chromosome condensation assays.International Journal of Radiation Biology, 1998. 74: 207-215.
- De Lemos Pinto MM, Santos NF, Amaral A. Current status of biodosimetry based on standard cytogenetic methods. Radiation and environmental biophysics, 2010. 49: 567-581.
- Vral A, Fenech M, Thierens H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis, 2011. 26: 11-7.
- Thierens H, De Ruyck K, Vral A, de Gelder V, Whitehouse CA, Tawn EJ, Boesman I. Cytogenetic biodosimetry of an accidental exposure of a radiological worker using multiple assays. Radiation Protection Dosimetry, 2005. 113: 408-414.
- Fenech M, Denham J, Francis W, Morley A. Micronuclei in cytokinesis-blocked lymphocytes of cancer patients following fractionated partial-body radiotherapy. Int J of Radiation Biol, 1990. 57: 373-383.
- Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1993. 31, 285: 35-44.
- Hoffmann GR, Sayer AM, Littlefield LG. Higher frequency of chromosome aberrations in late-arising first-division metaphases than in early-arising metaphases after exposure of human lymphocytes to X-rays in G 0. Int J Radiation Biol, 2002. 78: 765-772.
- Krishnaja AP, Sharma NK. Differential radiation effects in smokers–culture time dependence of the yield of gamma ray-induced chromosome damage in first division metaphases. Int J Radiation Biol, 2006. 82:363-377.
- Wilkins RC, Romm H, Kao TC, Awa AA, Yoshida MA, Livingston GK, Jenkins MS, Oestreicher U, Pellmar TC & Prasanna PG. Interlaboratory comparison of the dicentric chromosome assay for radiatiobiodosimetry in mass casualty events. Radiation Research, 2008. 169: 551-560.
- Lavin MF, Khanna KK. ATM: The protein encoded by the gene mutated in the radiosensitive syndrome ataxia-telangiectasia. Int J Radiation Biol, 1999. 75: 1201-1214.
Citation: Alotaibi MA, Alsuhaibani ES, Alsbeih GA (2018) Assessment of In vitro X-Ray Radiation Overexposure by Cytokinesis-Block Micronucleus Assay in Human Peripheral Blood Lymphocytes (HPBLs) in Saudi Population. Cell Mol Biol 64: 148.
Copyright: © 2018 Alotaibi MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3846
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2981
- PDF downloads: 865