Assessment of an Herbal Feed Additive on Reducing Gastrointestinal Nematodes in an Alpaca Operation
Received: 13-Jun-2022 / Manuscript No. jvmh-22-63751 / Editor assigned: 15-Jun-2022 / PreQC No. jvmh-22-63751 / Reviewed: 29-Jun-2022 / QC No. jvmh-22-63751 / Revised: 04-Jul-2022 / Manuscript No. jvmh-22-63751 / Accepted Date: 10-Jul-2022 / Published Date: 11-Jul-2022 DOI: 10.4172/jvmh.1000151
Abstract
With ever-growing drug resistance and significant global prevalence, gastrointestinal nematodes are an increasingly common cause of death on small ruminant and camelid operations, threatening the productivity and economic health of those industries. An alternative to traditional anthelmintics is an herbal feed additive called Early Bird. The formula includes constituents shown to be effective as nematicides, and addresses the comorbidities associated with acute and chronic parasitism. Thirty-six Huacaya alpacas of mixed genders and reproductive statuses were evaluated on the bases of reproductive demands, body condition score, FAMACHA score, and naturally occurring eggs per gram of multiple species on fecal testing, without any intervention. Daily feed delivery was dressed with the supplement in the form of a coarse powder rendered from twenty whole herbs, for a total dose of 500-2000mg/day over a 14 day period. This treatment was repeated a total of three times, with 14 days off between each treatment period. Alpacas were continuously evaluated for body condition score, FAMACHA score, quantitative fecal testing, and reproductive status. The population experienced an overall decrease in FAMACHA score, an overall increase in body condition score, and overall decrease in fecal egg counts of strongyle-type species. The percent of alpacas with a positive fecal egg count for strongyle-type eggs decreased from 33.02% to 2.44% during three treatment periods. Early Bird reduced strongyle eggs per gram and improved body condition score throughout the population during a time period of significant reproductive demands, eliminating the need for traditional anthelmintics.
Keywords
Introduction
Drug resistance following anthelmintic treatments in small ruminants leaves producers with few effective options to protect herds against gastrointestinal nematodes (GIN), which leads to economic losses and decreased productivity. Small ruminant and camelid industries need a sustainable alternative to anthelmintic usage – one that leads to healthier pastures and significant production improvements without the threat of drug resistance. An alternative method that prevents herd-wide rise in mean fecal egg counts (FEC) would allow these operations to use anthelmintics more judiciously, thereby delaying parasite resistance. Gastrointestinal nematodes such as Haemonchus contortus are the most prevalent threat to small ruminant and camelid operations worldwide. In the Mid-Atlantic region of the United States, one study detected Haemonchus contortus in 79% of the participating sheep and goat farms (Crook and O’Brien [1]). The National Animal Health Monitoring System named internal parasites to be the leading cause of non-predatory goat losses in the United States (Zajac and Garza [2]). Increased parasite populations are typically the result of casual overuse of commercial anthelmintics, ensuring that the organisms become drug resistant. As climate change affects global temperatures, GIN are expected to become more prevalent, and anthelmintic resistance is likely to expand (Kaplan and Vidyashankar [3]). Currently in the United States, there are six anthelmintic products available commercially, representing three drug classes. Haemonchus contortus is reported to be resistant to all three classes (Kotze and Prichard [4]). In the Mid-Atlantic, Crook and O’Brien [1] reported 100% resistance to benzimidazoles, 82% resistance to ivermectin, 24% resistance to levamisole, and 47% resistance to moxidectin. Some studies have identified growing drug resistance in alpaca herds in Australia and Germany (Rashid et al. [5]; Kultscher et al. [6]); - however, anthelmintics are not the only available method for parasite population control. The current strategy to manage GIN is to maintain the population’s naivety to anthelmintics. This is referred to as the preservation of “refugia,” and is the prevailing recommendation of the American Consortium for Small Ruminant Parasite Control (ACSRPC, 2020). Related practices include maintaining appropriate stocking density, pasture rotation, quarantining and biosecurity testing of new individuals, fecal testing surveillance, regular FAMACHA and body condition scoring, and appropriate nutritional plans; however, significant barriers in practicing appropriate pasture management exist in regions where acreage comes at a premium, or regions where veterinary oversight is not available. Anecdotal descriptions of botanical anthelmintics and antidiarrheals for parasitized livestock are found across the globe (Gray and Baker [7]). Tannin supplementation, which is frequently endorsed by livestock owners, has been shown to reduce fecal egg counts in goats infected with GIN and medicated with tanniferous extracts (Paolini and Bergeaud [8]). Similar studies have explored the efficacy of various plants and identified their mechanisms of action (Akkari and Hajaji [9]). Thyme, for example, is reported to have anthelmintic activity equivalent to thiabendazole and levimasole,with respect to H. contortus (Ferreria and Benincasa [10]). Veterinary parasitologists have been able to build a scientific foundation for the practical use of various herbs by elucidating the chemical structures of, and the pharmacokinetics of, herbal constituents (Mengistu and Hoste [11]).Available commercially, a well-known, single-ingredient herbal deworming tincture failed to control GIN in goats, and in some test populations, resulted in an increase of fecal egg counts (Burke and Wells [12]). Parasite infestations are multifactorial, engendering poor absorption of nutrients; poor gut motility, diarrhea, enteritis, poor red blood cell regeneration, and other comorbidities. It is reasonable to believe that products utilizing one or few ingredients fail to address all the concomitants of parasitism. An appropriate alternative dewormer may not only have anthelmintic properties, but it will also address nutrient malabsorption, mucosal damage, gastrointestinal inflammation, and bone marrow suppression in order to achieve complete recovery of the patient. A similar strategy employed during a field trial of neonatal lamb diarrhea utilized a treatment combining five medicinal plants with favorable results. The treated group experienced a shorter duration of clinical illness, a higher cure rate, lower mortality, and higher average body weight upon recovery.The formula used by Early Bird is comprehensive in that combines plants with anthelmintic, anti-diarrheal, appetite stimulating, anti-inflammatory, mucosalprotectant, red blood cell stimulating, and promotility constituents (Tables 1 and 2). It is prepared as dried, whole herbs in order to preserve a complex cellular structure similar to the fibrous roughage of a small ruminant’s natural diet. This trial aimed to evaluate the efficacy of a comprehensive herbal feed additive containing 20 whole, dried herbs, in improving overall GIN egg counts, body condition score, and FAMACHA score of alpacas experiencing different levels of reproductive demands.
Materials and Methods
Animal Use
All procedures were approved by the University of Vermont Institutional Animal Care and Use Committee (#20-371).
Animals and Diet
Thirty-six Huacaya alpacas (26 adult females, 9 intact males, and 1 gelding; Table 3) were used for novel supplement evaluation. This experiment utilized a one-way treatment structure where animals were grouped by sex. Alpacas were obtained from one fiber operation in Waretown, NJ and data were collected from July to November of 2020. Ten males were housed together on a 0.30-hectare dry lot soil pen containing mature pine trees. Twenty-six females were housed on a 0.45-hectare dry lot soil pen containing mature pine trees. The two areas were separated by 0.10 hectare and several visual barriers, including a tree line and barns. At the beginning of the trial, two females were lactating, two females were lactating while pregnant, and seven females were pregnant and not lactating. Nursing cria (n=5) were not included in this trial. All animals were fed 0.11 kg of Nutrena™ Llama and Alpaca Feed daily, 5.0 g of Stillwater Minerals daily, and had ad libitum access to second cutting orchard grass hay and fresh water daily. Nutrena™ Llama and Alpaca Feed was 14.0% CP, 1.22% NPN, 3.5% fat, and 16.0% crude fiber. Stillwater Minerals supplement contained 20% salt, 8% calcium, 6.5% phosphorus, 250,000 IU/lb Vitamin A, 30,000 IU/lb Vitamin D, and 7,000 IU/lb Vitamin E. This supplement also included manganese, zinc, iodine, cobalt, selenium, copper, potassium, and magnesium.
Experimental Supplement
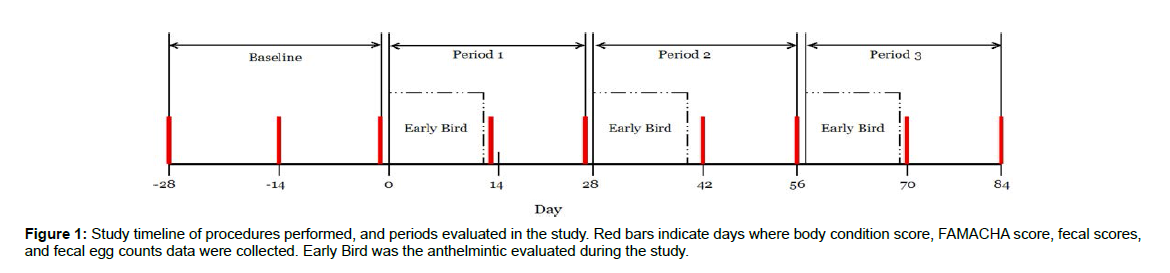
The experimental supplement consisted of dried, whole herbs that were rendered into a coarse powder. There are two formulas intended for delivery one immediately after another, called “Phase I” and “Phase II.” The first phase consisted of Quisqualis indica, Prunus mume, Atractylodes macrocephala, Codonopsis spp, Sophora flavenscens, Zingiber officinale, Torreya grandis, Raphanus sativus, and Omphalia lapidescens. The second phase consisted of Avena sativa, Astragalus membranaceous, Curcurbita pepo, Withania somnifera, Althaea officinalis, Urtica dioica, Medicago sativa, Centella asiatica, Foeniculum vulgare, Silybum marianum, and Calendula officinalis. Further information about each ingredient can be found in Tables 1 and 2. (Table 1) [13-38]. Supplement mixture was top dressed on grain in each individual’s feeding box once daily in the morning. The authors refer to the resulting product as Early Bird. The first phase was administered at a dose of 500 mg/alpaca. Once a day for two days, then increased to 1000mg/alpaca once daily for two days, and then increased to 2000mg/ alpaca once daily for three days. This was achieved by sprinkling the measured supplement on top of each animal’s daily grain allowance. The second phase was administered at a dose of 1000mg/alpaca once a day for three days, and then increased to 2000 mg/alpaca once a day for four days. Table 4 outlines the amount of supplement being added each day over the 14-d treatment period. Alpacas were fed the Early Bird supplement for a period of 14 days, and then provided a 14-day break in between (Table 2) [39-67]. The same basal diet was fed during the rest period. Figure 1 provides an illustration of timing when the product was administered to the basal diet.
| Taxonomic Name | Mechanism of Action | Scientific Reference(s) |
|---|---|---|
| Omphalia lapidescens | Anthelmintic | Zhang et al. [27], Chen et al.[28] , JinFu et al. [29] |
| Sophora flavescens | Anthelmintic | Tangalin, [30], Zhang et al.[31] , Li et al. [32] |
| Torreya grandis | Anthelmintic | Li et al.[33] |
| Prunus mume | Astringent | Li et al.[33] |
| Quisqualis indicum L. | Anthelmintic | |
| Codonopsis spp. | Promotes RBC generation | Sun et al. [34], Gao et al. [31] |
| Atractylodes macrocephala | Anti-inflammatory, carminative, prokinetic | Li et al.[33], Han et al.[35] |
| Zingiber officinale | Anthelmintic (in-vitro) | Qadir and Dixit [36] |
| Raphanus sativus L. | Anti-inflammatory, prokinetic | Sham et al.[37],Choi and Hwang [38] |
Table 1: Whole herb ingredient information included in the treatment product’s First Phase.
| Taxonomic Name | Mechanism of Action | Scientific Reference(s) |
|---|---|---|
| Avena Sativa | Antiparasitic, prebiotic | Sargautiene et al. [39], Doligalskia et al. [40], Liu et al.[41] |
| Curcurbita pepo | Anthelmintic, antioxidant | Zhang et al. [31], Li et al. 32] |
| Astragalus membranaceus | Immunomodulatory, antioxidant | Auyeung and Ko [42] |
| Withania somnifera | Adaptogenic, hematopoietic, anti-inflammatory, immunomodulatory | Bhattacharya et al. [43] Chandrasekhar et al. [44], Sharma et al. [45], Mishra [46]. |
| Althaea officinalis | Biofilm dissolution | Aminnezhad et al. [47] |
| Urtica dioica | Monocyte chemoattractant, HP-1 expression, MyD88/NF-κB/p38 signaling | Gülҫin et al. [48], Namazi and Esfanjani [49] |
| Medicago sativa | Hematopoietic | Bora and Sharma [50,51] Huyghe et al.[52], Vyas et al. [53] |
| Centella asiatica | Intestinal mucosal protectant, anti-inflammatory | Gohil at al. [54] Paocharoen [55], Sainath et al.[56] |
| Foeniculum vulgare | Anti-inflammatory, antispasmodic, galactagogue, carminative | Choi et al. [57] Bensch et al.[58] |
| Silybum marianum | Antifibrotic, antioxidant, hematopoietic | Abenavoli et al. [27], Kalantari et al.[59] |
| Calendula officinalis | Intestinal mucosal protectant, gastroprotectant, antispasmodic | Al-Snafi [60] ,Bashir et al.[61] Mehrabani et al.[62] |
Table 2: Whole herb ingredient information included in the treatment product’s Second Phase.
| Day | Adult Females (26) |
|---|---|
| -28 | 7 PR, 2 NRSPR, 2 NRS, 15 MNT |
| -14 | 7 PR, 2 NRSPR, 2 NRS, 15 MNT |
| 0 | 9 PR, 4 NRSPR, 13 MNT |
| 14 | 9 PR, 4 NRSPR, 13 MNT |
| 28 | 6 PR, 4 NRSPR, 3 NRS, 13 MNT |
| 42 | 6 PR, 4 NRSPR, 3 NRS, 13 MNT |
| 56 | 6 PR, 4 NRSPR, 3 NRS, 13 MNT |
| 70 | 5 PR, 6 NRSPR, 1 NRS, 14 MNT |
| 84 | 5 PR, 6 NRSPR, 1 NRS, 14 MNT |
Table 3: Inventory of animals experiencing different reproductive status¹ during study period. (¹Reproductive status abbreviations: MNT=maintenance, PR=pregnant, NRS=nursing, NRSPR= nursing while pregnant).
Data Collection
On day -28, all alpacas received an identifying colored collar and an individual number for the duration of the trial. At that time, each animal received a physical exam to ensure wellness prior to entering the study; no signs of infectious disease and no abnormalities were detected at that time. Individual body condition score (BCS), reproductive status (RDS), fecal scores, and FAMACHA© scores were recorded. Fecal samples were collected per rectum from each individual, stored in individual plastic cups with lids, refrigerated within 30 minutes of collection, and stored for 12-24 h prior to fecal egg count (FEC) analysis. Body condition scores were measured using a 1-5 scale where 1=emaciated, 3=optimal, and 5=obese (Penn State, 2013). Reproductive status was defined as alpacas in maintenance (MNT), nursing a single or pair of cria (NRS), pregnant (PR), or nursing while pregnant (NRSPR). Fecal scores were measured on a 1-5 scale, where 1=dry and firm, and 5=soft and unformed (adapted from Peltoniemi and Oliviero [13]). FAMACHA scores were evaluated using the 1-5 scale by evaluating the color of the conjunctival membranes as an indicator of anemia (Kaplan et al. [14]). Lower FAMACHA scores are indicative of minimal to negligible parasite burden, whereas higher FAMACHA scores indicate significant parasite burden, as pertaining to the parasite H. contortus. Fecal egg accounts were measured using the Modified McMaster fecal egg counting technique, which is described as follows. Four grams were taken from each individual’s sample, weighed using a digital scale rated for accuracy to 0.0001 g, and placed into a plastic up. Phoenix’s sodium nitrate fecal float solution, which represents a specific gravity between 1.25 and 1.30, was added to each representative sample in 28mL aliquots. Each sample was allowed to sit undisturbed in 28mL of solution for three minutes. Fecal pellets were then digitally agitated to form a homogenous mixture, and then the solution sat undisturbed for an additional three minutes. Two 4x4” 12-ply woven gauze sponges were laid across an additional cup, and the solution was poured over top of the gauze and into the new cup to achieve straining. The gauze and the material strained by the gauze were discarded. A pipette was used to transfer the solution into both chambers of the McMaster slide. Slides sat on a level surface undisturbed for ten minutes, and then promptly read. Eggs within grid lines were counted in both chambers, both totals were added together, and the sum was multiplied by 25 to achieve a figure of “eggs per gram” for each species of egg identified. On d -28, -14, and -1, RDS, BCS, fecal scores, FAMACHA score, and FEC were collected, and these collections will be described as the baseline period. After sample collection on d -1, alpacas began receiving Early Bird, which was delivered three times over the course of 84 d. These are described as the treatment periods (three treatment periods, 28 d each). Each treatment period begins with Early Bird Phase I being delivered over 7 d, Early Bird Phase II being delivered over 7 d, then 14 d without Early Bird (Figure 1, Table 4). Individual animal BCS, FAMACHA scores, fecal scores, RDS, and FEC were collected on days -1, 13, 27, 42, 56, 70, and 84. Days 13 and 27 were combined in treatment Period 1. Days 42 and 56 were used as treatment Period 2, and days 70 and 84 defined as treatment Period 3.
| Day of Treatment | First Phase | Second Phase |
|---|---|---|
| 1 | 500mg/head/day | - |
| 2 | 500mg/head/day | - |
| 3 | 1000mg/head/day | - |
| 4 | 1000mg/head/day | - |
| 5 | 2000mg/head/day | - |
| 6 | 2000mg/head/day | - |
| 7 | 2000mg/head/day | - |
| 8 | - | 1000mg/head/day |
| 9 | - | 1000mg/head/day |
| 10 | - | 1000mg/head/day |
| 11 | - | 1000mg/head/day |
| 12 | - | 2000mg/head/day |
| 13 | - | 2000mg/head/day |
| 14 | - | 2000mg/head/day |
Table 4: Schedule for treatment dosing amount and product phase being offered.
Statistical Analysis
Data were imported into a commercial software program (R Studio Team®-2020, Boston, MA). Multinomial cumulative link mixed models were used to evaluate distribution outcomes by study period. Strongyle-type eggs per gram were log-transformed after adding a count of 1 to all parasite counts to account for the 0 counts (log10(x+1)), and linear mixed models were used to evaluate strongyle-type eggs by study period. The multinomial and linear mixed models included fixed effects of study period, covariate for reproductive status, and random effect for repeated measures on individual alpaca. Differences exhibiting a P value ≤0.05 were considered statistically significant.
Results
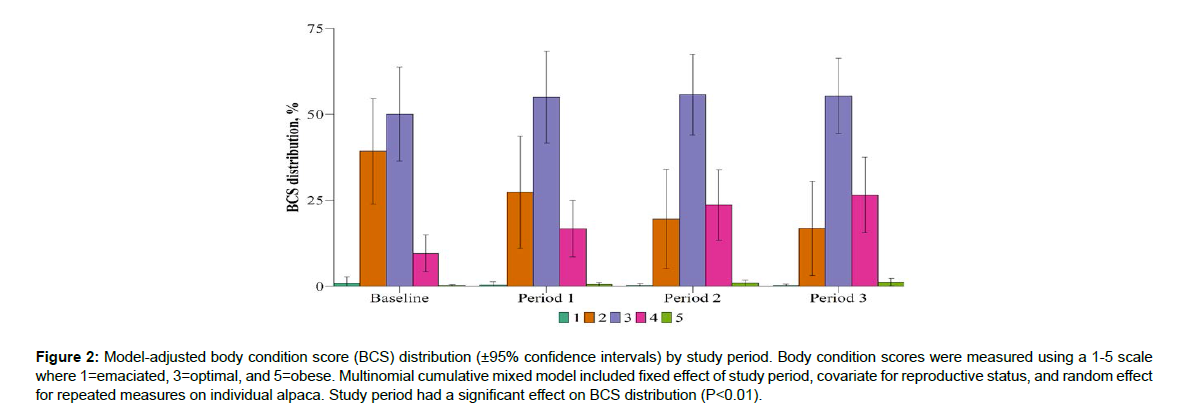
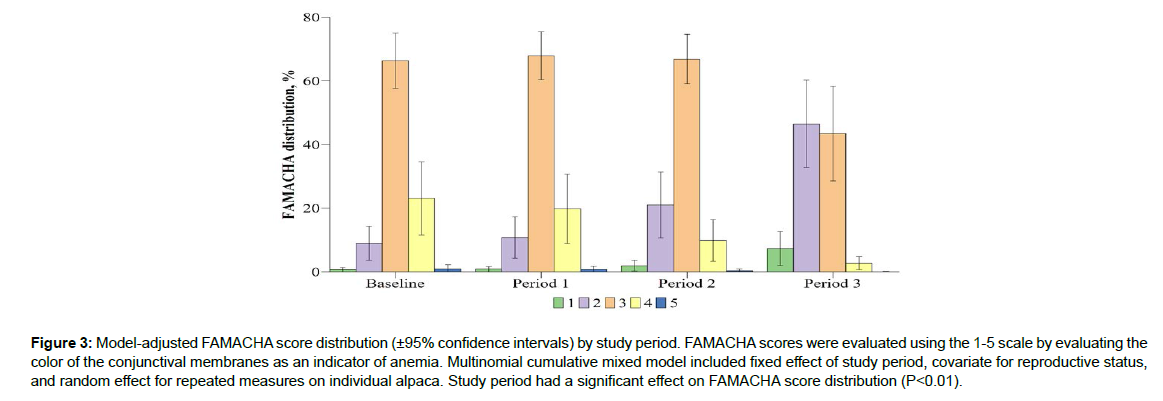
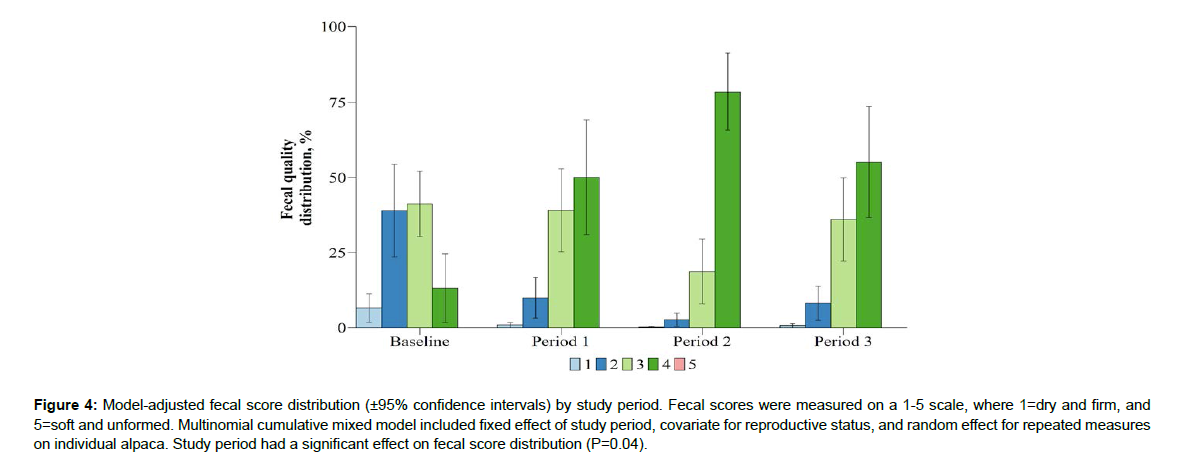
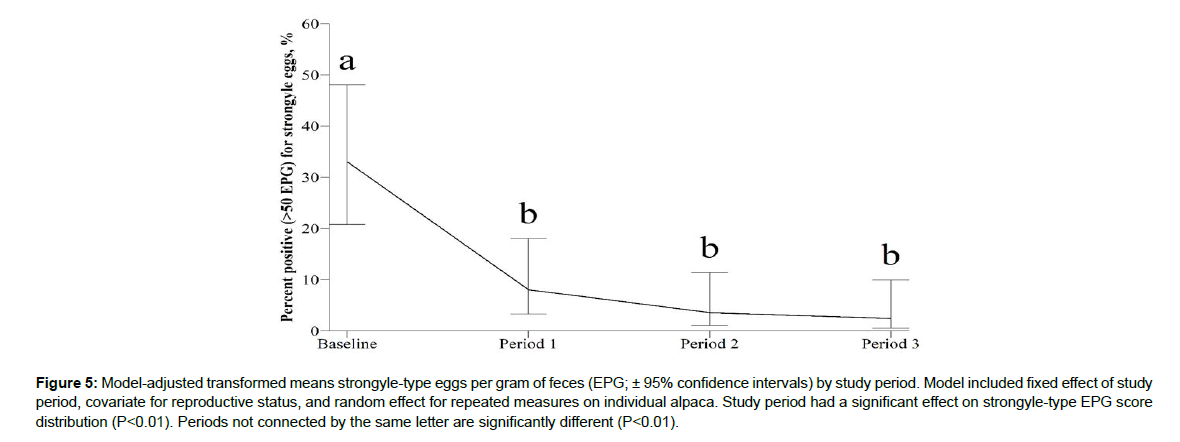
Study period had a significant effect on BCS distribution (P<0.01; Figure 2). Study period had a significant effect on FAMACHA score distribution (P<0.01; Figure 3). Study period had a significant effect on fecal score distribution (P=0.04; Figure 4). Study period has a significant effect on strongyle-type EPG score distribution (P<0.01; Figure 5). Baseline strongyle-type EPG was significantly greater (P<0.01) than Period 1, 2, and 3.
Figure 2: Model-adjusted body condition score (BCS) distribution (±95% confidence intervals) by study period. Body condition scores were measured using a 1-5 scale where 1=emaciated, 3=optimal, and 5=obese. Multinomial cumulative mixed model included fixed effect of study period, covariate for reproductive status, and random effect for repeated measures on individual alpaca. Study period had a significant effect on BCS distribution (P<0.01).
Discussion
The goal of this study was to evaluate the effectiveness of an herbal anthelmintic formula for control of parasite population in alpacas,based on measures previously used to evaluate anemia and parasite load. Traditionally, FAMACHA measures are used to identify animals that need to be treated for parasite infections, indicating varying levels of anemia in animals with scores of 3 or more (Cebra et al. [15]). Traditional parasite control studies report decreases in FAMACHA scores in animals treated with anthelmintics. Similarly, Early Bird was effective in decreasing herd FAMACHA scores, with favorable scores such as FAMACHA=2 increasing in distribution from 8.98% during the baseline period, to 46.51% at the cessation of Period 3. Conversely, unfavorable scores indicating anemia such as FAMACHA=4 decreased in distribution from a baseline value of 23.09%, to a value of 2.67% at the cessation of Period 3 (Figure 3). Based on these measures, the feed additive was successful in decreasing parasite loads in infected alpacas, as well as preventing further parasitism in an alpaca operation. This study also aimed to evaluate differences in product efficacy between alpacas experiencing different levels of production needs based on their reproductive status, as small ruminants are more susceptible to GIN during pregnancy and lactation (Gonzalez-Garduno et al. [16]). Previous reports have found pregnant small ruminants to be more susceptible to GIN, requiring 20-38% of females receive treatment; whereas the same studies reported highest susceptibility in lactating females, reporting 65-80% of females receive treatment (Rocha et al.[17]; Ruiz-Uitzil et al.[18]; Gonzalez-Garduno et al.[16]). These trials did report FEC greater than those reported in this trial but provide evidence that females experiencing pregnancy or lactation typically have higher incidence of parasite infection. Thus, it is favorable that Early Bird was equally effective in preventing or decreasing FEC in all production populations.The fecal score results are more difficult to interpret. Fecal quality is not commonly measured in anthelmintic assessment trials and was evaluated here to identify obvious differences in diarrhea for producers to use. Bentounsi et al. [19] reported that their diarrhea score (DISCO) was the most effective at identifying sheep requiring anthelmintic treatment, compared to FAMACHA and body weight changes. Those authors and other (Cabaret et al. [20]; Ouzir et al. [21]) reported the success of DISCO over other indicators, but sheep in those trials had smaller populations of strongyle-type eggs and were primarily infected with other species. Additionally, those trials used a smaller number of sheep to identify parasite species comprising the infection. In contract, alpacas in this trial had increasing fecal scores as fecal egg counts were decreasing (Figure 4) and at no point did any individual represent a fecal score of 5. The current trial differs from previous works regarding location, animal diet, age, parasite population make-up, and animal species, which could all be contributing factors to the different fecal quality results. Additionally, alpacas in maintenance appear to have the lowest fecal scores regardless of treatment period indicating that production stage may have greater effect on overall fecal quality. Thus, the relationship between parasite infection, Early Bird, and production stage warrants further investigation in alpacas in North America.
Figure 3: Model-adjusted FAMACHA score distribution (±95% confidence intervals) by study period. FAMACHA scores were evaluated using the 1-5 scale by evaluating the color of the conjunctival membranes as an indicator of anemia. Multinomial cumulative mixed model included fixed effect of study period, covariate for reproductive status, and random effect for repeated measures on individual alpaca. Study period had a significant effect on FAMACHA score distribution (P<0.01).
Since body condition and parasitism are undeniably associated (Baird and Pugh
[22]; Cebra et al. [15]; Gray and Baker [7]; Qamar et al. [23]), body condition was a very important parameter for this study. The physical demands of conception, gestation, and lactation in a healthy adult alpaca are expected to manifest as a decrease in body condition (Cebra et al. [15]). Burton [24] reported an average body weight loss of 9 kg in healthy adult dams from pre- to post-partum. During this trial, half of the females were classified as adults experiencing reproductive demands, one quarter of the females were growing weanlings, and one quarter of the females were in maintenance. From the baseline period to Period 3, the distribution of alpacas representing BCS=1 and BCS=2 declines, while the distribution of alpacas representing BCS=3, BCS=4, and BCS=5 increased. Alpacas representing BCS=4 experienced the largest distribution increase, beginning with baseline period of 9.50% and concluding with 26.55% at the cessation of Period 3. Meanwhile, alpacas representing BCS=2 began with baseline period of 39.0% and concluded with 16.84% at the cessation of Period 3 (Figure. 2). The ability to decrease GIN and support healthy BCS during periods of high energy demand makes this product a beneficial alternative to traditional dewormers. If a produce can reduce drug usage in instances where they would typically use it, then that producer is delaying drug resistance both on their own farm, and on the farms with which they trade (Emery and Hunt [25].Initial assessment of FEC aimed to measure how many animals had a positive fecal exam over time, as the percentage of animals with a positive exam were expected to decrease with administration of Early Bird. During the baseline period, 33.02% of the herd had a fecal exam positive for strongyle-type eggs, with a range of 100-600 eggs per gram. After Period 1, only 8.06% of the herd was positive for strongyle-type eggs, and after the third and final period, only 2.44% of the herd was positive for strongyle-type eggs on fecal exam. Because alpacas remained on the same pasture throughout the trial and were theoretically continually exposed to parasites, these results indicate that Early Bird was successful in inhibiting propagation of further infections. Thus, Early Bird is a valid management tool for the control of GINs in alpacas in North America, thus making judicious use of anthelmintics more attainable.The value of an additional parasite management tool cannot be overstated given how the topography of livestock is changing (Kaplan and Vidyashankar [3]). While prudent pasture practices are the keystone of parasite management, there are very real barriers to these strategies (Kotze and Prichard [4]). In underserved regions without access to large animal veterinarians, many farmers have not been historically judicious with drug usage, disarming themselves for the future (Kotze and Prichard [4]). In regions where development continues to creep into rural areas, the amount of acreage available for farmers decreases (Kaplan and Vidyashankar [3]). Delaying and/or decreasing the need for traditional dewormers, either in the short term or the long term, will achieve dividends in terms of overall production gains and pasture health (Gray and Baker [7]).There were several limitations with this study. The most notable shortcoming was the lack of a control group. The Northeast SARE’s Partnership Grant Program requires direct coordination with the research team, such that the novel treatment is implemented into the partner farmer’s operations. This poses limits in the form of population quantity and housing logistics, because the population available for the study is an established, closed herd that is privately owned. Their biosecurity and longevity are to be preserved. For these reasons, additional alpacas were not introduced in order to create a population size that would allow a true control. Instead, the -28 d to -1 d period monitored the naturally occurring parasite populations before treatment was introduced. The authors do not feel that the changes affected by the treatment would have been the same in a control group. This is because the reproductive demands of the herd would be expected to opposite trends across the measured parameters (FAMACHA, BCS, FEC).An additional limitation of this study was the usage of McMaster technique for egg detection without the added diagnostic support of larval culture, DNA detection, or peanut agglutination tagging. As a preliminary study seeking only to prove the validity of a new treatment, budgetary concessions were made when considering diagnostic approach. Overall FEC of alpacas in this trial were low compared to previous works reporting >1,000 eggs per gram. However, the current parasite loads are consistent with previous reports of camelids in the U.S. (Edwards et al. [26]). It may be beneficial to analyze this treatment method against a population challenged by identifiable H. contortus, and in higher numbers. Corollary questions to be addressed are the establishment of toxic doses, commodity withdrawals, and GIN resistance to this product.
Figure 4: Model-adjusted fecal score distribution (±95% confidence intervals) by study period. Fecal scores were measured on a 1-5 scale, where 1=dry and firm, and 5=soft and unformed. Multinomial cumulative mixed model included fixed effect of study period, covariate for reproductive status, and random effect for repeated measures on individual alpaca. Study period had a significant effect on fecal score distribution (P=0.04).
Figure 5: Model-adjusted transformed means strongyle-type eggs per gram of feces (EPG; ± 95% confidence intervals) by study period. Model included fixed effect of study period, covariate for reproductive status, and random effect for repeated measures on individual alpaca. Study period had a significant effect on strongyle-type EPG score distribution (P<0.01). Periods not connected by the same letter are significantly different (P<0.01).
Conclusion
This study was successful in showing that a comprehensive, herbal formula has a scientifically sound position alongside the typical methods of GIN control. Despite considerable reproductive demands, in the absence of any other medications or dewormers, treatment with this formula increased body condition scores, decreased FAMACHA scores, and decreased FEC in a statistically significant manner. This increases the overall health and sustainability of the herd, increases commodity yields, decreases mortality and expenses related to morbidity. Without the promise of any novel anthelmintics for the camelid industry, this could be the next logical step towards a solution for this particularly devastating problem.Declaration of Interest: Erin Masur and Alexia Tsakiris were the developers of the formula referred to as Early Bird, and as such have personal interest in its success.
Funding
This work was supported by The National Institute of Food and Agriculture, U.S. Department of Agriculture, through the Northeast Sustainable Agriculture Research and Education program under the subaward number ONE20-371.
References
- Crook EK O, Briena DJ, Howell SB, Storey BE, Whitley NC, et al. (2016) Prevalence of anthelmintic resistance on sheep and goat farms in the mid-Atlantic region and comparison of in vivo and in vitro detection methods. Small Rumin Res 143: 89-96.
- Zajac AM, Garza J (2020) Biology, epidemiology, and control of gastrointestinal nematodes of small ruminants.Vet Clin North Am Food Anim Pract 36: 73-87.
- Kaplan RM, Vidyashankar AN (2012) An inconvenient truth: Global worming and anthelmintic resistance. Vet Parasitol 186: 70-78.
- Kotze AC, Prichard RK (2016) Anthelmintic resistance to Haemonchus contortus: History, mechanisms and diagnosis. Adv Parasitol 93: 397-428.
- Rashid MH, Vaughan JL, Stevenson MA, Campbell AJ, Beveridge I, et al. (2018) Anthelmintic resistance in gastrointestinal nematodes of alpacas (Vicugna pacos) in Australia. Parasit Vectors 11: 388.
- Kultscher L, Hinney B, Schmäschke R, Joachim A, Wittek T (2019) Current anthelmintic treatment is not always effective at controlling strongylid infections in German alpaca herds. Parasit Vectors 12: 1-10.
- McLeod RS (2004) The economic impact of worm infections in small ruminants in Southeast Asia, India and Australia. Worm Control for Small Ruminants in Tropical Asia 23-33.
- Paolini V, Bergeaud JP, Grisez C, Prevot F, Dorchies P, et al. (2003) Effects of Condensed Tannins on Goats Experimentally Infected with Haemonchus Contortus. Vet Parasitol 113: 253-261.
- Akkari H, Hajaji S, B'chir F, Rekik M, Gharbi M (2015) Correlation of Polyphenolic Content with Radical-Scavenging Capacity and Anthelmintic Effects of Rubus Ulmifolius (Rosaceae) against Haemonchus Contortus. Vet Parasitol 221: 46-53.
- Ferreira LE, Benincasa BI, Fachin AL, França SC, Contini SSHT, et al. (2016) Thymus Vulgaris L. Essential Oil and its Main Component Thymol: Anthelmintic Effects against Haemonchus Contortus in Sheep. Vet Parasitol 228: 70-76.
- Mengistu G, Hoste H, Karonen M, Salminen J-P, Hendriks WH, et al. (2017) The InVitro Anthelmintic Properties of Browse Plant Species against Haemonchus Contortus is Determined by the Polyphenol Content and Composition. Vet Parasitol 237: 110-116.
- Burke JM, Wells A, Casey P, Kaplan RM (2009) Herbal Dewormer Fails to Control Gastrointestinal Nematodes in Goats. Vet Parasitol 160: 168-170.
- Peltoniemi OAT, Oliviero C (2015) The Gestating and Lactating Sow: Housing Management and Environment During Farrowing and Early Lactation. Wageningen Academic Publishes 231-252.
- Kaplan RM, Burke JM, Terrill TH, Miller JE, Getz WR, et al. (2004) Validation of the FAMACHA© Eye Color Chart for Detecting Clinical Anemia in Sheep and Goats on Farms in the Southern United States. Vet Parasitol 123: 105-120.
- Cebra C, Anderson DE, Tibary A, Van Saun RJ, Johnson LW, et al. (2014) Llama and Alpaca Care: Medicine, Surgery, Reproduction, Nutrition, and Herd Health. Elsevier Health Sciences.
- González-Garduño R, Torres-Acosta JFJ, Chay-Canul AJ (2014) Susceptibility of Hair Sheep Ewes to Nematode Parasitism during Pregnancy and Lactation in A Selective Anthelmintic Treatment Scheme Under Tropical Conditions. Res Vet Sci 96: 487-492.
- Rocha RA, Amarante AFT, Bricarello PA (2004) Comparison of the Susceptibility of Santa Inês and Ile De France Ewes to Nematode Parasitism Around Parturition and During Lactation. Small Rum Res 55: 65-75.
- Ruíz-Uitzil CA, Torres-Acosta JFJ, Cámara-Sarmiento R,Aguilar-Caballero AJ (2011) Uso De Vacunas Y Desparasitantes En Rebaños Ovinos Del Este De Yucatán. Memorias De La Reunión AMTEO.
- Bentounsi B, Meradi S, Cabaret J (2012) Towards Finding Effective Indicators (Diarrhoea and Anaemia Scores and Weight Gains) for the Implementation of Targeted Selective Treatment Against the Gastro-Intestinal Nematodes in Lambs in A Steppic Environment. Vet Parasitol 187: 275-279.
- Cabaret J, Gonnord V, Cortet J, Sauvé C, Ballet J, et al. (2006) Indicators for Internal Parasitic Infections in Organic Flocks: The Diarrhoea Score (DISCO) Proposal for Lambs.
- Ouzir M, Berrag B, Benjouad A, Cabaret J (2011) Use of Pathophysiological Indicators for Decision of Anthelmintic Treatment of Ewes Against Gastrointestinal Nematodes in Morocco. Vet Parasitol 180: 372-377.
- Baird A, Pugh D (2012) Sheep and Goat Medicine second edition. Maryland Heights Missouri: 106-107.
- Qamar MF, Maqbool A, Ahmad N (2011) Economic losses due to Haemonchosis in sheep and goats. Sci International 23: 321-324.
- Burton S, Robinson TF, Roeder BL, Johnston NP, Latorre EV, et al. (2003) Body condition and blood metabolite characterization of alpaca (Lama pacos) three months prepartum and offspring three months postpartum. Small Rum Res 48: 69-76.
- Emery DL, Hunt P, Le Jambre LF (2016) Haemonchus contortus: the then and now, and where to from here? Int J Parasitol 46: 755-769.
- Edwards EE, Garner BC, Williamson LH, Storey BE, Sakamoto K (2016) Pathology of Haemonchus contortus in New World camelids in the southeastern United States: a retrospective review. J Vet Diagn Invest 28: 105-109.
- Abenavoli L, Capasso R, Milic N, Capasso F (2010) Milk thistle in liver diseases: past, and future. Phytother Res 24: 1423-32.
- Chen L, Lu Z, Yang Y, Du L, Zhou X, et al. (2018) Effects of purified Omphalia lapidescens protein on metastasis, cell cycle, apoptosis, and the JAK-STAT signaling pathway in SGC-7901 human gastric cells. Oncol Lett 15: 41616-4170.
- JinFu L (2015) Efficacy of Omphalia lapidencens and praziquantel for treatment of mice infected with pleroceroids. Modern Prev Med Vol 42:2409-2411.
- Tangalin MGG (2011) Anthelmintic effects of processed mature betel nut as dewormer to native chicken and small ruminants. Asian J Health 1: 230-243.
- Gao SM, Liu JS, Wang M, Cao TT, Qi YD, et al. (2018) Traditional uses, phytochemistry, pharmacology, and toxicology of Codonopsis: A review. J Ethnopharmacol 219: 50-70.
- Li H, Zhu J, CheY, Dao T (2013) Efficacy of pumpkin seeds in combination with areca in treatment of 204 taeniasis cases of Blang Ethnic group. China Tropical Med 13: 1027-1028.
- Li S, Cui D, Wang S, Wang H, Huang M, et al. (2015) Efficacy of an herbal granule as treatment option for neonatal Tibetan lamb diarrhea under field conditions. Livestock Sci 172: 79-84.
- Sun QL, Li YX, Cui YS, Jiang SL, Dong CX, et al. (2019) Structural characterization of three polysaccharides from the roots of Codonopsis pilosula and their immunomodulatory effects on RAW264.7 macrophages. Int J Biol Macromol 130: 556-563.
- Han KH, Park JM, Jeong M, Han YM, Go EJ, et al. (2017) Heme oxygenase-1 induction and anti-inflammatory actions of Atractylodes macrocephala and Taraxacum herb extracts prevented colitis and was more effective than sulfasalazine in preventing relapse. Gut Liver 11: 655-666.
- Qadir S, Dixit AK, (2010) Use of medicinal plants to control Haemonchus contortus infection in small ruminants. Vet World 3: 515-518.
- Sham TT, Yuen AC, Ng YF, Chan CO, Mok DK, et al. (2013) A review of the phytochemistry and pharmacological activities of raphani semen. Evid Based Complement Alternat Med: 636194.
- Choi E, Hwang J (2004) Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 75: 557-565.
- Sargautiene V, Nakurte I, Nikolajeva V (2018) Broad prebiotic potential of non-starch polysaccharides from oats (Avena sativa L.): an in vitro study. Pol J Microbiol 67: 307-313.
- Doligalska M, Jóźwicka K, Donskow-Lysoniewska K, and Kalinowska M (2017) The antiparasitic activity of avenacosides against intestinal nematodes. Vet. Parasit. 241: 5-13.
- Liu M, Panda S K, Luyten W (2020) Plant-based natural products for the discovery and development of novel anthelmintics against nematodes. J Biomolecules 10: 426.
- Auyeung K, Han K (2016) Astragalus membranaceous: A review of its protection against inflammation and gastrointestinal cancers. American J Chinese Med 44: 1-22.
- Bhattacharya S K ,Muruganandam AV (2003) Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacology Biochem 75:547-55.
- Chandrasekhar K, Kapoor J and Anishetty S (2012) A prospective, randomized, double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychological Med. 34(3), 255-62.
- Al-Snafi A (2015) The chemical constituents and pharmacological effects of Calendula officinalis – a review. Indian J Pharm Sci. 5: 172-185.
- Aminnezhad S, Kermanshahi RK, Ranjbar R (2016) Effect of Althea officinalis extract on growth and biofilm formation in Pseudomonas aeruginosa. J Pure and Appl Microbio 10:1857-1863
- Bashir S, Janbaz KH, Jabeen Q and Gilani, AH (2006) Studies on spasmogenic and spasmolytic activities of Calendula officinalis flowers. Phytotherapy Res 10: 906-910.
- Bensch K, Tiralongo J, Schmidt K, Matthias A, Bone KM, et al. (2011) Investigations into the antiadhesive activity of herbal extracts against Campylobacter jejuni. Phytotherapy Res. 25, 1125-32.
- Bora KS and Sharma A (2011) Evaluation of antioxidant and cerebroprotective effect of Medicago sativa linn against ischemia and reperfusion insult. Evid Based Complement Alternat Med
- Bora K S and Sharma A (2011) Phytochemical and pharmacological potential of Medicago sativa: A Review. Pharm Bio 49:211-20.
- Choie KC, Cho SW, Kook SH, Chun SR, Bhattarai G, et al. ( 2016) Intestinal anti-inflammatory activity of the seeds of Raphanus sativus L in experimental ulcerative colitis models. J Ethnopharmacology 179: 55-65.
- Gohil KJ, Patel JA , Gajjar AK (2010) Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J Pharm Sci 72: 546-556.
- Gülҫin I, Küfrevioğlu Ӧİ, Oktay M, Büyükokuroğlu ME (2004) Antioxidant antimicrobial antiulcer and analgesic activities of nettle (Urtica dioica L). J Ethnopharmacology 90: 205-215.
- Huyghe C, Bertin E, Landry N (2007) Medicinal and nutraceutical uses of alfalfa (Medicago sativa L.). Advances in Medicinal Plant Res 147-172.
- Kalantari H, Shahshahan Z, Hejazi SM, Ghafghazi T,Sebghatolahi V, et al. (2011) Effects of Silybum marianum on patients with chronic hepatitis C. J of Med Sci 16:287-290.
- Liu F, Chen JF, Wang Y, Guo L, Zhou QM, et al. (2019) Cytotoxicity of lanostane-type triterpenoids and ergosteroids isolated from Omphalia lapidescens on MDA-MB-231 and HGC-27 cells. Biomed Pharmacother 118:109-273.
- Mehrabani D, Ziaei M, Hosseini SV, Ghahramani L, Bananzadeh AM , et al (2011) The effect of Calendula officinalis in therapy of acetic acid induced ulcerative colitis in dogs as an animal model. Iran Red Crescent Med Journal 13: 884-90.
- Mishra LC, Singh BB, Dagenais S (2000) Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review Altern Med Rev 5:334-46.
- Namazi N, Esfanjani AT, Heshmati J, Bahrami A (2011) The effect of hydro alcoholic nettle (Urtica dioica) extracts on insulin sensitivity and some inflammatory indicators in patients with Type 2 Diabetes: a randomized double-blind control trial. J Bio Sci 14: 775-779.
- Paocharoen V (2010) The efficacy and side effects of oral Centella asiatica extract for wound healing promotion in diabetic wound patients. J Med Assoc Thai 93: 166-170.
- Penn State Extension (2013) Body Condition Scoring of Llamas and Alpacas.
- Sainath SB, Meena R, Supriya C, Reddy KP, Reddy PS (2011) Protective role of Centella asiatica on lead-induced oxidative stress and suppressed reproductive health in male rats. Environ Toxicol Pharmacol 32: 146-154.
- Sharma AK, Basu I, Singh S (2018) Efficacy and safety of ashwagandha root extract in subclinical hypothyroid patients: a double-blind, randomized placebo-controlled trial J Altern Complement Med 24: 243-248.
- Tibary A, Johnson LW, Pearson LK, Rodriguez JS (2014) Lactation and neonatal care. Llama and Alpaca Care 286-297.
- Váradyová Z, Mravčáková D, Babják M, Bryszak M, Grešáková Ľ, et al (2018) Effects of herbal nutraceuticals and/or zinc against Haemonchus contortus in lambs experimentally infected. BMC Vet Res 14: 78.
- Vyas S, Collins SM, Bertin E, Davys GJ, Mathur B (2013) Leaf concentrate as an alternative to iron and folic acid supplements for anaemic adolescent girls: a randomized controlled trial in India. Public Health Nutr 13: 418-423.
- In: Organic Congress (2006) Organic Farming and European Rural Development Odense Denmark May 30:552-553.
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Masur E, Tsakiris A, Bruno K, Theurer M, Gerb S (2022) Assessment of an Herbal Feed Additive on Reducing Gastrointestinal Nematodes in an Alpaca Operation. J Vet Med Health 6: 151. DOI: 10.4172/jvmh.1000151
Copyright: © 2022 Masur E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2977
- [From(publication date): 0-2022 - Apr 16, 2025]
- Breakdown by view type
- HTML page views: 2551
- PDF downloads: 426