Research Article Open Access
Assessment of Coastal Water Habitat with Reference to the Variability of Plankton during Spawning Season of Indian River Shad in Greater Noakhali-Bangladesh
Md. Jahangir Sarker*, Farhana Binte Rashid and Mehedi Hasan TanmayDepartment of Fisheries and Marine Science, Noakhali Science and Technology University, Bangladesh
- *Corresponding Author:
- Jahangir Sarker Md
Department of Fisheries and Marine Science
Noakhali Science and Technology University
Bangladesh
Tel: +8801733910237, +880-321-71487
Fax: +880-321-62788
E-mail: swaponj@yahoo.com
Received date June 11, 2016; Accepted date July 02, 2016; Published date July 11, 2016
Citation: Sarker MJ, Rashid FB, Tanmay MH (2016) Assessment of Coastal Water Habitat with Reference to the Variability of Plankton during Spawning Season of Indian River Shad in Greater Noakhali-Bangladesh. J Ecosys Ecograph 6:197. doi:10.4172/2157-7625.1000197
Copyright: ©2016 Sarker MJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
The present study was conducted to quantify the water quality parameters and plankton concentrations of the Meghna river estuary during the spawning season of Hilsa (Tenualosa ilisa) and also to establish knowledge about the habitat. Hilsa (Tenualosa ilisha) is one of the flagship anadromous fish species of Bangladesh that migrate downstream for spawning purposes only through the Ganges-Meghna river system route. The study period constitutes two spawning seasons of Hilsa (Tenualosa ilisha) (June 2014 and February, March 2015). The water quality parameters and plankton concentrations were measured and analyzed by standard methods. A total of 50 genera of plankton identified from the water body belonging to the group Bacillariophyceae, Cyanophyceae, Chlorophyceae, Dinophyceae, Coscinodiscophyceae, Copepoda, Rotifera, Cladoceran, Protozoa, Crustacean larvae and Meroplankton. The phytoplankton density was varied from 2.93×103 to 7.94×103 cells/L and zooplankton density of 1.15×103 cells/L to 1.8×103 cells/L. The plankton concentrations were strongly correlated with the fluctuations of water quality. The water temperature and phytoplankton density was positively correlated (r = 0.75), on the other hand, negative correlation was found between phyplankton and transparency (r = - 0.84). Bacillariophyceae was the most dominant group contributing 78% of phytoplankton and among zooplankton, Copepoda contributes 36%. Shannon-Weiner species diversity index (Hʹ) used as an indicator of water quality. It ranged from 2.07 to 2.74 (phytoplankton) and 1.82 to 2.38 (zooplankton). The mean value of phytoplankton was 2.42 ± 0.19 and zooplankton was 2.14 ± 0.16 and it was within the range of 1 to 3, so the water body is moderately polluted. The phytoplankton cell density is a good indicator to determine the trophic status of a particular water body. The mean cell density of phytoplankton was 5372 cells/L and could be classified as oligotrophic. Oligotrophic water body characters lack nutrients which resulted in the lowest density of plankton. Based on plankton density it can be concluded that during spawning season, plankton profile is low which might be hindered Hilsa to migrate this spawning ground.
Keywords
Indian river shad; Plankton; Coastal water habitat; Noakhali; Bangladesh
Introduction
Estuaries considered as the most productive aquatic ecosystem which plays an important role in breeding and nursery ground for wide variety fish species. In Bangladesh, the Meghna river estuary is the largest estuarine ecosystem and support diverse fisheries communities in comparison to others. It is one of the prominent habitats of Hilsa (Tenualosa ilisha) fishery which is used for their spawning purpose. Hilsa is a migratory fish species and in the spawning season it migrates to the lower reaches of the Meghna River in the estuarine zone. According to Ward and Whipple [1], Hilsa is primarily planktivore species and its food items are diatoms, blue-green algae, copepods, desmids, cladocerans, rotifers etc. It is very important to understand the feeding habit of Hilsa like the presence of their preferable food items with abundance. The qualitative and quantitative abundance of plankton and its relation to environmental condition consider as a prerequisite to manage an aquatic ecosystem successfully. In most cases, the abundance of planktonic organisms proved as beneficial for fish production. However, if the plankton abundance causes bloom then it exerts negative effect likely severe economic loss to aquaculture, fisheries operation and causing major environmental and human health related problem. Due to rapid industrialization and urbanization in the country, at the same time, river erosion which is thoroughly associated with heavy surface runoff causing siltation and have an effect on growth and production rate of Hilsa and exerts influence on primary production in the eastern region. So, the monitoring program of plankton during the spawning season of Hilsa will provide up-todate information on that habitat. The pollution status of aquatic habitat can detect through the species diversity index, which is one of the best ways to evaluate the impact of pollution on aquatic communities [2]. In spite of having overwhelming importance, no research has not yet been conducted to date on the assessment of ecological conditions of the Meghna estuary for Hilsa fishery. The present study was undertaken to study monthly variations of plankton with some water quality parameters and assessing the status of this habitat.
Materials and Methods
The study area
The research work was conducted in the Meghna estuary, Char Alexender, Ramgati Upazilla of Lakshmipur district, Bangladesh (Figure 1). The Hatiya Island situated in the east, Bhola to the west, greater Noakhali to the north and the Bay of Bengal to the south around the study area. The study was carried out over three season winter (February 2015), pre-summer (March 2015) and summer (June 2014) covered two spawning seasons of Hilsa from June-July and January- March. Water sampling was done from six stations of the Meghna river estuary, which is the most important estuarine ecosystem in the southeast coastal portion of Bangladesh.
Water quality parameters
Surface water samples were collected and analyzed in situ to determine salinity (ppt), temperature (°C), transparency (cm) and pH using a Multiparameter analyzer. Water temperatures were recorded directly on the spot by a Celsius thermometer and pH by a digital pH meter. Water transparency values were measured by a simple Secchi disc. Salinity was measured by using Refractometer (NewS-100, TANAKA, Japan) and expressed as the parts per thousand (ppt).
Phytoplankton study
The phytoplankton quantitative and qualitative estimations were taken for each month of the study period. Plankton samples (20 L) were collected from the surface water of estuary by passing water samples through fine-meshed plankton net (25 μm meshes sized). The samples were preserved immediately with 10% buffered formalin in plastic bottles. A Sedgwick–Rafter (S–R) cell was used under a luminous microscope (XSZ21-05DN, made in China) for phytoplankton counting. Phytoplankton was identified to genus level and counted using the formula proposed by Stirling [3] and was expressed as the number of cells per litter of water.
Zooplankton study
Zooplankton quantitative and qualitative estimations were taken in during sampling month of the study period. Plankton samples (20 L) were collected from the surface water of estuary by passing water samples through fine-meshed plankton net (40 μm meshes sized). The samples were preserved immediately with 10% buffered formalin in plastic bottles. A Sedgwick–Rafter (S–R) cell was used under a luminous microscope (XSZ21-05DN, made in China) for zooplankton counting. Zooplankton was identified to genus level and counted using the formula proposed by Stirling [3] and was expressed as the number of cells per litter of water.
Species richness, diversity and evenness index calculation
Species richness index (d):
Margalef index (d) [2] was used to measure species richness by using the following formula:
d = (S-1)/ ln N
Where,
d = Species richness index,
S = Number of species in a population,
N = Total number of individuals in S species.
Species diversity index (H): Shannon-Weiner diversity index [4- 6] considers the number of species and the distribution of individuals among species. The Shanon-Weiner diversity was calculated by the following formula.
H = -ΣPi ln Pi
Where,
H = Diversity index,
i = Counts denoting the ith species ranging from 1-n,
Pi = Proportion that the ith species represents in terms of numbers of individuals with respect to the total number of individuals in the sampling space as the whole.
Evenness index (j): Buzas and Gibson’s [7] was measured by using the following formula:
j = H / ln S
Where,
j = Equitability index,
H = Shannon and weaver index,
S = Number of species in a population.
Statistical analysis
For all sampling techniques, three replicates were analyzed and means and standard deviations were calculated and expressed as mean (± SD). Paleontological Statistics (PAST) version 3.15, a software package for paleontological data analysis written by Ryan et al. [8] was used to run the analysis.
Results and Discussion
Water quality parameters
In the present study, water salinity of February and March were varied in the ranges of (2-4) ppt where in June salinity varies between (1-2) ppt (Table 1). The dropping of salinity in June was might be due to freshwater inflow from the surrounding land area. According to McErlean et al. [9] salinity of an estuary ranged between 0.50 and 35 ppt. Surface water pH value varies between 7.0 (February, 2015) – 7.5 (March, 2015). The mean water pH found maximum in March (7.3) and minimum one was in June (7.08). More or less similar results found by Hossain et al. [10] where water pH values vary between 7.7 to 6.9 in the Meghna estuary. As salinity was considerably higher in winter and freshwater influx was low as compared to summer period, hence it might be due to greater concentration of available alkali metals in their ionic forms pH in winter was higher than in summer months. Surface water temperature (°C) showed maximum in June (27°C) in the summer period and a minimum in February (20°C) during winter. Maximum average water temperature occurred 24.92 ± 1.93 in June and minimum average one was resulted 22.42 ± 1.74 in February (Table 1). It can be said that the higher surface water temperature was observed in sampling month June due to strong solar irradiance as the sample collection day was sunny. Patra and Azadi [11] studied on the planktonic organisms of Halda river recorded highest water temperature during summer and lowest in winter months. The highest water transparency was recorded in the sampling month February and March (32 cm) where the minimum value observed (19 cm) during June. The water transparency ranged between (28-32 cm) in February and March, (19-25) cm (Table 1) in June during the study period. Water transparency was lower in June might be due to raining when river erosion occurs and makes the water body turbid by carrying large amount of sand and silt. Reid and Wood reported that water transparency depends on several factors likely silting, plankton density, suspended organic matter, latitude, season and the angle and intensity of incident light.
Qualitative and quantitative plankton count
Phytoplankton: In the current study period, 31 genera of phytoplankton were identified which belonged to five major groups likely Bacillariophyceae, Chlorophyceae, Coscinodiscophyceae, Cyanophyceae and Dinophyceae (Table 2). Therefore, 31 phytoplankton genera were divided as 22 belonged to Bacillariophyceae, 5 to Chlorophyceae, 1 to Coscinodiscophyceae, 3 to Cyanophyceae and 1 to Dinophyceae (Table 2). A study of Halda River recorded the phytoplankton population as algal flora under the classes Chlorophyceae, Cyanophyceae, Bacillariophyceae and Myxophyceae [11]. The findings of the present study can be compared with Shah et al. [12] studied on the seasonal variations of phytoplankton communities in the southwest coastal waters of Bangladesh recorded a total of 31 phytoplankton species; where 17 to Bacillariophyceae, 7 to Cyanophyceae, 5 to Chlorophyceae, and 2 to Dinophyceae. During the present study period for total abundance of plankton population phytoplankton was contributed about 79%. Shafi et al. [13] reported higher percentage composition of phytoplankton (76.0 ‚?ź 93.6) % from the Meghna river. Similar findings were found in the recent investigation by Ahsan et al. [14]studied on the plankton abundance of the Meghna river, observed that phytoplankton formed 90% of the total plankton abundance.
Phytoplankton counts (number of cells per liter of water) were 2.93-4.62×103 cells/L, 4.50-5.93×103 cells/L, and 5.50-7.93×103 cells/L in February, March, and June respectively. Maximum phytoplankton density was also observed in June (175.8×103 cells/L) by Shah et al. [12] where the minimum number in September (12.0×103 cells/L) in Shibsa river, southwest coast of Bangladesh. These higher densities of Shibsa river were attributed due to continuous discharge of sewage water during the rainy periods. The phytoplankton densities can be compared with Boonyapiwat [15] who worked in the coasts of the Gulf of Thailand and east coast of Peninsular Malaysia recorded 214-33520 cells/L and 178-14223 cells/L of phytoplankton. However, another study of Boonyapiwat et al. [16] was also recorded lower (2800-4380 cells/L) density of phytoplankton using 80 μm mesh size of the net.
| Station | St-1 | St-2 | St-3 | St-4 | St-5 | St-6 | |
|---|---|---|---|---|---|---|---|
| February | Temperature (°C) | 21 | 22.5 | 23 | 20 | 23 | 25 |
| Transparency (cm) | 31 | 30 | 32 | 32 | 28 | 32 | |
| pH | 7.2 | 7.2 | 7.1 | 7 | 7.3 | 7.1 | |
| Salinity (ppt) | 2 | 3 | 2 | 2 | 4 | 2 | |
| March | Temperature (°C) | 23 | 24 | 22.5 | 24.5 | 22 | 25 |
| Transparency (cm) | 31 | 31 | 32 | 28 | 32 | 30 | |
| pH | 7.3 | 7.5 | 7.4 | 7.1 | 7.3 | 7.2 | |
| Salinity (ppt) | 2 | 4 | 4 | 2 | 3 | 2 | |
| June | Temperature (°C) | 24 | 27 | 25 | 24.5 | 26 | 23 |
| Transparency (cm) | 21 | 19 | 20 | 22 | 20 | 25 | |
| pH | 7 | 7.1 | 7.1 | 7.2 | 7 | 7.1 | |
| Salinity (ppt) | 1 | 1 | 2 | 2 | 2 | 1 | |
| St-1 = Station-1 and respectively | |||||||
Table 1: Water quality parameters observed in three different months in the Meghna river estuary during the present study.
| Bacillariophyceae | Chlorophyceae | Coscinodiscophyceae | Cyanophyceae | Dinophyceae |
|---|---|---|---|---|
| Amphora Bacillaria Biddulphia Chaetoceros Coscinodiscus Cyclotella Diploneis Fragilaria Melosira Nitzschia Pleurosigma Rhizosolenia Stephanophysis Surirella Thalassionema Cymbella Synedra Gyrosigma Licmophora Cocconeis Pseudo-nitzschia Tabellaria sp. |
Ulothrix Spirogyra Closterium Characium Monoraphidium |
Hemisdiscus | Oscillatoria Anabaena Microcystis |
Procentrum |
Table 2: List of phytoplankton genera recorded from the surface water of the Meghna river estuary throughout the experimental period.
The seasonal phytoplankton community indicated that diatoms especially the Rhizosolenia sp. as most dominant species during summer (June) while some other diatoms including Coscinodiscus sp., Thalassionema sp., Melosira sp., Amphora sp., Characium sp. also found more or less throughout the study period. Blue-green algae, especially Oscillatoria sp. were most abundant in February.
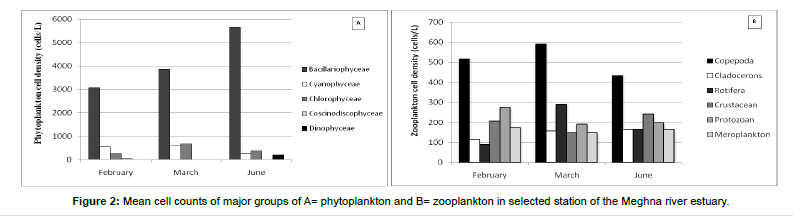
In the present study, Bacillariophyceae was the largest group by quality and quantity which comprised 78% (approx.) of total phytoplankton abundance. Similar findings by Shah et al. [12] where Bacillariophyceae comprising 75% of total cell counts. The dominance of diatoms in estuarine and marine aquatic environment was also agreed Boonyapiwat [15], Sevindik [17] and Al-Hashmi et al. [18]. It showed availability throughout the present study period, the dominant species in this included Melosira sp., Coscinodiscus sp., Nitzschia sp., Fragilaria sp., Rhizosolenia sp. and Thalassionema sp. Coscinodiscus sp. comprised about 23% and Melosira sp. 17% approximately of Bacillariophyceae. Among diatoms, Pseudo-nitzschia sp. was showed maximum density in February, and Tabellaria sp. was only present in June where cell density 63 cells/L. The highest density of Bacillariophyceae was observed during summer (June at station-2) about 6.375×103 cells/L and lowest density in winter (February at station-6) 2.625×103 cells/L. In the present study, mean cell density becomes higher from February (3073.166 cells/L) to March (3864.833 cells/L) to June (5656.666 cells/L) (Figure 2).
Among all phytoplankton groups, Cyanophyceae is the second most dominant group in term of abundance. The group was dominated by Oscillatoria sp. and other species like Anaebena sp. and Microcystis sp. Cyanophyceae contributed approximately 9% of total phytoplankton abundance which was recorded larger amount in pre-summer (March) about 875 cells/L at sampling station-1. The mean abundance of Cyanophyceae 552.5, 604.333 and 271.166 cells/L in February, March and June respectively (Figure 2).
On the other hand, Chlorophyceae was ranked as third among phytoplankton group in accordance of abundance. Among Chlorophytes, the different species in order of abundance Characium sp., Ulothrix sp., Closterium sp., Spirogyrasp., and Monoraphidium sp. The highest number of species was presented in March. Like Cyanophyceae highest cell density was also observed in March about 938 cells/L at station-4. The average cell density was 260.5, 667.166 and 375.333 cells/L in February, March and June respectively (Figure 2).
In the present study, Dinophyceae was only found in the month of June and it constituted approximately 1% of total phytoplankton abundance. The highest cell density 563 cells/L in station-2 of June. The genera in this group were Procentrum. The mean abundance of dinophyceae was 198 cells/L in June (Figure 2).
Another class was found in the present study which is Coscinodiscophyceae, only species belonged to this group was Hemisdiscus sp. This was only present in February and highest density was 125 cells/L at station-3. It constituted <1% of total phytoplankton abundance. The average density of this group was 41.833 cells/L in February (Figure 2).
The dominance of Bacillariophyceae suppresses the abundance of other phytoplankton groups. According to abundance phytoplankton group was found in the following order:
Bacillariophyceae > Cyanophyceae > Chlorophyceae > Dinophyceae > Coscinodiscophyceae
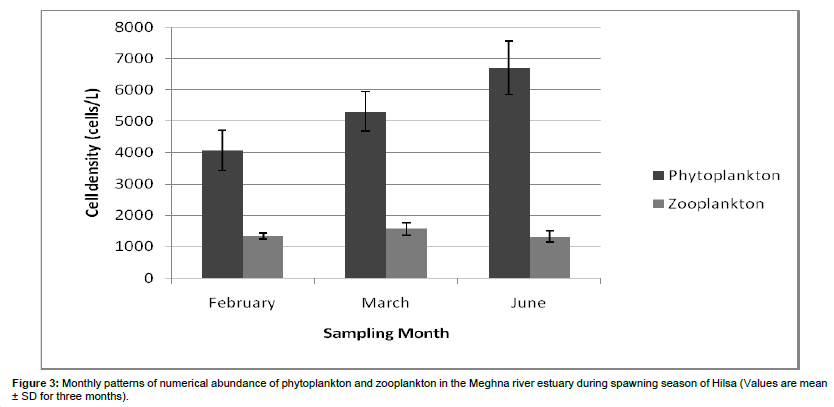
Zooplankton: The recorded zooplanktonic organisms were belonging to the group of rotifers, copepods, cladocerons, crustacean larvae and meroplankton. A total of 18 genera were identified from the zooplankton community where 8 belong to Copepoda, 2 to Rotifera, 2 to Cladocera, 3 to Protozoa and 3 to Meroplankton (Table 3). No genera were identified under the group of crustacean larvae. In the current investigation, zooplankton contributed about 21% of total plankton abundance which differs from Ahsan et al. [14] where they observed zooplankton population about 10% of total plankton. It can be said that in a lotic waterbody low population of zooplankton is not uncommon. Sundar et al. [19] reported the major contribution of phytoplankton (>97.0%) and lower concentration of zooplankton (0.13 ‚?ź 2.4) % at three stations in the Guala river of Uttar Pradesh, India. In the present study, zooplankton counts (number of cells per liter of water) were ranging from 1.20-1.50×103 cells/L, 1.25-1.80×103 cells/L, and 1.15-1.60×103 cells/L in February, March, and June respectively. The highest zooplankton production was recorded in March 1.80×103 cells/L and minimum one was 1.15×103 cells/L in June. The average zooplankton density of February, March and June was 1391.66 ± 142.88 cells/L, 1575.67 ± 201.86 cells/L and 1275.33 ± 117.26 cells/L respectively (Figure 3). Islam et al. [20] recorded the highest density of zooplankton in January (1350 cells/L). The peak of zooplankton in winter may be due to the favourable conditions of the physicochemical parameters and the availability of nutrients. Also, zooplankton showed their abundance in winter might be due to the lesser abundance of phytoplankton in winter.
Copepods were dominated throughout the study period, constituted approximately 36% of total zooplankton abundance. The present study can be compared with Gutkowska et al. [21] stated that in the saline region, copepods (45%) were the predominant species. Higher percentage found by Omondi et al. [22] studied on Lake Baringo, Kenya also observed Copepoda as a dominant group formed (60-72) % of the total zooplankton. The percentage variation might be due to a presence of nutrients in an aquatic habitat. The representative species of copepod was recorded from all sampling stations of the entire study period. The mean abundance of copepods was 513.55 ± 109.55 cells/L. The highest cell density was 700 cells/L which recorded in March at sampling station-4 and lowest density was observed in June station-2 and February station-6 (350 cells/L). Cyclops sp. was the most dominant species of copepods and occupied approximately 36%. Other dominant species of copepods were Cyclops nauplius and Diaptomus sp.
Crustacean larvae were the second dominant group and contributed 14% (approx.) of the total zooplankton population. It varied from 100 cells/L to 350 cells/L. The highest cell density was observed in June (station-2) and lowest in March (station-1 and 3). The mean abundance of crustacean larvae was 200 ± 70.71 cells/L.
| Copepods | Cladocerons | Rotifer | Crustacean larvae | Protozoan | Meroplankton |
|---|---|---|---|---|---|
| Candacia Cyclops Diaptomus Calanus Onacaea Oithona Eucalanus Cyclops nauplius | Moina Diaphanosoma | Brachionus Rotaria | Nil | Volvox Arcella Actinophrys |
Nauplius Copepodite Zoea |
Table 3: List of zooplankton genera recorded from the surface water of the Meghna river estuary throughout the experimental period.
Protozoa group constituted the third dominant and comprised about 16% (approx.) of the total zooplankton population. Volvox, Arcella, and Actinophyrs genera have identified in this study (Table 2). Actinophyrs sp. was the most dominant protozoan. The cell density of protozoa ranged from 100 cells/L to 400 cells/L. Density was higher in February (station-2) and lower in March (station-2) and June (station-6). The mean protozoa cell density was 222.22 ± 82.64 cells/L.
Meroplankton was the fourth dominant group constituting 12% (approx.) of total zooplankton abundance and ranged from 100 to 200 cells/L. The mean abundance of meroplankton was 163.88 ± 65.98 cells/L.
Cladocerans were the fifth, dominant group of total zooplankton. It was formed 10% of total zooplankton abundance. The highest density of Cladocerans was found at station-4 of March (250 cells/L) and lowest in February at station-1 and station-2 (50 cells/L). The average density of Cladocerans was 147.22 ± 49.40 cells/L.
Rotifers were the least dominant among total zooplankton groups. They contributed to 10% of zooplankton population. Brachionus and Rotaria were the identified genera in this study period. Lowest total cell density (50 cells/L) was observed in June and highest total cell density (400 cells/L) was recorded in March (station-1).
Seasonal zooplankton population indicated that copepods especially the Cyclops sp. Were dominant in three months and also found in abundance. The mean (± SD) zooplankton variations showed that highest density was in March, then it became lowest gradually to February and then June (Figure 3).
Relationship of plankton density with water quality parameters
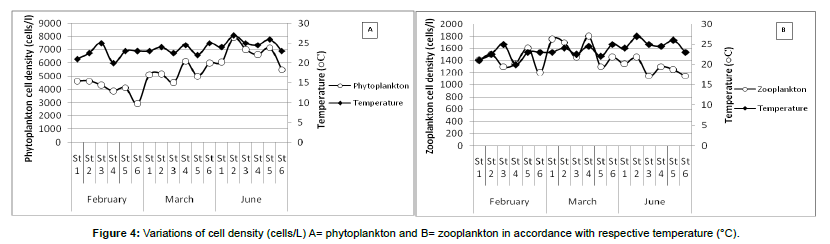
Variation of plankton density with Temperature: During the study period, three months (February, March, and June) cover six sampling stations with three replicates in total of eighteen had an individual temperature with the individual cell density of phytoplankton and zooplankton showed in Figure 4.
In the month of June, station-1 upholds the water temperature 24°C and respective phytoplankton cell density was 6063 cells/L, wherein station-2 with increasing temperature, 27°C phytoplankton cell density also caught the increasing trend, 7939 cells/L. In the present study period, water temperature was positively correlated (r = 0.75) with phytoplankton cell density (Table 4). On the other hand, zooplankton cell density picked the decreasing trend with increasing temperature. Radhakrishnan et al. [23] studied in the phytoplankton population in relation to the fluctuation of a physicochemical characteristic of Muthannankulam pond, the phytoplankton population was positively correlated with temperature (°C).
In the sampling station-3, water temperature was recorded 25°C and zooplankton cell density was 1150 cells/L, wherein station-4 water temperature was decreased to 24.5°C but zooplankton cell density was increased, 1300 cells/L. This inverse relationship of zooplankton and water temperature was found by Patra and Azadi [11].
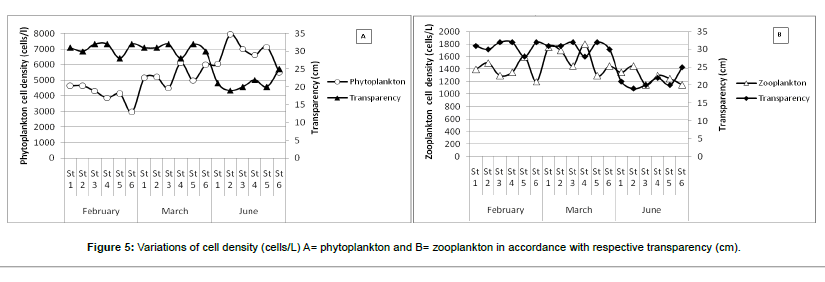
Variation of plankton density with Transparency: Phytoplankton and zooplankton cell density had shown the different value with the variation of water transparency (Figure 5). There is a significant correlation between the growth of plankton and transparency in different aquatic ecosystems recorded by several authors Çetin et al. [24]; Hossain et al. [25]; and Begum et al. [26].
In the month of June, lowest water transparency (19 cm) was recorded during the entire study period. This lowest water transparency was recorded at sampling station-2 where phytoplankton cell density showed the maximum value about 7939 cells/L. On the other hand, when water transparency was increased (20 cm at the station-3) phytoplankton cell density decreased (7000 cells/L). In the present study, water transparency had an inverse relationship with phytoplankton abundance and negatively correlated (r = -0.84) (Table 4).
In the case of zooplankton, transparency also showed a negative relationship with cell density. The mean water transparencies of March was 31 cm (approx.) and mean zooplankton density was observed about 1575 cells/L. In June mean water transparency was recorded lower (21 cm) where the lowest zooplankton cell density was observed (1275 cells/L). On the other hand, transparency showed the positive relationship (r = 0.31) with zooplankton abundance (Table 4). Zooplankton cell density showed a negative relationship with the individual month, but with overall consideration, it exerts positivity might be due to the presence of phytoplankton in larger densities in the sampling period.
| pH | Salinity | Temperature | Transparency | Phytoplankton | Zooplankton | |
|---|---|---|---|---|---|---|
| pH | 1 | |||||
| Salinity | 0.717 | 1 | ||||
| Temperature | -0.019 | -0.250 | 1 | |||
| Transparency | 0.343 | 0.413 | -0.666 | 1 | ||
| Phytoplankton | -0.097 | -0.351 | 0.751 | -0.845 | 1 | |
| Zooplankton | 0.495 | 0.315 | -0.022 | 0.317 | -0.047 | 1 |
Table 4: Interactions (in terms of the values of the correlation coefficient r) between different physico-chemical properties of water and phytoplankton and zooplankton density.
Diversity indices
Diversity (or biodiversity) is typically measured by a species count (richness) and sometimes with an evenness index; it may also be measured by a proportional statistic that combines both measures (e.g. Shannon–Wiener index H`) [27]. In the present study, Species richness index (d), Shannon‚?źWeiner diversity (H`), and Evenness (J`) were used to describe the diversity in a community and also used to assess water quality of the habitat. The mean (± SD) plankton diversity indices (Species richness, Shannon-Weiner, and Evenness) are presented in Table 5. Among phytoplankton, Bacillariophyceae showed highest species diversity about 1.95 ± 0.13, 2.16 ± 0.11 and 2.22 ± 0.19 in February, March and June respectively (Table 5). Others group of phytoplankton recorded much lower diversity (<1) than Bacillariophyceae. It might be due to the higher abundance of one group which resulted in higher diversity can lower the diversity of others. Shannon-Weiner species wise diversity index (H`) ranged from 0.37 to 2.46. Coscinodiscophyceae and Dinophyceae resulted in no diversity in the study period because during observation only one species was recorded for both groups for that it nullify species diversity index. Ahsan et al. [14] studied on plankton composition, abundance and diversity in Hilsha (Tenualosa ilisha) migratory rivers of Bangladesh during spawning season found the diversity index (H`) ranged from 1.500 to 3.334 with the mean value of 2.717 ± 0.147.
| Plankton | Group | Months | Evenness | Margalef | Shannon |
|---|---|---|---|---|---|
| Phytoplankton | Bacillariophyceae | February March June |
0.90 ± 0.03 0.92 ± 0.02 0.87 ± 0.06 |
2.19 ± 0.22 2.65 ± 0.21 3.10 ± 0.45 |
1.95 ± 0.13 2.16 ± 0.11 2.22 ± 0.19 |
| Cyanophyceae | February March June |
0.55 ± 0.45 0.47 ± 0.40 0.57 ± 0.46 |
0.24 ± 0.19 0.30 ± 0.14 0.38 ± 0.32 |
0.38 ± 0.31 0.32 ± 0.27 0.49 ± 0.40 |
|
| Chlorophyceae | February March June |
0.65 ± 0.50 0.82 ± 0.23 0.65 ± 0.41 |
0.32 ± 0.18 0.65 ± 0.24 0.68 ± 0.50 |
0.45 ± 0.34 0.85 ± 0.36 0.76 ± 0.59 |
|
| Coscinodiscophyceae | February March June |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
|
| Dinophyceae | February March June |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
|
| Zooplankton | Copepoda | February March June |
0.95 ± 0.04 0.96 ± 0.03 0.94 ± 0.04 |
1.11 ± 0.22 1.26 ± 0.29 1.13 ± 0.51 |
1.30 ± 0.14 1.42 ± 0.18 1.25 ± 0.38 |
| Cladoceron | February March June |
0.00 ± 0.00 0.63 ± 0.49 0.49 ± 0.53 |
0.00 ± 0.00 0.29 ± 0.22 0.22 ± 0.24 |
0.00 ± 0.00 0.44 ± 0.34 0.34 ± 0.36 |
|
| Rotier | February March June |
0.00 ± 0.00 0.53 ± 0.42 0.00 ± 0.00 |
0.00 ± 0.00 0.27 ± 0.20 0.00 ± 0.00 |
0.00 ± 0.00 0.27 ± 0.20 0.00 ± 0.00 |
|
| Protozoan | February March June |
0.89 ± 0.09 0.56 ± 0.44 0.77 ± 0.38 |
0.62 ± 0.06 0.39 ± 0.30 0.53 ± 0.26 |
0.62 ± 0.06 0.28 ± 0.22 0.53 ± 0.26 |
|
| Meroplankton | February March June |
0.63 ± 0.49 0.65 ± 0.50 0.65 ± 0.50 |
0.41 ± 0.02 0.45 ± 0.35 0.36 ± 0.18 |
0.44 ± 0.34 0.29 ± 0.22 0.54 ± 0.27 |
|
| Crustacean larvae | February March June |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 |
Table 5: Monthly variations of plankton diversity indices in February, March and June.
Species richness index (d) was also higher in Bacillariophyceae because there were about 22 species recorded belonged to this group and deliberately increased richness index. The species richness index (d) of Bacillariophyceae ranged from 2.00 (February, station-3) to 3.71 (June, station-4) with the highest mean value which was observed in June (3.10 ± 0.45) (Table 5). The richness index (d) becomes lower where a recorded number of species were lower.
Evenness index (J`) was used to determine how evenly species are distributed in the community. The highest mean value of evenness was observed in Bacillariophyceae, 0.92 ± 0.02 (March) and lowest in Cyanophyceae, 0.47 ± 0.40 (March) (Table 5).
.In zooplankton, highest species diversity (H`) was recorded in Copepoda and it ranged from 0.64 to 1.76. The mean value of Copepoda species diversity was 1.30 ± 0.14, 1.42 ± 0.18 and 1.25 ± 0.38 in February, March, and June respectively (Table 5). It has resulted from the highest species richness and evenness index. No species were identified from the group Crustacean larvae so it was given no species richness index, no evenness and ultimately, no species diversity index.
The ecosystem of water bodies is continuously changing with an environment which was affecting the composition of the biota. Rivers, reservoirs, and estuaries are constantly going into detour condition due to lack of awareness during discharge of pollutants, river erosion, heavy fishing pressures, navigation, etc. Diversity index is a good indicator to determine the pollution status of these aquatic ecosystems. Species diversity within aquatic communities is closely related to the trophic state of the water body [28]. Being properly identified, measured on the unified basis and monitored variability (or stability) of structural and functional parameters (such as Shannon–Wiener index H´) of plankton communities in the estuaries, can serve as an indicator for the modification of ecosystems under the eutrophication/pollution stress [28].
Balloch et al. [29] found the Shannon–Wiener diversity index to be a suitable indicator of water quality. Hendley [30] used the Shannon– Wiener diversity index as pollution index in diatom communities and put forward the following scale: of 0–1 for high pollution, of 1–2 for moderate pollution, of 2–3 for small pollution and of 3–4 for incipient pollution. In the present study, diversity index of diatom ranged from 1.82 to 2.46 and the mean value was 2.10 which indicate small pollution in the water body. But the total phytoplankton diversity ranged from 2.07 to 2.74 during the entire study period with a mean value of 2.42 ± 0.19. Species diversity index of zooplankton ranged from 1.82 to 2.38 with a mean value of 2.14 ± 0.16. The diversity index value of plankton greater than 3.00 indicates clean water. Values in the range of 1.00 to 3.00 are characteristics of moderately healthy conditions and values less than 1.00 characterize heavily deterioration condition. It can be said that diversity index values of plankton are within the range of 1-3 indicates the moderately polluted environment of the Meghna River from the present study. There is a similarity found by Ahsan et al. [14] where the pollution status of Hilsa migratory river resulted moderate pollution.
The phytoplankton cell density is a good indicator to determine the trophic status of a particular water body. The mean cell density of phytoplankton was 5372 cells/L and could be classified as oligotrophic. According to the classification scheme proposed by Siokou-Frangou et al. [31]; Pagou [32], oligotrophic systems are defined by this scheme to be systems with phytoplankton cell densities less than 6000 cells/L. Kiteresi et al. [33] studied on the Ramisi-Vanga system with phytoplankton cell densities ranging only from 194.96 cells/L to 3919.6 cells/L and classified as oligotrophic. In an oligotrophic system, water is in the clear state, but in the Meghna River turbidity was present. The water turbidity can occur due to the sandy bottom, river erosion, navigation. In the study area extreme river erosion occurs and it might be the reason of the turbid water body.
Conclusion
It may be concluded that cell density of phytoplankton was very low in the present study comparing to the productive ecosystem (104-105 cells/ml). Growth of plankton depends on the availability of nutrients (N, P, and Si) but in the present study phytoplankton cell density was less than 6000 cells/L and by considering that the Meghna estuary considered as Oligotrophic. Bacillariophyceae and Copepoda in phytoplankton and zooplankton respectively, were found in dominant number and showed highest density. According to value of Shannon-Weiner diversity index (H) the Meghna estuary considered as moderately polluted environment.
References
- WardHB, WhippleCC (1959) Freshwater Biology (2nded.). John Willy and Sons Inc., New York, London. pp: 1248.
- Margalef R (1958) Information theory in ecology. Gen Systems 3: 36-71.
- StirlingHP (1985) Chemical and biological methods of water analysis for aquaculturists. Institute of Aquaculture, University of Striling, Scotland. pp: 119.
- ShannonCE (1984) Communication in the presence of noise. In: Proceedings of the IIEE 72: 1192-1201.
- ShannonCE,WeaverW (1963) The Mathematical Theory of Communication. The University of Illinois Press, Illinois.pp: 144.
- Ramos S,CowenRK,ReP,BordaloAA (2006) Temporal and Spatial distribution of larval fish assemblages in the Lima estuary (Portugal). Estuarine, Coastal and Shelf Science. 66:303-314.
- Harper DAT (1999) Numerical Palaeobiology: Computer-based modelling and analysis of fossils and their distributions. John Wiley & Sons, USA. pp:478.
- Ryan PD, Harper DAT, Whalley JS (1995) PALSTAT, Statistics for paleontologists. Chapman & Hall, Kluwer Academic Publishers.
- McErlean AJ, O’Connor SG, Mihursky JA, Gibson CI (1973) Abundance, diversity and seasonal patterns of estuarine fish populations. Estuarine, Coastal and Marine Science 1: 1936.
- Hossain MS, Das NG, Sarker S, Rahman MZ (2012) Fish diversity and habitat relationship with environmental variables at Meghna river estuary, Bangladesh. The Egyptian Journal of Aquatic Research 38: 213-226.
- PatraRWR, AzadiMA (1987) Ecological studies on the planktonic organisms of the Halda River. Bangladesh JZool15:109-123.
- ShahMMR, HossainMY,BegumM,AhmedZF,OhtomiJ, et al. (2008) Seasonal Variations of Phytoplanktonic Community Structure and Production in Relation to Environmental Factors of the Southwest Coastal Waters of Bangladesh. J Fish AquatSci3:102-113.
- ShafiM,QuddusMMA,IslamN (1978) Maturation and spawning of Hilsailisha (Hamilton-Buchanan) of the river Meghna. Dacca UnivStudB26: 63-71.
- AhsanDA,KabirAKMN,RahmanMM,MahabubS,YesminR, et al. (2012) Plankton composition, abundance and diversity in Hilsa (Tenualosailisha) migratory rivers of Bangladesh during spawning season. Dhaka UnivJBiolSci21:177‚?ź189.
- Boonyapiwat S (1997) Distribution, abundance and species composition of phytoplankton in the South China Sea, Gulf of Thailand and Peninsular Malaysia. In: Proceedings of the 1st Technical Seminar on Marine Fishery Resources Survey in the South China Sea, Gulf of Thailand and East Coast of Peninsular Malaysia. Bangkok, Thailand. pp: 111-134.
- Boonyapiwat S, Maungyai S, Chantarasakul W (1984) A study on phytoplankton in relation to oceanographic conditions in the lower Gulf of Thailand. Report of the Thai-Japanese-SEAFDEC Joint Oceano and Fish Sur in the Gulf of Thailnd on Board Nagasaki-Maru. SEAFDEC, Samutprakarn. pp: 27-43.
- SevindikTO (2010) Phytoplankton composition of Caygoren reservoir, Balikesir-Turkey. TurkJFishAquatSci10: 295-304.
- Al-Hashmi K, Al-Azri A, Calerebudt MR,Pinotkovski S, Amin SMN (2013) Phytoplankton community structure of a Mangrove habitat in the arid environment of Oman: The dominance of Preidiniumquinguecorne. JFishAquatSci8: 595-606.
- SunderS,RainaHS,MohonM,SinghB (1995) Ecology and fisheries potentials of the Gaulariver with special reference to proposed impoundment (Jamrani dam) on the system. J Inland FishSoc27:33-45.
- Islam MN, Khan TA, Bhuiyan AS (2000) Ecology and seasonal abundance of some zooplankton of a pond in Rajshahi. Univ j zoolRajshahiUniv 19:25- 32.
- Gutkowska A, Paturej E, Kowalska E (2012) Qualitative and quantitative methods for sampling zooplankton in shallow coastal estuaries. Ecohydrology and Hydrobiology 12: 253-263.
- Omondi R, Yasindi AW, Magana A (2011) Spatial and temporal variations of zooplankton in relation to some environmental factors in Lake Baringo, Kenya. Eger J SciTechnol 11: 29-50.
- RadhakrishnanS,SaravanaBhavanP,VijayanP,KannanS,KarpagamS (2009) Assessment of phytoplankton population in a pond water. ResEnvironLife Sci2:77-82.
- Cetin AK, Sen B (2004) Seasonal distribution of phytoplankton in Orduzu Dam Lake (Malatya, Turkey). Turk J Bot 28: 279-285.
- Hossain MY, Jasmine S, Ibrahim AHM, Ahmed ZF, Ohtomi J, et al. (2007) A preliminary observation on water quality and plankton of an earthen fish pond in bangladesh: Recommendations for future studies. Pakistan Journal of Biological Sciences 10: 868-873.
- Begum M, Hossain MY, Wahab MA, Ahmed ZF, Alam MJ, et al. (2007) Effects of iso-nutrient fertilization on plankton production in earthen ponds of Bangladesh. Pak J BiolSci 10: 1221-1228.
- StirlingG, WilseyB (2001)Empirical relationships between species richness, evenness, and proportional diversity. The American Naturalist158: 286-299.
- TeleshIV (2004) Plankton of the Baltic estuarine ecosystems with emphasis on Neva Estuary: a review of present knowledge and research perspectives. Marine Pollution Bulletin 49: 206-219.
- BallochD,DaviesCE,JonesFH (1976) Biological assessment of water quality in three British rivers: the North Esk (Scotland), the Ivel (England) and the Taff (Wales). Water Pollution Control 75: 92-114.
- HendleyNI (1977) The species diversity index of some in-shore diatoms communities and its use in assessing the degree of pollution insult on parts of the North Coast of Cornwall. In: CrammeJ(ed.) Fourth Symposium on recent and fossil marine diatoms.pp: 355-378.
- Siokou-FrangouI, PagouK (2000) Assessment of the trophic conditions and ecological status in the Inner Saronikos Gulf. Technical Report for the Ministry of Environment, Planning and Public Works, NCMR, Athens. Greece. pp: 43.
- Pagou K (2000) Assessment of the trophic conditions in the inner Thermaikos Gulf. Technical Report for the Ministry of Environment, Planning and Public Works, NCMR, Athens. pp: 11.
- KiteresiLI,OkukuEO,MwangiSN,OhowaB,WanjeriVO, et al. (2012) The influence of land based activities on the phytoplankton communities of shimoni-vanga system, Kenya. IntJEnvironRes6:151-162.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 12417
- [From(publication date):
June-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11219
- PDF downloads : 1198