Research Article Open Access
Assessment of Drug Interaction Potential between LCZ696, an Angiotensin Receptor Neprilysin Inhibitor, and Digoxin or Warfa rin
| Surya Ayalasomayajula1*, Pierre Jordaan, MBChB2, Parasar Pal3, Priyamvada Chandra1, Diego Albrecht2, Thomas Langenickel2, Iris Rajman2and Gangadhar Sunkara1 | |
| 1DMPK, Novartis Institutes for BioMedical Research (NIBR), East Hanover, NJ, USA | |
| 2Translational Medicine, Novartis Institutes for BioMedical Research (NIBR), Basel, Switzerland | |
| 3Biostatistical Sciences, IDFR, Novartis Healthcare Pvt Ltd, Hyderabad, Telangana, India | |
| Corresponding Author : | Surya Ayalasomayajula Novartis Institutes for BioMedical Research (NIBR), DMPK One Health Plaza 438/3413, East Hanover, NJ 07936, USA Tel: +1-862-778-0217 E-mail: surya.ayalasomayajula@novartis.com. |
| Received: October 01, 2015; Accepted: October 08, 2015; Published: October 14, 2015 | |
| Citation: Ayalasomayajula S, Jordaan P, MBChB, Pal P, Chandra P, Albrecht D et al. (2015) Assessment of Drug Interaction Potential between LCZ696, an Angiotensin Receptor Neprilysin Inhibitor, and Digoxin or Warfarin. Clin Pharmacol Biopharm 4:147. doi:10.4172/2167-065X.1000147 | |
| Copyright: © 2015 Ayalasomayajula S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
LCZ696 (sacubitril/valsartan) is a first-in-class angiotensin receptor neprilysin inhibitor that simultaneously inhibits neprilysin and blocks the angiotensin II receptor. LCZ696 has been recently approved for treatment of HF and likely be co-administered with digoxin or warfarin. The drug interaction potential between LCZ696 and digoxin or warfarin was evaluated because of their potentially shared metabolic/elimination pathways. Two separate drug-drug interaction studies were conducted in healthy subjects: LCZ696 200 mg twice daily was co-administered with digoxin 0.25 mg once daily (n=24) and warfarin 25 mg single dose (n=26), respectively. The pharmacokinetic profiles of the LCZ696 analytes (sacubitril, LBQ657 and valsartan), digoxin and R- and S-warfarin, the pharmacodynamic effects of warfarin and the safety and tolerability of the investigational drugs were assessed. The geometric mean ratio (GMR) and 90%confidence interval (90% CI) for Cmax and AUCs of R- and S-warfarin, digoxin, and pharmacologically active LCZ696 analytes were within the bioequivalence range of 0.8-1.25 when co-administered. The GMR and 90% CI of warfarin pharmacodynamics effects were also within 0.8-1.25 range when co-administered with LCZ696. LCZ696 was generally safe and welltolerated when administered alone or in combination with digoxin/warfarin. No drug-drug interaction was observed upon co-administration of LCZ696 with digoxin/warfarin in healthy subjects.
| Keywords |
| LCZ696; Digoxin; Warfarin; Drug-Drug interaction; Neprilysin; Angiotensin-II receptor |
| Abbreviations |
| AE: Adverse Event; AUC: Area Under the Plasma Concentration-Time Curve; AUCinf: Area Under the Plasma Concentration−Time Curve With the Last Concentration Extrapolated; AUClast: Area Under the Plasma Concentration−Time Curve Up To the Last Measurable Concentration; AUC0-12h: Area Under the Plasma Concentration−Time Curve from Time Zero to the End of the Dosing Interval (0-12 hours); AUC0-24h: Area Under the Plasma Concentration−Time Curve from Time Zero to 24 Hours; b.i.d: twice daily; BMI: Body Mass Index; CI: Confidence Interval; Cmax, ss: Maximum (peak) Observed Steady-State Drug Concentration; CYP: Cytochrome P450; GMR: Geometric Mean Ratio; INR: International Normalised Ratio; MRP2: Multidrug Resistance-Associated Protein 2; OATP: Organic Anion Transporting Polypeptide; Pgp: P-Glycoprotein; PT: Prothrombin Time; q.d: Once Daily; SAE: Serious Adverse Event; SD: Standard Deviation; Tmax: Time to Reach Maximum (peak) Concentration Following Drug Administration; t1/2: Half-Life |
| Introduction |
| LCZ696 (sacubitril/valsartan) is a first-in-class angiotensin receptor neprilysin inhibitor that simultaneously inhibits neprilysin and blocks the angiotensin II receptor 1 (AT1). LCZ696 is developed for the treatment of heart failure [1,2] and has now been approved to reduce the risk of cardiovascular death and hospitalisation for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction [3]. Following oral administration of LCZ696, systemic exposure to sacubitril (pro-drug), LBQ657 (neprilysin inhibitor) and valsartan (AT1 receptor blocker) is observed. Peak concentrations of sacubitril, LBQ657 and valsartan are observed within a median time (Tmax) of 1-3 hours [4]. LBQ657 is predominantly eliminated by the renal route, whereas valsartan is primarily eliminated by the biliary route. In vitro studies have indicated that sacubitril, LBQ657 and valsartan are not metabolised by cytochrome P450 (CYP) enzymes to a significant level. Sacubitril exhibits a weak inhibition potential for CYP 2C8 (IC50: ~15 μM) and CYP 2C19 (IC50: ~20 μM), and LBQ657 exhibits a weak inhibition potential for CYP 2C9 (IC50 ~40 μM; Data on file), whereas valsartan shows no inhibition potential for any CYP enzyme [5,6]. In vitro data from Caco-2 cells suggest that P-glycoprotein (Pgp) is implicated in the transport of sacubitril [7]. Valsartan is transported by organic anion transporting polypeptide 1B1 (OATP1B1), 1B3 (OATP1B3) and multidrug resistance-associated protein 2 (MRP2) [8]. |
| Guideline-recommended treatment of heart failure includes administration of renin−angiotensin−aldosterone system inhibitors, aldosterone antagonists and beta blockers [9] In addition, digoxin maybe co-prescribed as it is considered potentially beneficial in patients with heart failure with reduced left ventricular ejection fraction, and has been shown to decrease hospitalisation rates [9]. Indeed, in the recently concluded PARADIGM-HF trial about 30% of participating subjects were administered with digoxin as a concomitant medication [2] In patients with chronic heart failure with atrial fibrillation or other additional risk factors for cardio-embolic stroke (e.g., history of hypertension, diabetes mellitus, previous stroke or transient ischemic attack or age ≥75 years), oral anticoagulants such as warfarin maybe prescribed [9]. Both digoxin and warfarin are narrow therapeutic index drugs. Digoxin undergoes Pgp-mediated transport in the intestine and kidney [10-12] and have a variable bioavailability rate of 60%–85% [13] It has been shown that concomitant treatment with drugs sharing the same transport or elimination mechanism, or those that inhibit Pgp, affect the disposition of digoxin [10,12] Pharmacokinetic interaction data for digoxin obtained from healthy subjects have been previously used to support prescription guidance [14-16]. |
| The S-enantiomer of warfarin is primarily metabolised by CYP2C9, whereas the R-enantiomer is metabolised by CYP1A2, CYP3A4, CYP2C19 [17]. Co-administration with compounds that inhibit these CYP enzymes could potentially increase the R- and S-warfarin concentrations, thereby increasing their anticoagulant effect and risk of bleeding [18]. Pharmacokinetic and pharmacodynamic interaction data for warfarin obtained from healthy subjects have been previously used in various studies to support prescription guidance [14,19]. |
| Based on in vitro observations, the potential for drug interaction between LCZ696 and digoxin via a Pgp mechanism and between LCZ696 and warfarin via a CYP2C9 interaction exists. Therefore, two separate clinical studies were conducted in healthy subjects to determine the potential drug interaction between LCZ696 and digoxin or warfarin. |
| Methods |
| Subjects |
| Healthy men and women between 18 and 45 years of age were included in both the clinical studies. Men using acceptable contraception during the studies and women of non-childbearing potential were eligible to participate in the studies. The major exclusion criteria for both the studies included history of smoking, use of any |
| prescription drugs or herbal supplements within 4 weeks prior to the initial dosing, use of over-the-counter medication or dietary supplements (vitamins included) within 2 weeks prior to the initial dosing and a history or presence of cardiovascular disease, including arrhythmias. |
| The studies were conducted at Triumpharma LLC (Amman, Jordan) in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki, following approval from the local institutional review board at the University of Jordan and the University Hospital, Amman, Jordan. All participants provided written informed consent before participating in the study. |
| A total of 24 subjects were enrolled in LCZ696–digoxin drug interaction study to ensure that at least 19 subjects complete the study to detect a change of at least 25% in digoxin pharmacokinetics with 80% power. A total of 26 subjects were enrolled in LCZ696–warfarin drug interaction study to ensure that at least 20 subjects complete the study to detect a change of at least 15% in warfarin pharmacokinetics with 90% power. The magnitude of change in exposure that can be detected with adequate power is appropriate for these narrow therapeutic index drugs. |
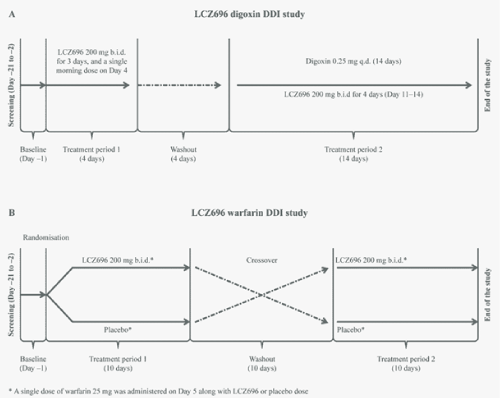
| LCZ696–digoxin drug interaction study: This was an openlabel, two-period, single-sequence study in healthy subjects (Figure 1A). After screening (Days −21 to −2) and baseline measurements (Day−1), in treatment period 1, the subjects were domiciled for 5 days and received LCZ696 200 mg twice daily (b.i.d.) for 3 days and a single (morning) dose of LCZ696 200 mg on Day 4. Pre-dose blood samples were collected on Day 3 (morning and evening dose) and Day 4 to measure LCZ696 trough levels. On Day 4, serial blood samples were collected for up to 12 hours to determine the pharmacokinetics of LCZ696 analytes (sacubitril, LBQ657, valsartan). The subjects were discharged from the study centre on Day 5 after the 24-hour sample of Day 4. |
| After a washout period of 4–7 days and baseline measurements, in treatment period 2, the subjects were domiciled for 15 days and received digoxin 0.25 mg once daily (q.d.) for 14 days, with LCZ696 200 mg b.i.d. co-administered from Day 11–14. Pre-dose blood samples were collected on Days 8, 9, 12 and 13 to measure digoxin trough levels. Serial post-dose blood samples were collected on Day 10 for 24 hours to determine the steady-state pharmacokinetics of digoxin. Pre-dose samples were collected on Days 13 (prior to the morning and evening LCZ696 doses) and 14 (prior to the morning LCZ696 dose) to measure trough levels of LCZ696 analytes. On Day 14, serial blood samples were collected for 12 and 24 hours post-dose to determine the pharmacokinetics of LCZ696 and digoxin, respectively. |
| LCZ696–warfarin drug interaction study: This was a single-blind, randomised, two-period, cross-over study in healthy subjects (Figure 1B). The subjects underwent a screening period (Days −21 to −2), two baseline periods (Day −1) and two treatment periods separated by a washout period of 10−14 days. In each period, the subjects were domiciled for at least 11 days and received either LCZ696 200 mg b.i.d. or matching placebo for 10 days with a single dose of warfarin 25 mg co-administered on Day 5. Pre-dose blood samples for LCZ696 were collected on Day 3 (morning and evening LCZ696/placebo dosing) and on Day 4 (morning only) to measure trough levels of LCZ696 analytes. Post-dose serial blood samples were collected on Day 4 for up to 12 hours post-morning dose to determine the pharmacokinetics of LCZ696 and on Day 5 and for up to 144 hours thereafter to determine the pharmacokinetic and pharmacodynamic profiles of warfarin. |
| Pharmacokinetic assessments |
| The plasma samples were analysed using validated liquid chromatography–tandem mass spectrometry with a lower limit of quantification at 1.0 ng·mL−1 for LCZ696 analytes (sacubitril, LBQ657 and valsartan) [20] and 0.1 ng·mL−1 for digoxin and 2.0 ng·mL−1 for warfarin enantiomers [14]. |
| The plasma pharmacokinetic parameters were determined using the non-compartmental method (WinNonlin Pro; version 5.2). The pharmacokinetic parameters for LCZ696 analytes and digoxin were the maximum observed steady-state drug concentration (Cmax,ss), Tmax and area under the plasma concentration−time curve (AUC) from time zero to the end of the dosing interval (0 to 12 hours [AUC0-12h] for LCZ696 analytes and from 0 to 24 hours [AUC0-24h] for digoxin). Pharmacokinetics parameters for warfarin enantiomers were Cmax, AUC up to the last measurable concentration (AUClast), AUC with the last concentration extrapolated (AUCinf) and half-life (t1/2). |
| Pharmacodynamic assessments |
| Pharmacodynamic parameters were assessed in the LCZ696– warfarin drug interaction study. Blood samples were collected in 3.8% sodium citrate-containing tubes at screening, at baseline (periods 1 and 2), just prior to warfarin administration (pre-dose, Day 5) and at 4, 8, 12, 24, 36, 48, 60, 72, 96, 120 and 144 hours post warfarin dose of each treatment period. The PT (seconds) and international normalised ratio (INR) values were determined [14] The mean PT (AUCPT/144; AUC for PT divided by time interval 144 hours [seconds]), peak PT (the maximum observed PT over 144 hours), mean INR (AUCINR/144; AUC for PT divided by time interval 144 hours [seconds]) and peak INR (the maximum observed INR over 144 hours) were determined from the PT and INR values. |
| Safety and tolerability assessments |
| Safety assessments consisted of recording all adverse events (AEs) and serious AEs (SAEs), with their severity and relationship to the study drugs. Physical examinations and regular monitoring/ assessment of haematology, blood chemistry urine, vital signs and electrocardiograms were conducted. |
| Statistical analysis |
| For statistical analysis, co-administration of digoxin or warfarin with LCZ696 was considered as the test treatment, whereas the individual treatments (LCZ696, digoxin or warfarin) were considered as reference. The primary analysis was conducted on log-transformed pharmacokinetic parameters. The point estimates and confidence limits were back-transformed to the original scale as geometric mean ratios (GMRs) and the corresponding 90% confidence interval (CI) limits. |
| In the LCZ696–digoxin drug interaction study, digoxin steadystate pharmacokinetic parameters were compared between period 2 Day 14 (LCZ696 + digoxin) and period 2 Day 10 (digoxin alone). Similarly, LCZ696 steady-state PK parameters were compared between period 2 Day 14 (LCZ696 + digoxin) versus period 1 Day 4 (LCZ696 alone). |
| In the LCZ696–warfarin drug interaction study, the LCZ696 steady-state pharmacokinetic parameters were compared between Day 5 (LCZ696 + warfarin) and Day 4 (LCZ696 alone), considering only the period where warfarin was administered. All the above comparisons were made using a paired t-test on log-transformed PK data and the difference estimate and 90% CI was back-transformed to obtain GMR and 90% CI of GMR. Since warfarin pharmacokinetic parameters for warfarin + LCZ696 and warfarin alone were collected in 2 × 2 cross-over set-up, a mixed model analysis of log-transformed pharmacokinetic data to obtain the difference estimates and 90% CI was used. The model included sequence, treatment and period as fixed effects and random effects from subject nested in sequence. GMR and 90% CI were obtained by back-transformation. |
| The pharmacodynamic analysis of warfarin (mean PT, peak PT, mean INR and peak INR) was conducted on log-transformed pharmacodynamics parameters using a linear mixed-effects model. Sequence (1, 2), period (1, 2) and treatment (factors were included in the model as fixed effects and subjects as a random effect). |
| No drug interaction was concluded if the GMR and the corresponding 90% CIs were within the bioequivalence range of 0.8- 1.25. If 90% CI bounds were outside of this range, the clinical relevance of a change in GMR was evaluated. |
| Results |
| Baseline characteristics and subject disposition Of the 24 subjects enrolled in the LCZ696–digoxin drug interaction study, 23 (95.8%) subjects completed the study and one subject discontinued due to an AE. In this study, all subjects were Caucasian males with a mean (± standard deviation [SD]) age of 26.9 ± 5.80 years and a body mass index (BMI) of 23.1 ± 3.0 kg/m2. |
| In the LCZ696–warfarin drug interaction study, a total of 26 subjects were enrolled; of these, 24 (92.3%) subjects completed the study and two subjects discontinued due to AEs. All the subjects enrolled in this study were Caucasian males with a mean (± SD) age of 27.3 ± 6.4 years and a BMI of 23.6 ± 3.2 kg/m2. In both studies, none of the AEs leading to discontinuations were suspected to be related to the study treatments. AEs are further described in the safety and tolerability section. |
| Assessment of steady-state concentrations of LCZ696 analytes and digoxin |
| The trough concentrations of the LCZ696 analytes as measured on Days 3 and 4 of the treatment in LCZ696-digoxin drug interaction study and Days 3-5 in LCZ696-warfarin drug interaction study indicate that steady state was achieved by Day 3 in both the studies following b.i.d. dosing (Table 1). The results of trough concentrations for digoxin as measured on Days 8–10 of the treatment also indicated that steadystate concentrations were achieved by Day 8 of the treatment as the Ctrough (mean ± SD) on Days 8, 9 and 10 were 0.5 ± 0.1, 0.5 ± 0.1 and 0.5 ± 0.1 ng·mL−1, respectively. |
| Effect of digoxin on the pharmacokinetics of LCZ696 analytes |
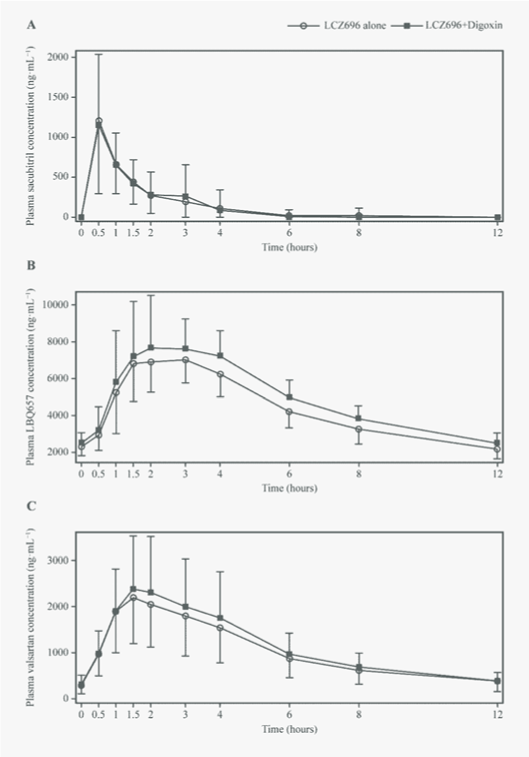
| The plasma concentration–time profiles of LCZ696 analytes were not altered when LCZ696 was co-administered with digoxin (Figure 2). The corresponding pharmacokinetic parameters for LCZ696 analytes are summarised in Table 2. LCZ696 analytes (sacubitril, LBQ657 and valsartan) were rapidly absorbed into systemic circulation when administered alone (median values of Tmax: 0.5, 2.5 and 1.5 hours, respectively) or in the presence of digoxin (median values of Tmax: 0.5, 2.0 and 1.5 hours, respectively). The mean Cmax,ss of sacubitril decreased by 2% in the presence of digoxin, based on the estimated GMR (90% CI) of 0.98 (0.8, 1.25). The 90% CIs and respective GMRs for Cmax,ss for LBQ657 and valsartan, and AUC0-12h for LBQ657, sacubitril and valsartan in the presence of digoxin were within the noeffect range of 0.8-1.25 (Table 2). |
| Effect of warfarin on the pharmacokinetics of LCZ696 analytes |
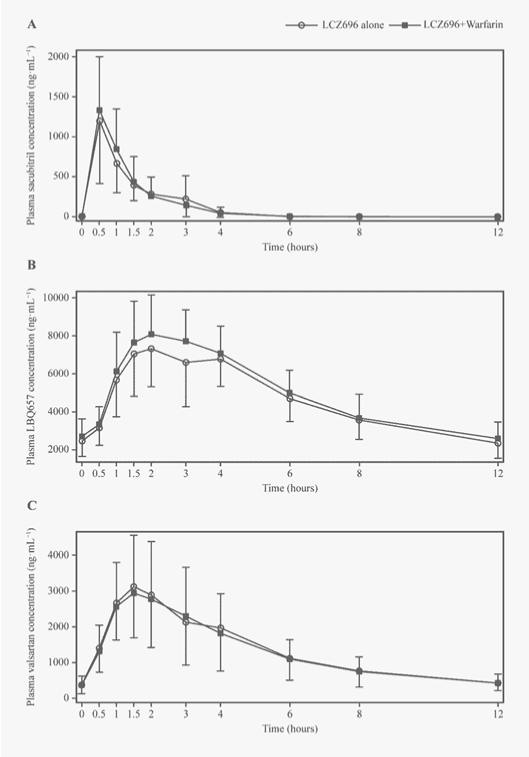
| The plasma concentration−time profiles of the LCZ696 analytes were not altered when co-administered with warfarin (Figure 3). The median values of Tmax were 0.5, 2.0 and 1.5 hours for sacubitril, LBQ657 and valsartan, respectively, and remained unchanged in the presence of warfarin. The mean Cmax,ss and AUC0-12h for LBQ657 and valsartan, and the mean AUC0-12h for sacubitril were similar to those observed following individual treatments (within the no-effect range of 0.8−1.25). The mean Cmax,ss for sacubitril increased by 18% in the presence of warfarin, with the upper limit of 90% CI being 1.5 (Table 2). |
| Effect of LCZ696 on the pharmacokinetics of digoxin |
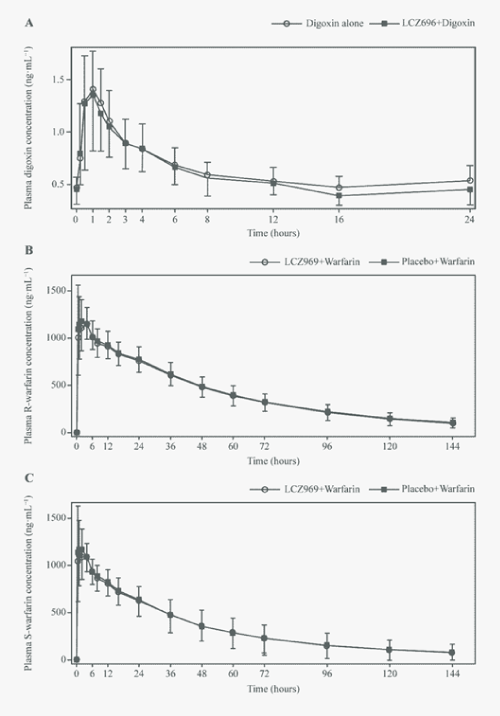
| The mean Cmax,ss of digoxin was 1.5 ng·mL−1 (GMR [90% CI]: 0.96 [0.9, 1.0]) at approximately 1 hour when administered alone or in combination with LCZ696 (Figure 4A). The mean AUC0-24h for digoxin was similar when administered alone (15.3 ng·h.mL−1) or in the presence of LCZ696 (14.2 ng·mL−1; GMR [90% CI]: 0.9 [0.9, 0.96]; (Table 3). |
| Effect of LCZ696 on the pharmacokinetics and pharmacodynamics of warfarin |
| The plasma concentration–time profile of R- and S-warfarin (Figure 4B, Figure 4C) showed that Cmax was achieved within 2 hours when warfarin was administered alone (median Tmax: 2.0 and 1.0 hours for R- and S-warfarin, respectively), and remained unchanged in the presence of LCZ696. LCZ696 did not affect the pharmacokinetic parameters of warfarin enantiomers, including Cmax, AUClast and AUCinf. The 90% CIs of the estimated GMRs were within the no-effect range of 0.8-1.25 (Table 4). Co-administration of warfarin and LCZ696 and co-administration of warfarin and placebo were associated with similar effects on pharmacodynamics of warfarin PT, maximal PT, INR and maximal INR (Figure 5). The adjusted GMRs (90% CI) between the LCZ696 + warfarin and LCZ696 + placebo treatments for mean PT, maximal PT, INR and maximal INR remained within the no-effect range of 0.8-1.25 (Table 5). |
| Safety and tolerability |
| All of the AEs reported with LCZ696 in combination with digoxin or warfarin were mild-to-moderate in severity, except one incidence of severe AE of headache in the LCZ696–digoxin drug interaction study. There were no SAEs or deaths in either of the studies. |
| In the LCZ696–digoxin drug interaction study, the proportion of subjects with at least one AE with LCZ696 (29.2%) was similar to digoxin (25.0%) and was slightly higher during the co-administration treatment phase (i.e. period 2; 41.7%). The most frequently reported AEs were diarrhoea, dyspepsia, headache and abdominal pain. Diarrhoea was more commonly reported during co-administration of LCZ696 and digoxin (29.2%) compared with LCZ696 (4.2%) or digoxin (0%) alone, whereas dyspepsia was more common with LCZ696 administration (12.5%) compared with digoxin (4.2%) or their combination (0%). One subject discontinued the study due to an AE of vomiting, which was not considered to be related to the study drug by the study investigator, and the event resolved on the same day without treatment. |
| In the LCZ696–warfarin drug interaction study, 40.0%, 24.0%, 32.0% and 19.2% of subjects on LCZ696, warfarin + LCZ696, warfarin + placebo and placebo, respectively, had AEs. Headache and back pain were the most commonly reported AEs. Headache was reported by five subjects (20.0%) each on LCZ696 + warfarin and warfarin + placebo treatments and two subjects (8.0%) on LCZ696 alone. Back pain was more common in subjects taking LCZ696 alone (20.0%) compared with the other treatments (none on LCZ696 + warfarin and placebo + warfarin and 7.7% with placebo). Two subjects discontinued the study due to AEs, one subject due to facial swelling secondary to a tooth abscess and another subject due to a mild wound due to a cut. The facial swelling and cut resolved 10 and 22 days after the last study medication, respectively. A relationship between these events and study drug was not suspected by the study investigator. |
| Discussion and conclusions |
| Digoxin and warfarin are narrow therapeutic index drugs that are commonly used to treat patients with heart failure and reduced ejection fraction [8]. In patients with mild-to-moderate heart failure, digoxin has been shown to improve health-related quality of life and exercise tolerance, regardless of the underlying rhythm, causes of heart failure or concomitant therapy [9] Warfarin is commonly prescribed in patients with chronic heart failure and permanent or paroxysmal atrial fibrillation and additional risk factors for cardioembolic stroke [9] Therapeutic drug monitoring of warfarin, which involves regular monitoring of its INR, is indicated [21] as the INR can be affected by numerous factors including diet [22] LCZ696 is being developed for the treatment of heart failure at a proposed dosing regimen of 200 mg b.i.d. As there is a potential for co-administration of LCZ696 with either digoxin or warfarin in this patient population, two clinical studies were conducted to evaluate the drug interaction potential between LCZ696 and digoxin, and LCZ696 and warfarin. |
| Following twice-daily administration of LCZ696 200 mg, steadystate levels of LCZ696 analytes were achieved by Day 3. The median values of Tmax for all LCZ696 analytes were similar when LCZ696 was administered alone or in combination with digoxin or warfarin. The GMRs and 90% CIs for both Cmax and AUCs of LBQ657 and valsartan were within the bioequivalence range of 0.8-1.25 when LCZ696 and digoxin were co-administered, suggesting that digoxin has no effect on the pharmacokinetics of LCZ696. Similarly, the GMRs and 90% CIs of Cmax and AUCs of LBQ657 and valsartan were within the bioequivalence range of 0.8-1.25 when LCZ696 and warfarin were coadministered, suggesting that the steady-state pharmacokinetics of LCZ696 were not changed when co-administered with a single dose of warfarin. In contrast, the Cmax,ss of sacubitril increased by 2% and 18% when LCZ696 was co-administered with digoxin and warfarin, respectively. This is not considered to be clinically relevant because 1) the inter-subject variability of sacubitril Cmax,ss exceeded the observed changes (coefficient of variation: 41%–56%), 2) sacubitril is a prodrug and not biologically active, and 3) Cmax and AUC of LBQ657, the active neprilysin inhibitor, were not affected. |
| Digoxin transport is mainly mediated by Pgp [10,11] and a coadministered drug with a similar transport mechanism or an inhibitor of Pgp may interfere with the disposition of digoxin [10,12]. The present study indicated that co-administration of LCZ696 does not alter the steady-state levels and pharmacokinetics of digoxin. The GMRs and 90% CIs for both Cmax and AUCs of digoxin were within the bioequivalence range of 0.8-1.25, suggesting that co-administration with LCZ696 has no effect on the pharmacokinetics of digoxin and thus does not influence the PgP-mediated transport of any PgP substrate. |
| The more potent S-enantiomer of warfarin is primarily oxidised by CYP2C9, whereas the R-enantiomer is oxidised by CYP1A2, CYP3A4 and CYP2C19 [17]. The clinical effects of warfarin are primarily assessed by measuring PT and its derived standardised parameter, INR [23,24]. In vitro assessment demonstrated inhibition of CYP 2C19 by sacubitril (IC50 of ~20 μM) and inhibition of CYP 2C9 by LBQ657 (IC50 ~40 μM) (Data on file). The GMRs and 90% CIs of Cmax and AUCs of R- and S-warfarin and the peak and mean parameters of PT and INR values were within the bioequivalence range of 0.8-1.25, suggesting that LCZ696 has no impact on the pharmacokinetics and pharmacodynamics of warfarin. These results indicate that LCZ696 at the proposed therapeutic dose does not inhibit CYP2C9. |
| Overall, LCZ696 was safe and well tolerated both as monotherapy and in combination with either digoxin or warfarin. The reported AEs were mostly mild to moderate in severity and the AEs leading to study discontinuation were not related to the study drugs. |
| In conclusion, the present studies in healthy subjects indicate that co-administration of LCZ696 200 mg b.i.d. and warfarin 25 mg q.d. or digoxin 0.25 mg q.d. are not subject to a drug–drug interaction, and that LCZ696 does not interact with PgP-mediated drug transport or CYP2C9-mediated drug metabolism. |
| Acknowledgements |
| The authors acknowledge Arvind Semwal (Novartis, Hyderabad, India) for providing medical writing assistance in developing the manuscript. |
| Author contributions |
| Study design: P Jordaan, S Ayalasomayajula, P Pal, T Langenickel, I Rajman, G Sunkara; Study conduct: P Jordaan, S Ayalasomayajula, Chandra P; Data collection: P Pal; Data analysis: S Ayalasomayajula, P Jordaan, P Pal; Data interpretation: P Jordaan, S Ayalasomayajula, P Pal, T Langenickel, I Rajman, G Sunkara; Drafting manuscript and revising contents: All the authors; Approving final version: All the authors; Took responsibility for integrity of data analysis: All the authors. |
| Financial Disclosure: This study was funded by Novartis Pharma AG, Basel, Switzerland. |
| Conflict of Interest: Surya Ayalasomayajula, Pierre Jordaan, Parasar Pal, Diego Albrecht, Thomas Langenickel, Iris Rajman and Gangadhar Sunkara are employees of Novartis and are therefore eligible for Novartis stock options. Priyamvada Chandra was an employee of Novartis at the time of study completion and data interpretation. Priyamvada Chandra currently works at Genentech, Inc., Clinical Pharmacology, South San Francisco, CA, USA. |
References
- Solomon SD, Zile M, Pieske B, Voors A, Shah A, et al. (2012) The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 380: 1387-1395.
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, et al. (2014) Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). European journal of heart failure 16: 817-825.
- Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, et al. (2010) Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J ClinPharmacol 50: 401-414.
- Nakashima A, Kawashita H, Masuda N, Saxer C, Niina M, et al. (2005) Identification of cytochrome P450 forms involved in the 4-hydroxylation of valsartan, a potent and specific angiotensin II receptor antagonist, in human liver microsomes. Xenobiotica 35: 589-602.
- Taavitsainen P, Kiukaanniemi K, Pelkonen O (2000) In vitro inhibition screening of human hepatic P450 enzymes by five angiotensin-II receptor antagonists. Eur J ClinPharmacol 56: 135-140.
- Knutter I, Kottra G, Fischer W, Daniel H, Brandsch M (2009) High-affinity interaction of sartans with H+/peptide transporters. Drug MetabDispos 37: 143-149.
- Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y (2006) Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug MetabDispos 34: 1247-1254.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: e240-327.
- deLannoy IA, Silverman M (1992) The MDR1 gene product, P-glycoprotein, mediates the transport of the cardiac glycoside, digoxin. BiochemBiophys Res Commun 189: 551-557.
- Hori R, Okamura N, Aiba T, Tanigawara Y (1993) Role of P-glycoprotein in renal tubular secretion of digoxin in the isolated perfused rat kidney. J PharmacolExpTher 266: 1620-1625.
- Wessler JD, Grip LT, Mendell J, Giugliano RP (2013) The P-glycoprotein transport system and cardiovascular drugs. Journal of the American College of Cardiology 61: 2495-2502.
- Joseph G. Murphy MAL (2008) Mayo Clinic Cardiology Concise Textbook 3rd Edition ed: Mayo Clinic Scientific Press :1233.
- Yan JH, Meyers D, Lee Z, Danis K, Neelakantham S, et al. (2014) Pharmacokinetic and pharmacodynamic drug-drug interaction assessment between pradigastat and digoxin or warfarin. J ClinPharmacol 54: 800-808.
- Dieterich HA, Kemp CSV, CM Y (2006) Aliskiren, the first in a new class of orally effective renin inhibitors, has no clinically significant drug interactions with digoxin in healthy volunteers. ClinPharmacolTher 79: 111-124.
- Vaidyanathan S, Camenisch G, Schuetz H, Reynolds C, Yeh CM, et al. (2008) Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: the role of P-glycoprotein in the disposition of aliskiren. J ClinPharmacol 48: 1323-1338.
- Wittkowsky AK (2003) Warfarin and other coumarin derivatives: pharmacokinetics, pharmacodynamics, and drug interactions. SeminVasc Med 3: 221-230.
- Serlin MJ, Breckenridge AM (1983) Drug interactions with warfarin. Drugs 25: 610-620.
- Dieterle W, Corynen S, Mann J (2004) Effect of the oral renin inhibitor aliskiren on the pharmacokinetics and pharmacodynamics of a single dose of warfarin in healthy subjects. Br J ClinPharmacol 58: 433-436.
- Gan L, Jiang X, Mendonza A, Swan T, Reynolds C, et al. (2015) Pharmacokinetic drug-drug interaction assessment of LCZ696 (an angiotensin receptor neprilysin inhibitor) with omeprazole, metformin or levonorgestrel-ethinyl estradiol in healthy subjects. ClinPharmacol Drug Dev.
- Kuruvilla M, Gurk-Turner C (2001) A review of warfarin dosing and monitoring.Proc (BaylUniv Med Cent) 14: 305-306.
- Horton JD, Bushwick BM (1999) Warfarin therapy: Evolving strategies in anticoagulation. AmFam Physician 59: 635-646.
- Kirkwood TB (1983) Calibration of reference thromboplastins and standardisation of the prothrombintime ratio. ThrombHaemost 49: 238-244.
- Johnston M, Harrison L, Moffat K, Willan A, Hirsh J (1996) Reliability of the international normalized ratio for monitoring the induction phase of warfarin: Comparison with the prothrombin time ratio. J Lab Clin Med 128: 214-217.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 15158
- [From(publication date):
November-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10437
- PDF downloads : 4721
