Research Article Open Access
Assessing the Impact of Diagnostic Imaging at the End of Life: A Single-Center Retrospective Cohort Study
Myriam Irislimane1, Fran&ccedi 1;ois Lamontagne2*, John J You3, Daren K Heyland4 and Lucie Brazeau-Lamontagne11Department of Diagnostic Radiology, University Hospital of Sherbrooke, Sherbrooke, Canada
2Research Center CHU de Sherbrooke and Université de Sherbrooke, Sherbrooke, Canada
3Departments of Medicine, and Clinical Epidemiology & Biostatistics, McMaster University, Hamilton, Canada
4Clinical Evaluation Research Unit, Kingston General Hospital, Canada
- *Corresponding Author:
- François Lamontagne, MD, MSc
Research Center CHU de Sherbrooke and Université de Sherbrooke
Sherbrooke, Canada
Tel: +(819)346-1110, ext: 74977
Fax: (819) 820-6406
E-mail: francois.lamontagne@usherbrooke.ca
Received Date: July 06, 2016; Accepted Date: August 22, 2016; Published Date: August 25, 2016
Citation: Irislimane M, Lamontagne F, You JJ, Heyland DK, Lamontagne LB (2016) Assessing the Impact of Diagnostic Imaging at the End of Life: A Single-Center Retrospective Cohort Study J Palliat Care Med 6:279. doi:10.4172/2165-7386.1000279
Copyright: © 2016 Irislimane M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Objectives: Goals of care discussions allow seriously ill patients to opt out of technology-laden care, which can improve quality of life at the end of life. In a group of patients with metastatic cancer, we sought to document situations where diagnostic testing might have been avoided. Methods: In this single-center retrospective cohort study, we reviewed the medical records of patients with a known diagnosis of metastatic cancer that were hospitalized between January 1st 2012 and December 31st 2012 and underwent a pulmonary angioscan. We documented level of care prescriptions and treatment plans before and after the test postulating that patients who refused anticoagulation despite a diagnosis of pulmonary embolism might have also refused the pulmonary angioscan if goals of care discussions had encompassed diagnostic procedures. Results: We reviewed the charts of 43 patients who met eligibility criteria. Before the pulmonary angioscan, explicit levels of care were documented for 8 patients (19%). This number increased to 25 (58%) after the test. Of 8 documented levels of care before the pulmonary angioscan, 7 were modified to "comfort measures only" after the test. Three of nine patients (33%) with a pulmonary embolism did not receive anticoagulation. In 2 of the 43 patients (5%), documented discussions about end of life preferences encompassed diagnostic procedures. Conclusions: In a population at high risk of death, documented levels of care were infrequent at hospital admission. Having earlier discussions about end of life preferences encompassing diagnostic procedures may reduce unwanted tests at the end of life.
Keywords
Diagnostic procedures; End of life care; Goals of care; Advance care planning
Introduction
Goals of care discussions are fundamental to ensure that care plans are consistent with patients’ values and preferences [1,2]. By allowing seriously ill patients to opt out of technology-laden end-of-life care, the quality of end-of-life care can be improved [3-7]. While goals of care discussions most commonly focus on preferences regarding therapeutic interventions, they could also focus on diagnostic procedures that may be associated with discomfort [8] and complications [9].
Moreover, encompassing diagnostic procedures into goals of care discussions provide patients who seek comfort-oriented care an opportunity to express that they would refuse further life-sustaining therapy regardless of the results of a diagnostic test. The primary objective of this study was to evaluate the impact of a specific diagnostic test (i.e. pulmonary angioscan) on documented goals of care and treatment plans.
In a group of patients with metastatic cancer, we sought to document situations where diagnostic testing might have been avoided because treatment plans were not contingent upon pulmonary angioscan results.
Methods
Design and data source
We conducted a retrospective cohort study at a 677-bed tertiary care center. After obtaining approval from the research ethics board (IC-2013-002107), we screened and reviewed eligible medical records using the hospital database. This database includes detailed information on primary and secondary diagnoses, coded from hospital discharge forms according to the International Classification of Diseases, 10th revision (ICD-10) and the International Classification of Disease for Oncology, 3rd Edition (ICD-O-3), for every patient admitted to the hospital [10]. The principal investigator (MI) then reviewed individual records to confirm eligibility and collect data. The study period ranged from January 1st 2012 to December 31st 2012. Outcomes were ascertained up to March 2014 to account for deaths occurring after the index hospital admission. Individual patient consent was not required for this retrospective chart review.
Study population
The study population consisted of patients with known metastatic cancer who underwent a pulmonary angioscan between January 1st 2012 and December 31st 2012. We estimated that this population was most likely to have clearly stated end of life preferences before undergoing the diagnostic test. Pulmonary angioscans imply a clinical suspicion of pulmonary embolism, a condition with a unique therapeutic option (anticoagulation) that is easily ascertained from medical records [11]. We postulated that patients with metastatic cancer who refused anticoagulation despite a diagnosis of pulmonary embolism might have also refused the pulmonary angioscan if goals of care discussions had encompassed diagnostic procedures. We excluded patients who received a diagnosis of metastatic cancer after the pulmonary angioscan. We also excluded patients if the temporal relationship between the diagnosis of metastatic cancer and pulmonary angioscan was uncertain.
Data collection
We documented the primary cancer type, distribution of metastases, and the medical specialty of the physician who prescribed the pulmonary angioscan. We collected the radiologist's interpretation regarding the diagnosis of pulmonary embolism (lobar, segmental, subsegmental emboli) as well as the progression of the tumour burden since this may also have prompted a reassessment of the goals of care. Reviewing dedicated forms and general medical orders, we documented the medical prescriptions regarding each patient's level of care and the timing of these prescriptions relative to the pulmonary angioscan. These prescriptions fall in 4 mutually exclusive categories: disease modifying/curative treatment including cardiopulmonary resuscitation (level IV); disease modifying/curative treatment excluding cardiopulmonary resuscitation (level III); palliative care consisting of vigorous care and symptom management (i.e. does not exclude selected curative treatments) (level II); and comfort care (level I).
Following the pulmonary angioscan, we collected information on subsequent therapeutic interventions. Specifically, we documented whether therapeutic anticoagulation followed diagnoses of pulmonary emboli. We extracted information on relevant clinical outcomes including complications from therapeutic anticoagulation and mortality. We also sought written documentation of any discussion about the patients' end of life preferences regarding diagnostic procedures. To that end, we reviewed medical notes, hospital discharge summaries and the hospital's dedicated level of care form.
The sample size was not determined a priori. Instead, we incorporated data from every eligible medical record over the course of a predetermined 1-year period.
Statistical analysis
We report binary variables as frequencies and proportions and continuous variables as medians and interquartile ranges (IQR) or means and standard deviations (SD) as appropriate for all patients.
Results
Over a 12 month period 31741 patients received in-hospital care at our institution. Of these, 1415 (4.5%) had metastatic cancer, 78 of whom (5.5%) underwent a pulmonary angioscan during this episode. We ascertained that the diagnosis of metastatic cancer preceded the prescription of the pulmonary angioscan for 43 patients in the study. The median age was 64 years (IQR: 56-73) and 21 patients (49%) were women. Patients’ characteristics are described in Table 1.
| Median age (interquartile range) | 64 (56, 73) |
| Sex (male) | 22 (51%) |
| Cancer Type (12) | |
| Lung | 15 (35%) |
| Breast | 8 (19%) |
| Prostate | 4 (9%) |
| Colorectal | 5 (12%) |
| Thyroid | 1 (2%) |
| Nasopharyngeal | 1 (2%) |
| Oesophagus | 2 (5%) |
| Bladder | 1 (2%) |
| Uterine | 1 (2%) |
| Testis | 1 (2%) |
| Ovarian | 3 (7%) |
| Lymphoma | 1 (2%) |
| Distributions of metastases | |
| Bone | 17(40%) |
| Lymph nodes | 17 (40%) |
| Lung | 13 (30%) |
| Liver | 12 (28%) |
| Brain | 4 (9%) |
| Peritoneal carcinomatosis | 5 (12%) |
| Cutaneous | 4 (9%) |
| Adrenal | 3 (7%) |
| Kidneys | 1 (2%) |
| Ovarian | 1 (2%) |
| Pleural | 2 (5%) |
| Spleen | 1 (2%) |
Table 1: Baseline characteristics.
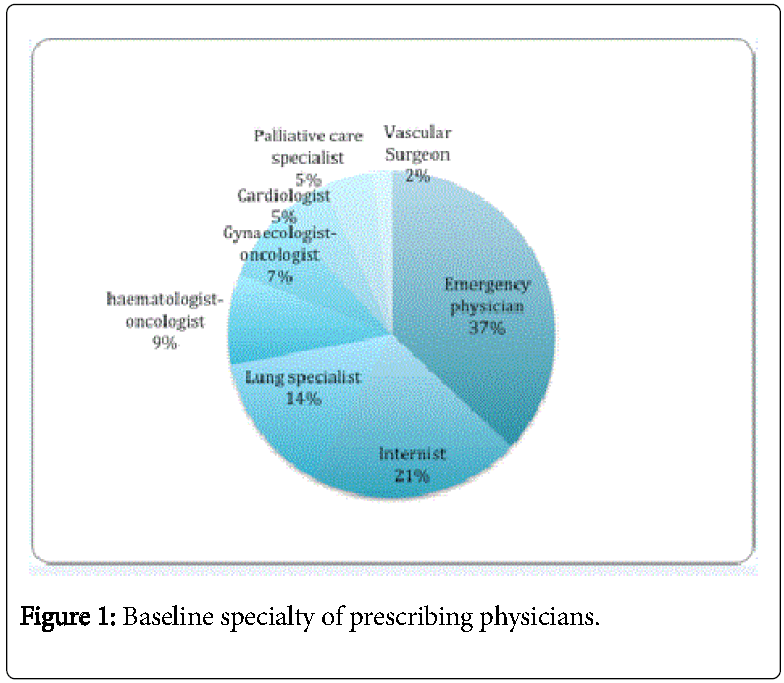
Thirty-six patients (84%) were admitted after presenting to the emergency room and, for 16 patients (37%), the pulmonary angioscan was ordered when the patient was still in the emergency room.
The background specialties of the physician who wrote the prescription appear in Figure 1. In 5 cases (12%), residents ordered the test outside working hours.
Prescribed levels of care and end of life preferences
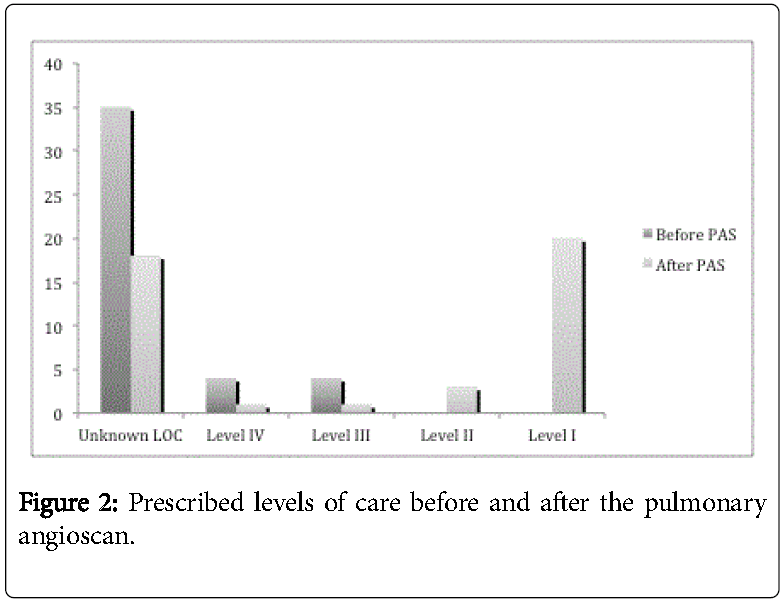
For 35 patients (81%), explicit levels of care were not prescribed before the test. After the test, the number of patients with no explicit level of care order fell to 18 (42%). Of 8 documented goals of care before the pulmonary angioscan, 7 were modified after the test.
Ultimately, comfort measures (level I) were ordered for 20 of 25 patients (80%) (Figure 2 and Table 2).
| Distribution of LOC Before and After Pulmonary Angioscan | ||||||
|---|---|---|---|---|---|---|
| After Before | No LOC | IV | III | II | I | |
| No LOC | 18 | 1 | 0 | 3 | 13 | |
| IV | 0 | 0 | 0 | 0 | 4 | |
| III | 0 | 0 | 1 | 0 | 3 | |
| II | 0 | 0 | 0 | 0 | 0 | |
| I | 0 | 0 | 0 | 0 | 0 | |
Table 2: Prescribed levels of care before and after the pulmonary angioscan.
Progression of tumour burden was clearly stated in the radiologist report in 32/43 (74%) patients.
Documentation regarding the patients' end of life preferences was found in medical notes (24 patients), hospital discharge summaries (6 patients) and the hospital's dedicated form (4 patients). These notes explicitly mentioned diagnostic procedures on only 2 occasions (5%). In both instances, this documentation took place after the pulmonary angioscan and patients expressed their wishes to avoid future diagnostic procedures.
Anticoagulation, complications and mortality
The pulmonary angioscan revealed a pulmonary embolism in nine patients (21%). Six patients received therapeutic anticoagulation one of whom suffered a haemorrhagic stroke and died. Three patients did not receive anticoagulation.
Overall, 9 patients (21%) died in hospital, including 1 patient who received anticoagulation. Of the 34 patients who were discharged alive, 21 were reported deceased in the hospital database before March 2014 (ascertainable 2-year mortality rate 70%). Among decedents, the median number of days from hospital admission to death of our cohort was 75 days (25-422).
Discussion
The results of this retrospective study on pulmonary angioscans prescribed to patients suffering from metastatic cancer suggest that 1) explicit levels of care were seldom documented before the index acute care episode; 2) in a small number of cases, the procedure was potentially superfluous since anticoagulation was declined despite a diagnosis of pulmonary embolism; 3) explicit levels of care were commonly prescribed after the pulmonary angioscan, most commonly to deescalate levels of care; and 4) documentation of end of life preferences most commonly do not address diagnostic procedures.
Our data are concordant with other studies indicating healthcare professionals who work in acute care hospitals often fail to consider the patients end of life preferences and to inquire about previous advance care planning efforts [12]. In fact, assuming that clinical teams are most aware of advance care planning interventions in the context of cancer, this study likely underestimates the burden of all diagnostic procedures (i.e. beyond radiological tests) that are potentially misaligned with patient preferences if one considers the broader population of patients with non-cancer diagnoses.
Assessing the relevance of diagnostic procedures at the end of life has a few implications. First, a process that would impose a reflection on the therapeutic implications (e.g. anticoagulation in case of pulmonary embolism) may help avoid superfluous testing [13,14]. One could imagine that simple context-specific prompts asking physicians who are prescribing diagnostic tests if they have explained the therapeutic implications of a positive result may constitute a reasonable first step. Similar measures reminding physicians of the costs [15,16] and risks [17,18] of interventions influence prescription patterns. Second, most pulmonary angioscans in this study were not unnecessary. Judging from medical orders for levels of care and documented end of life discussions that occurred after this test, radiological procedures may constitute a powerful trigger to realign acute care with patient preferences. However, this raises a question about prerequisites for end of life discussions. A recent editorial suggests that “our unwillingness to live with uncertainty can result in overtesting […], overdiagnosis […]), or overtreatment ...” [15]. It is conceivable that patients who are severely ill may be more comfortable with uncertainty than their treating physicians and willing to make end of life decisions without additional testing.
Strengths of this study include the systematic and comprehensive review of individual medical records and the selection of a diagnostic procedure with therapeutic implications that could be ascertained retrospectively. Limitations include the retrospective design, which limits our ability to capture undocumented end of life discussions as well as communications between clinical and radiology teams. Our inclusion criteria were very restrictive and defined a patient population for whom medical teams show considerable restraint before ordering tests. It is plausible that diagnostic procedures are frequently ordered for patients who suffer from illnesses other than cancer despite their clear preference for strictly palliative care. As such, our findings probably underestimate the number diagnostic procedures that may be avoided if goals of care discussions occurred consistently before diagnostic procedures. Because we did not review medical records of cancer patients who did not undergo pulmonary angioscans, we cannot comment on the prevalence of explicit level of care prescriptions, their effect on decisions regarding prescriptions for diagnostic tests, nor on the occurrence of communications with radiology teams. Notwithstanding this limitation, our perspective was that the end of life preferences of metastatic cancer patients should be systematically documented and influence clinical decisions. The review of medical charts was not duplicated and the reproducibility of our methods was not ascertained. Finally, this study was conducted in a single center which provides care to a dominantly Caucasian, Christian (practicing or not) population. The results may not apply in more culturally diverse settings or in centers where tools efficiently trigger the systematic documentation of end of life preferences. We view these results as hypothesis-generating and believe that they warrant a larger prospective observational study assessing how diagnostic procedures may constitute opportunities to provide better care at the end of life.
Conclusion
In a population at high risk of death, documented levels of care were infrequent at hospital admission. Earlier discussions about end of life preferences encompassing diagnostic procedures may reduce unwanted tests at the end of life and constitute an opportunity to better align acute care with patient preferences.
References
- You JJ, Fowler RA, Heyland DK (2014) Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ 186:425-32.
- Sinuff T, Dodek P, You JJ, Barwick D, Tayler C, et al. (2015) Improving End-of-Life Communication and Decision Making: The Development of Conceptual Framework and Quality Indicators. J Pain Symptom Manage 49:1070-1080.
- Heyland D, Dodek P, Rocker G, Groll D, Gafni A, et al. (2006) What matters most in end-of-life care: perceptions of seriously ill patients and their family members. CMAJ 174:627-33.
- Michael N, O'Callaghan C, Baird A, Hiscock N, Clayton J (2014) Cancer Caregivers Advocate a Patient- and Family-Centered Approach to Advance Care Planning. J Pain Symptom Manage 47:1064-1077.
- Wright AA, Zhang B, Ray A, Mack JW, Trice E, et al. (2008) Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300:1665-1673.
- Cook D, Rocker G, Heyland D (2013) Enhancing the quality of end-of-life care in Canada. CMAJ 185:1383-1384.
- Detering KM, Hancock AD, Reade MC, Silvester W (2010) The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 340:c1345.
- Hackstein N, Schneider C, Eichner G, Rau WS (2007) Effect of i.v. injection of radiographic contrast media on human renal blood flow. Am J Roentgenol 188:1367-1372.
- Dhamanaskar KP, Figueira KS, Jerome SC, Yemen BL (2013) Test bolus technique for detection of pulmonary emboli at 64-slice multidetector computed tomography angiography. Can AssocRadiol J 64:226-228.
- Lamontagne F, Garant MP, Carvalho JC, Lanthier L, Smieja M, et al. (2008) Pneumococcal vaccination and risk of myocardial infarction. CMAJ 179:773-777.
- Farge D, Debourdeau P, Beckers M, Baglin C, Bauersachs RM, et al. (2013) International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J ThrombHaemost 11:56-70.
- Heyland DK, Barwich D, Pichora D, Dodek P, Lamontagne F, et al. (2013) Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med 173:778-787.
- Gunderman RB, Olack SB (2012) Providing, withholding, and withdrawing treatment. J Am CollRadiol9:461-462.
- Lin E (2011) Radiology 2011: the big picture. Am J Roentgenol 196:136-139.
- Aspelin P (2011) Toward providing effective, efficient, and equitable care: how much care can we afford? J Am CollRadiol 8:828-829.
- Kelly AM, Cronin P (2011) Rationing and Health Care Reform: Not a Question of If, but When. J Am CollRadiol 8: 830-837.
- Klok FA, Mos IC, Kroft LJ, de Roos A, Huisman MV (2011) Computed tomography pulmonary angiography as a single imaging test to rule out pulmonary embolism. CurrOpinPulm Med 17:380-386.
- Glasziou P, Moynihan R, Richards T (2013) Too much medicine; too little care. BMJ 346: f4247.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 10993
- [From(publication date):
September-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10140
- PDF downloads : 853