Assessing the Antioxidants and the Anti-Inflammatory Activities of Methanolic, Ethyl Acetate and Petroleum Ether Extracts of the Stem Bark of Heisteria parvifolia
Received: 07-Jan-2020 / Accepted Date: 23-Jan-2020 / Published Date: 30-Jan-2020 DOI: 10.4172/2167-065X.1000191
Abstract
This research assessed the antioxidants and anti-inflammatory activities of methanolic, ethyl acetate and petroleum ether extracts of the stem bark of Heisteria parvifolia. The phytochemical screening identified several important phytochemical constituents among the various solvent used. The various extracts were also tested for their abilities to scavenge a stable 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical by way of determining the plant antioxidant properties gave the IC50 values as, 46.98 μg/mL, 132.65 μg/mL, 2784.21 μg/mL for methanolic, ethyl acetate and petroleum ether respectively and 27.26 μg/mL for the standard drug (ascorbic acid). The ant-inflammatory activities of the plant extracts were determined which also gave promising carrageenan induced inflammation inhibition, methanolic extract against carrageenan induced inflammation were 59.12%, 54.62% and 51.55%. The ethyl acetate extract showed significant percentage inhibitions of 52.38%, 46.92% and 42.34%, while the petroleum ether only showed a percentage inhibition of chicks paw size of 42.85%. These results have justified the relevance of this plant in its wide traditional usage in wound healing.
Keywords: Antioxidant; Anti-inflammatory; Free radicals; P-values; DPPH; Total antioxidant capacity; Carrageenan; Foot paw size; Heisteria parvifolia; Dunnet’s multiple comparision test
Introduction
Nature has provided possible drug agents for millions of years which led to the development of new pharmaceutical preparations. Plant medicines are the primitive source of health care practice by man. In other underdeveloped nations, a huge number of the population depends on herbal practitioners and herbal products to cure their health needs [1]. The World Health Organization (WHO) report, 2008 estimates about 80% of the world human population of underdeveloped nation’s reliance on herbal preparations for basic health care needs. Our fore fathers continued to prepare herbal concoctions as remedy for the treatment of various forms of diseases without knowing the active principles behind the remedy because they lacked the knowledge of modern science which could have even helped isolate and purify these preparations to make them more efficacious and less toxic to the consumers [2]. Modern scientific practices have helped unveiled the possible phytoconstituents present in these medicinal plants that help them in performing the activities they do in traditional practice [3].
Plants, since the pre-colonial era are used as a source of medicines throughout the world and is still currently the main source for novel drug discovery and form part of treatment option in our current medical system. Medicinal plants can therefore be defined as any plant with the ability to reduce, cure, prevent or change the physiological and pathological conditions of an individual [4].
Medicinal plants generate a wide array of phytochemical constituents that contributes to the improvements of total health [5]. These phytochemical constituents or compounds act individually, in combination or in synergy to elicit a pharmacological activity. They act in isolation if the constituent or constituents is or are in the right proportion to give the needed therapeutic effect. Also, they act in synergy if the constituents in the plant are not in the proportion adequate to elicit the desired activities and need to support each other using different mechanisms of actions to produce the same results [6]. A lot of mechanisms can be stimulated by a plant compound for example, a plant substance could aid digestion, treat inflammation, or treat oxidative stresses generated by free radicals, prevent bacterial and fungal invasions and other substances that help release unwanted products from the body [6].
Natural products can be described as molecules or compounds obtained from biological organisms, plants, animals, fungi, insects and other marine organisms [7]. These organic molecules possess pharmacological or biochemical activities which warrant their usage in pharmaceutical drug discovery and drug formulation. These molecules or compounds are referred to as phytochemicals or metabolites of which they are grouped into two main classes, primary and secondary metabolites [8].
The primary metabolites are common compounds or molecules which are used in normal cell development and functioning. They are widespread in all plants which include and not limited to carbohydrates, proteins, vitamins, lipids, and other mineral nutrients. On the other hand, are the secondary metabolites whose role is for the total maintenance of the plant life [8]. Normally these phytoconstituents are produced in small amount and special to some plant species. Phytoconstituents can be used as agents of pollinators in plants and soldiers against predators and pathogens.
With the high demands for medicinal plants products, there is a need for thorough scientific investigations into these medicinal plants for their health benefits and the likely possible toxic effects [9]. Among the vast range of medicinal plants used for medications is Heisteria parvifolia which is used in the treatment of many health conditions in Ghana [10].
Heisteria parvifolia is a shrub or small tree that grows up to about 20 to 60 m tall and has a bole up to about 60cm in average diameter with slight fluttering at the base and thin buttress. The genus Heisteria comprises about 65 species in tropical Africa. Some species of this plant include, Heisteria trillesiana, Heisteria zimmereri and many others in tropical Africa [11]. Heisteria parvifolia occurs in different types of habitats, evergreen moist rainforest, coastal and riverine forest and primary upland forest, seasonally flooded forest and can also occur in savanna and secondary forest [12], in Ghana it is strongly associated with acid and base-poor soils. It is a dicotyledonous plant belonging to the family Olacaceae. The specie can also be found in Senegal, South- West Mali, Democratic Republic of Congo (DRC), Angola, Uganda, South Sudan and many other African countries [13]. The wood of Heisteria parvifolia is used for building poles, piles, palisades and tool handle. The flexibility of the stem and wood make them suitable for making bows, the twigs are used as chew-sticks and arrows. It has a glossy, dark green leaves with enlarged scarlet calyx persisting on the developing fruits. In some areas, the fruits are eaten fresh, the oilrich seeds are eaten fresh or roasted or cooked before eaten [14]. The plant is known for its medicinal uses in various countries for instance in Ghana, the ground roots are used as enema against stomach-ache, stem bark is also used for treating diarrhoea, convulsion and as a cough medication [15]. In Democratic Republic of Congo, the root bark is used against migraine by dropping it into the nose and into the eyes to treat painful or infected eyes and against cough [15]. The stem bark is applied to circumcision wounds in Gabon. In Cote d’Ivoire the leaves are taken against convulsion [16].
The seed kernel contains about 50% fat and a nice flavor scent [17]. The fatty acid composition is reported as mainly long chain saturated fatty acid (18.5%) with carbon length of C16-C28 and 31% of oleic acid with other mono and di-enoic fatty acids [18].
The plant is of great prospects and most likely to remain a producer of timber, fruits, kernels and as source of food for local use. The seed oil is on a high demand as a source of rare fatty acids [18]. There is however, limited scientific research into the medicinal importance of this plant despite its widespread traditional usage.
Formation of free radicals
When an unstable bond breaks it results in the formation of unpaired molecules known in other words as free radicals [19]. These free-radicals in their search for stability react rapidly with the nearby stable compounds or molecules. Normally, these unstable molecules attack the closest stable compounds by either losing or gaining electron from the stable compound to attain stability. When the stable compound or molecule loses an electron, it becomes a free radical because it now has unpaired electrons and this process can persist until it is terminated by another molecule with unpaired electron [20]. Despite the biological mechanisms or activities that generate free radicals, atmospheric or environmental factors such as pollution, chemical radiation, smoke from tobacco, agricultural chemicals can also produce free radicals in the human system. In the absence of a terminating agent to stop the proliferation of these free radicals, the persistent formation of free radicals can cause damage to tissues and cells in the body and any other biological molecule that undergoes oxidation [20]. There is growing interest in the activities of free radicals in the body, food rich in fats such as polymer and the mechanisms inhibiting the damages caused by terminating agents such as antioxidants [21]. Cell metabolic products directly support cell development and also increase the pathogenesis produced by atherosclerosis, thus low-density protein oxidation. Free-radicals can cause damages to the human brain associated with other diseases such as Alzheimer’s disease. However, research has demonstrated that the length of damage caused by these free radicals can be altered by dietary modifications and pharmaceutical therapy [22].
Natural products as antioxidants
Antioxidants are compounds, molecules or substances that are capable of protecting cells against damages caused by free radicals. They react with the free radicals by removing the free lone pair of electrons in its outermost shell there by stabilizing the free radicals which goes a long way to reduce the damages caused by these free radicals. Free radicals can cause diseases such as cancer and other degenerative conditions [23]. Examples of compounds and molecules that can help stabilize these free radicals are beta-carotene, vitamins A, C, lycopene and other common vegetables with phenolic content [24]. Oxidation in biological system is a reaction process involving the transfer of electrons from one molecule to another molecule known as the oxidizing agent. These oxidative processes can generate unstable molecules which are the starters of chain reaction leading to the damage of cells. Antioxidants act by terminating these chain reactions initiated by free radicals through the removal of the intermediates created by free radicals and also terminate the oxidation processes by oxidizing themselves. By way of their interactions, antioxidants are mostly reducing agents such as vitamin C, polyphenols and thiols [23]. Oxidation processes are very important processes in human life but can sometimes be very damaging to cells and tissues hence, living things maintain complex systems of antioxidants which include glutathione, ascorbic acid and other enzymes such as superoxide dismutase, catalase and various peroxidases. Limited amount of antioxidants or its inhibition of important enzymes causes oxidative stress that can lead to cell death [25]. The oxidative menace impose on human tissues and cells by free radicals can be a contributing factor to human diseases and is therefore important to encourage the pharmacological activities of antioxidants in treating chronic health conditions such as cancers, stroke and neurodegenerative illnesses. The health benefits of antioxidants in plants have necessitated manufacturers of dietary supplements to include them as part of dietary supplements in the quest to maintain and improve the health of consumers [26]. Although preliminary studies have shown that antioxidants supplements in food can promote health, a more detailed clinical research have demonstrated that excessive use of antioxidants can produce harmful effects in the body. Chemists over the years have suggested that oxidations caused by free radicals can be terminated by terminating agents such as antioxidants [26].
Mechanisms of inflammation
The mechanisms underpinning the protrusions of inflammation are enormous both natural and environmental because of the complexity associated with biological systems. Inflammation is a tightly controlled cascade of physiological and behavioral processes arranged by dissolvable immune signaling molecules referred to as cytokines [27]. The initials of inflammatory cascade are the recognitions of infections. This can be obtained through detection of pathogen-associated molecular patterns and specifically directed towards the known distinctive molecules as expressed by pathogens survival. Infections associated with molecular patterns are molecules or compounds which show necrosis and can be recognized by innate immune system. The merit of detecting the signals is that it helps reduce the targeting of host cells and tissues. As it is seen in other adaptive immunity, the natural immune system is deficient in differentiating between strains of pathogens and to ascertain the harmfulness of strains to host cells and tissues [27].
Affected cells are noticed by germ-line protected receptors such as transmembrane Toll-like receptors and intracellular nucleotide binding domain [28]. After the recognition of ligands, the transmembrane toll-like receptors trigger signaling pathways which help in the activation of nuclear factor kappa-light-chain-enhancer of activated B cells. The transcription factor is present in all cells in an inactive form linked to an inhibitor, kappa-light-chain-enhancer of activated B cells [28]. Immediately after the transduction of the signal, kappa-light-chain-enhancer of activated B cell is released from kappalight- chain-enhancer of activated B cell and moves to the center of the cell, nucleus, and a place where transcription is controlled by linking to target genes. The activation of nuclear factor kappa-light-enhancer of activated B cells need the synthesis of proteins. The nuclear factor kappa-light-enhancer of activated B cells signaling system is strange but there is evidence that the control of immune functions through a pathway arise independently [28]. The inducible display of proinflammatory cytokines such as tumor necrosis occurs as a result of the transcription and translation of genes in the third stage of the cascade of inflammation which occurs in conjunction with the attractants and various molecules that help in the generations of effector cells that include neutrophils and monocytes at the disturbed area. A cytotoxic environment is created by neutrophils through the release of poisonous chemicals from cytoplasmic granules. The quick outpour of these chemicals requires the intake of both oxygen and glucose through a process known as respiratory burst [25]. The most poisonous chemicals released included reactive oxygen species and reactive nitrogen species respectively and some forms of proteinases. The toxic chemicals generated are damaging to both the host and the pathogens which essentially induce a primer of the cascade of inflammations. The five main processes of inflammations are (1) Tissue injury or pathogens induce inflammation. (2) The transmembrane toll-like receptors bind to Pathogen- associated molecular pattern. (3) The toll-like receptors activate a signal transduction pathway involving the phosphorylation of the inhibition of kappa-light-activated B cell protein. (4) Different pro-inflammatory cytokines and attractants are generated and released to boost effector functions of inflammation. (5) The blood-borne neutrophils and monocytes then move to the disturbance site through chemotaxis and specifically pass through the endothelial cells to reach their target areas or sites [24]. The inward movement of fluids in cells is accompanied by protein-rich fluids which is referred to as exudate which promote swelling. Mast cells and tissue-resident macrophages assist this movement by releasing histamine, prostaglandins and leukotriene which have quick effects on the vasculature in addition to vasodilation increasing vascular permeability [29].
Materials and Methods
Chemicals and reagents used
The following chemicals/reagents were secured from the indicated sources, methanol, petroleum ether (Fisher Scientific, UK), chloroform (Fisher Scientific, UK), hexane (Fisher Scientific, UK), ethanol (Sigma Aldrich, U.K), ferric chloride, Meyers’ reagent, hydrochloric acid (Sigma Aldrich, U.K), sulphuric acid (Sigma Aldrich, U.K), diethyl ether (Fisher Scientific, UK), Fehling solution I and II (prepared Inhouse), acetone (Fisher Scientific, UK), acetic anhydride (Chemiphase, UK), Tragacanth (Oxoids Limited, Basingsingstoke, Hampshire, England), Folin-Ciocalteu (Oxoids Limited, Basingsingstoke, Hampshire, England) disodium hydrogen phosphate (BDH chemical limited, poole, England), sodium carbonate and ascorbic acid (BDH Chemical laboratory U.K), carrageenan (Greenwich University, U.K), and Diclofenac sodium (Sara Export Limited, batch No; DFS- 0316250, Assay value; 99.7% (w/w).
Equipment and apparatus used
The following pieces of equipment and apparatus were used for the experiments; autoclave, incubator and oven (Gallenkamp, U.K), water bath and electronic balance (Gallenkamp, U.K), fume chamber (Gallenkamp, U.K), rotary evaporator (R-210 Buchi, Switzerland), micropipette (Anumbra, U.K), separatory funnel (Anumbra, U.K), beakers and test tubes (Oxoids Limited, Basingsingstoke, Hampshire, England), syringes (Quickfit limited, England), fridge (Systec Wattenberg, Switzerland), Whatman No.1 filter paper, micro-titre plate (Synergy H5, No: 29302), UV-Visible Spectrophotometer (T90+ UV-VIS Spectrometer, PG Instruments LTD).
Authentication of collected sample
The stem bark of Heisteria parvifolia was harvested from some farm lands in Mampong-Akwapim (Eastern Region of Ghana) and authenticated by Mr. Asare of the Department of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Science and was assigned the voucher specimen number KNUST/HMI/002 and deposited at the Faculty’s herbarium for record purposes.
Sample Preparations
The stem bark of Heisteria parvifolia was harvested, washed and chopped into smaller pieces, air-dried under room temperature for about two weeks and pulverized into fine powder. The powdered sample (150g) was extracted with methanol, ethyl acetate and petroleum ether by cold maceration for three days (72 h) separately. The macerated sample was filtered and concentrated to dryness which gave the following yields, 6.56g for methanol extract, and 5.45g for ethyl acetate and 3.11g for petroleum ether. The extracts were stored in a deep fridge before use.
Methods
Evaluation of free radical scavenging activity of Heisteria parvifolia
The determination of the free radical scavenging activity of the extracts (methanolic, ethyl acetate and petroleum ether) was carried out using the DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay as described by (Mensor et al., 2001) with a slight modification. Different concentrations of 500, 250, 125, 62.50 and 31.25 μg/mL of ascorbic acid and sample extracts in methanol were prepared. DPPH (2 mL) in methanol was added to 1 mL solution of the extracts and the ascorbic acid and kept at room temperature in a dark chamber for 30 min. The change in colour from deep violet to light yellow was then measured at 517 nm by a UV-Visible Spectrophotometer. The decrease in absorbance was then converted to percentage radical scavenging antioxidant activity (% RSA) using the formula. RSA%= {[(Abs control- Abs sample) x100] / Abs control}. Methanol was used as the blank (1 mL) plus sample solution (1 mL), DPPH solution (2 mL, 0.002%) was used as the negative control and ascorbic acid was used as the standard drug.
Evaluation of total antioxidant capacity (TAC) of Heisteria parvifolia
The total antioxidant capacity of the extracts (methanolic, ethyl acetate and petroleum ether) was evaluated by the Phosphomolybdenum method according to the procedure described by Prieto et al. The assay is based on the reduction of Molybdenum (Mo+6) (VI) to Molybdenum (Mo+5) (V) by the extracts and the formation of a green phosphate / Mo (V) complex at acidic pH. 1 mL each of the extract solution was mixed with 3 mL of reagent solutions (0.6 M sulfuric acid, 28 mM sodium phosphate and 4mM ammonium molybdate) and incubated in an oven at a temperature of 95℃ for 90 min. The absorbance of the reaction mixture was measured at 695 nm using a UV-Visible spectrophotometer. Methanol was used as the blank and ascorbic acid as the standard drug by establishing a calibration curve for the ascorbic acid using the concentrations (500, 250, 125, 62.50 and 31.25 μg/mL). The antioxidant activity of the extracts is expressed as the number of gram equivalent of ascorbic acid.
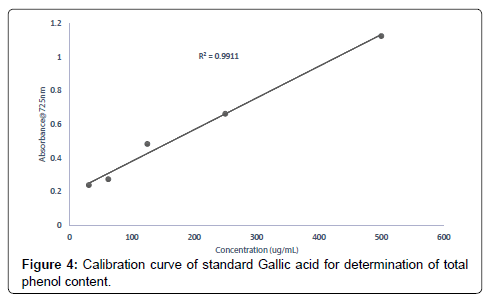
Evaluation of total phenolic content of Heisteria parvifolia The total phenolic content of the extracts (methanolic, ethyl acetate and petroleum ether) was determined using the method of (Mcdonald et al., 2001). Calibration curve was prepared by mixing ethanol solution of Gallic acid (1 mL; 31.25-500 μg/mL) with 0.5 mL of Folin- Ciocalteu reagent and sodium carbonate (3 mL, of 2.0%). Each extract solution (1 mL) was mixed with 3 mL Folin-Ciocalteu reagent solution and incubated for 30 min. The Absorbance values of the extract concentrations (500, 250, 125, 62.50, 31.25 μg/mL) were measured at 725 nm using a UV-Visible spectrophotometer to determine the total phenolic content in the extract (methanolic, ethyl acetate and petroleum ether) concentrations. Gallic acid equivalents (GAE) were calculated using the following formula;
T=C*V/M
Where T = total phenolic content, milligram per gram of sample extract, in GAE; C = the Concentration of Gallic acid established from the calibration curve, mg/mL; V = the volume of Extract in milliliter; M = the weight of sample extract (g).
In-vivo Anti-Inflammatory Activity Screening
The in-vivo anti-inflammatory potentials of the crude extracts of the stem bark of Heisteria parvifolia alongside with a standard drug (Diclofenac sodium) was assessed using the protocols [30].
A total of 100 chicks (seven-day old) were used for the antiinflammatory screening. The chicks were put into six groups with each group having five chicks for each extract. The chicks were weighed to help in determining the volume of extracts to be administered.
Tragacanth was used as the vehicle for dissolving the extracts with concentrations of 30 mg/kg, 100 mg/kg and 300 mg/kg for each of the extracts and concentrations of 10 mg/kg, 30 mg/kg and 100 mg/kg for the standard drug (Diclofenac sodium).
Suspension (10 μL of 2%) in 0.9% saline of carrageenan was prepared. The initial foot paw of the chicks was taken using digital calipers before the therapeutic analysis was done.
The carrageenan was injected into the left foot paw of the chicks for 30 min to 1 h after which the extracts and the standard drug (Diclofenac sodium) were simultaneously administered orally. The change in foot paw of the chicks was monitored hourly for six hours to determine the effects of the extracts and the standard drug.
Results
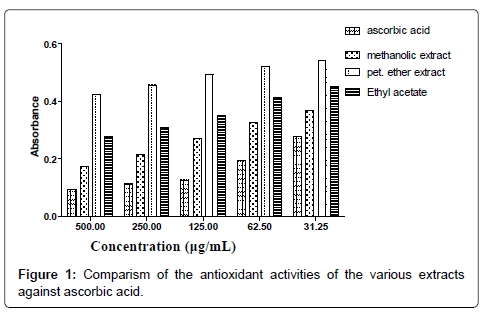
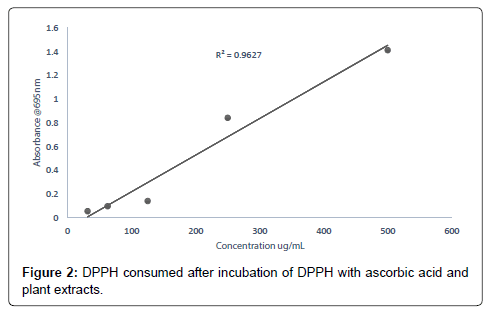
The smaller the IC50 value the more potent the sample is in terms of terminating free radicals (Tables 1-7 and Figure 1). As the concentration of the extract increases the free radical decreases (Figures 2-8).
| Sample | IC50 in µg/mL |
|---|---|
| Methanol extract | 46.98 |
| Ethyl acetate extract | 132.65 |
| Petroleum ether extract | 2784.21 |
| Ascorbic Acid | 27.26 |
Table 1: DPPH scavenging activities of the Extracts and ascorbic acid (standard drug).
| Sample (Extract) | Mean ± standard deviation |
|---|---|
| Methanol | 12.84 ± 2.512 |
| Ethyl acetate | 11.17 ± 1.896 |
| Petroleum ether | 3.97 ± 3.174 |
Table 2: Total antioxidants capacity expressed as ascorbic acid Equivalent (Mean ± standard Deviation).
| Sample (Extract) | Mean ± standard deviation |
|---|---|
| Methanol | 23.05 ± 1.123 |
| Ethyl acetate | 17.21 ± 1.421 |
| Petroleum ether | 12.31 ± 2.564 |
Table 3: Total phenol content expressed as Gallic acid Equivalent (Mean ± standard deviation).
| Sample | Concentration μg/mL | Absorbance | % Inhibition |
|---|---|---|---|
| Methanol | 500 | 0.13 ± 0.010 | 74.95 |
| 250 | 0.15 ± 0.010 | 71.18 | |
| 125 | 0.21 ± 0.017 | 60.45 | |
| 62.50 | 0.48 ± 0.011 | 39.60 | |
| 31.25 | 0.62 ± 0.021 | 17.51 |
Table 4: Concentration, Absorbance and % Inhibition of DPPH by Methanolic extract of Heisteria parvifolia.
| Sample | Concentration μg/mL | Absorbance | % Inhibition |
|---|---|---|---|
| Ethyl acetate | 500 | 0.174 ± 0.023 | 67.23 |
| 250 | 0.245 ± 0.005 | 53.86 | |
| 125 | 0.286 ± 0.022 | 46.14 | |
| 62.5 | 0.522 ± 0.15 | 28.87 | |
| 31.25 | 0.727 ± 0.271 | 10.11 |
Table 5: Concentration, Absorbance and % Inhibition of DPPH by Ethyl acetate extract of Heisteria parvifolia.
| Sample | Concentration μg/mL | Absorbance | %Inhibition |
|---|---|---|---|
| Petroleum ether | 500 | 0.47 ± 0.076 | 39.45 |
| 250 | 0.87 ± 0.0563 | 26.56 | |
| 125 | 0.96 ± 0.521 | 18.45 | |
| 62.50 | 1.94 ± 0.045 | 7.73 | |
| 31.25 | 2.07 ± 0.563 | 4.69 |
Table 6: Concentration, Absorbance and % Inhibition of DPPH by Petroleum ether extract of Heisteria parvifolia.
| Sample | Concentration μg/mL | Absorbance | %Inhibition |
|---|---|---|---|
| Ascorbic acid | 500 | 0.11 ± 0.001 | 93.45 |
| 250 | 0.13 ± 0.007 | 89.67 | |
| 125 | 0.19 ± 0.003 | 84.93 | |
| 62.50 | 0.36 ± 0.011 | 75.67 | |
| 31.25 | 0.53 ± 0.023 | 65.34 |
Table 7: Concentration, Absorbance and % Inhibition of DPPH by Ascorbic acid (standard drug).
| Concentration (mg/kg) | Percentage (%) Inhibition if Chicks Foot Paw Size | ||||
|---|---|---|---|---|---|
| Plant extract | Diclofenac sodium | Methanol | Ethyl acetate | Petroleum ether | Diclofenac sodium |
| 300 mg/kg | 100 mg/kg | 59.12 | 52.38 | 42.85 | 83.67 |
| 100 mg/kg | 30 mg/kg | 54.62 | 46.92 | 36.23 | 78.34 |
| 30 mg/kg | 10 mg/kg | 51.55 | 42.34 | 20.90 | 74.54 |
Table 8: Anti-inflammatory activities of Heisteria parvifolia extracts compared to standard drug (Diclofenac sodium).
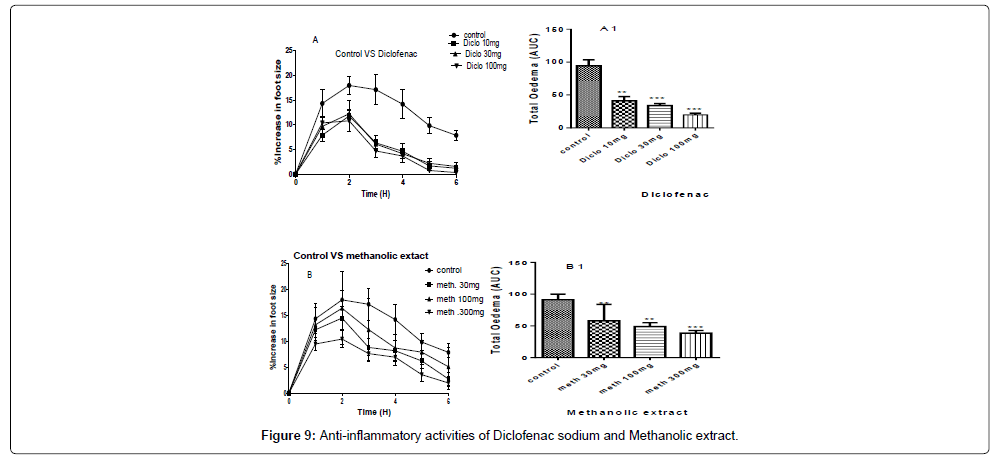
A and B are the inhibitions of the carrageenan induced paw of the chicks by diclofenac sodium and methanolic extract at concentrations of 100 mg/kg, 30 mg/kg and 10 mg/kg for the standard drug (Diclofenac sodium) and 300 mg/kg, 100 mg/kg and 30 mg/kg for the methanolic extract (Table 8).
A1 and B1 are the areas under the curve (total oedema) with statistical p-values ranging from, * for P<0.05 , ** for P<0.01, *** for P<0.001, showing the degree of significance of the activities of the extract on the paw size using ANOVA (one-way) and Dunnet’s multiple comparism test (Figure 9).
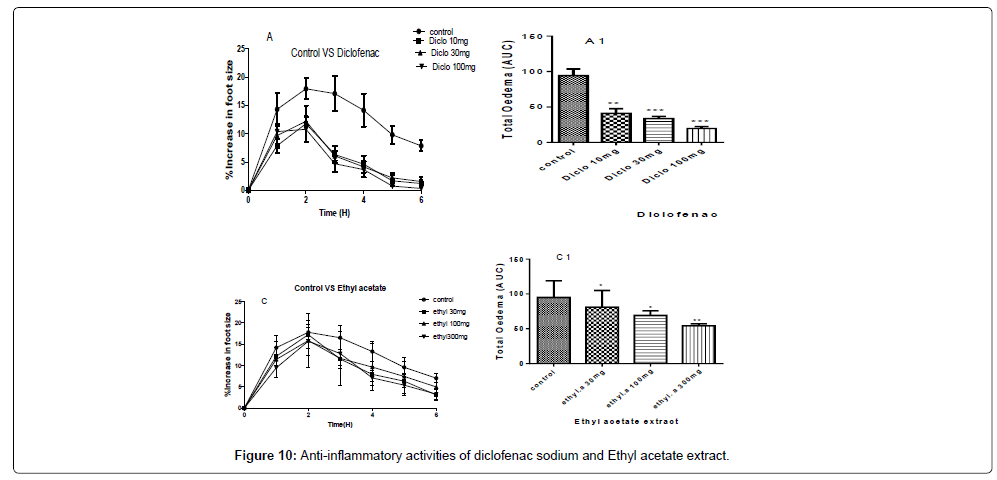
A and C- are the inhibitions of the carrageenan induced paw of the chicks by diclofenac sodium and ethyl acetate extract at concentrations of 100 mg/kg, 30 mg/kg and 10 mg/kg for diclofenac sodium and concentrations of 300 mg/kg, 100 mg/kg and 30 mg/kg for the ethyl acetate extract.
A1 and C1 are the areas under the curve (total oedema) with statistical p-values ranging from, * for P<0.05, ** for P<0.01, *** for P<0.001, showing the degree of significance of the extract activities on the paw size using one way ANOVA and Dunnet’s multiple comparism test (Figure 10).
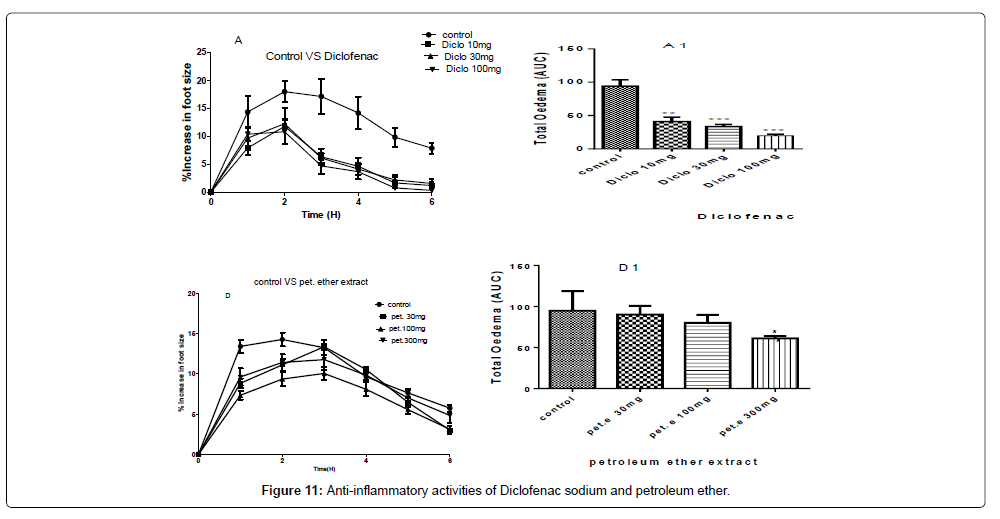
A and D- are the inhibitions of the carrageenan induced paw of the chicks by diclofenac sodium and the petroleum ether extract at concentrations of 100 mg/kg, 30 mg/kg and 10 mg/kg of diclofenac sodium and concentrations of 300 mg/kg, 100 mg/kg and 30 mg/kg of the petroleum ether extract.
A1 and D1 are the areas under the curve (total oedema) with statistical p-values ranging from, * for P<0.05, ** for P<0.01, *** for P<0.001, showing the degree of significance of the extract activities on the paw size using one way ANOVA and Dunnet’s multiple comparism test (Figure 11).
Discussion
Antioxidant assay
The methanol, ethyl acetate and petroleum ether extracts were assessed for their ability to scavenge a stable free radical, DPPH; the total antioxidant capacity and the total phenol content were also evaluated.
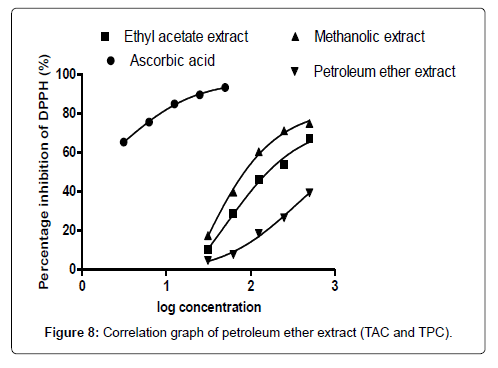
The DPPH scavenging activity evaluated from the antioxidant screening shows that there is a direct relationship between the extract concentration and the antioxidant activity as shown in Figure 1. Thus, when the extract concentration is increased, a corresponding higher antioxidant activity was observed. From the assessment of the antioxidant activity of the extracts, it was observed that as the concentration of the extracts increased, there was a corresponding decrease in the absorbance of the stable DPPH free radicals due to the mopping up of the free radicals by the plant extracts. The IC50 (which is the concentration of the sample needed to decrease the initial DPPH by 50%) values obtained were 27.26 μg/mL (ascorbic acid, standard drug), 46.98 μg/mL (methanol extract), 132.65 μg/ mL (ethyl acetate extract), and 2784.21 μg/mL (petroleum ether). The higher antioxidant activity exhibited by the methanol extract as compared to the ethyl acetate may be due to the concentration and the type of phytochemical constituents in the extracts. Siddique et al., has explained that the variation in antioxidant is due to the nature of the phenolic compounds (thus, from phenolic acids to flavonoids) and not necessarily the content of phenolic compounds, because the activities of phenolic compounds is directly dependent on the chemical structure such as the hydroxyl group in the molecules [30]. The low antioxidant activity in the petroleum ether extract may be due to the absence of flavonoids and tannins (because petroleum ether which is a non-polar solvent cannot extract polar compounds) which are known to have demonstrated some antioxidant activities [31]. Showed that antioxidant activities of medicinal plants are due to the presence of phenolic compounds such as flavonoids and tannins [31]. Mensor et al. [32], has established that phenolic compounds scavenge free radicals by breaking the chain of reaction either by the transfer of hydrogen atom or using single electron transfer based on the structures of the compound, their solubility and coefficient of partition, the bond energy of dissociation and the ionization potential of the compounds [32].
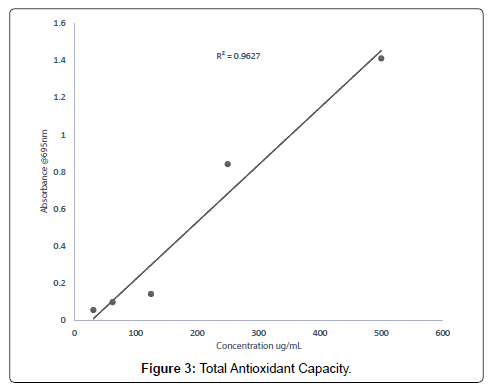
The total antioxidant capacity of the extracts (methanolic, ethyl acetate and petroleum ether) was conducted to assess their ability to reduce Molybdenum (VI) to Molybdenum (V) [33]. The total antioxidants capacity of the methanolic, ethyl acetate and petroleum ether extracts evaluated were 16.37 mg/g, 12.92 mg/g and 5.87 mg/g respectively. From the results obtained, it can be deduced that for every 1 g of methanolic extract, 16.37% was able to scavenge the free radical DPPH, for every 1 g of the ethyl acetate extract, 12.92% of it was able to scavenge the free radical, DPPH and for every 1 g of the petroleum ether extract 5.87% was able to scavenge the free radical DPPH used as compared to the standard drug, ascorbic acid. Report [34], state that, medicinal plants with appreciable free radical scavenging abilities can be used in the management of oxidative stress related conditions in the aged. Research conducted by states that medicinal plants with polyphenols such as tannins, flavonoids, and coumarins demonstrate good antioxidants activities [35].
Total phenol content of the extracts (methanolic, ethyl acetate and petroleum ether) was assessed based on the extracts (methanol, ethyl acetate and petroleum ether) ability to reduce Folin-Ciocalteu reagent. The results obtained from the total phenol evaluations from the extracts were 23.06 mg/g, 18.26 mg/g and 9.34 mg/g for methanolic, ethyl acetate and petroleum ether extracts respectively. It can therefore be inferred that, for every 1 g of the methanolic extract measured 23.05% will react with the reagent Folin-Ciocalteu, for every 1 g of ethyl acetate extract taken 18.26% reacted with the Folin-Ciocalteu and every 1 g of petroleum ether measured 9.34% reacted with the reagent Folin-Ciocalteu as an equivalent to the standard Gallic acid [36]. The variations in the results of the total phenol content of the methanolic, ethyl acetate and petroleum ether extracts is due to the different abilities of the extracts to reduce Folin-Ciocalteu reagent which is a reliable and a common reagent used in accessing antioxidant activities.
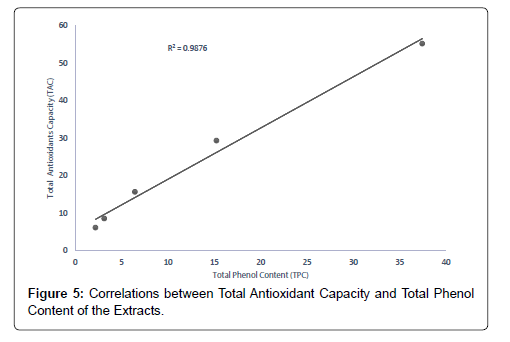
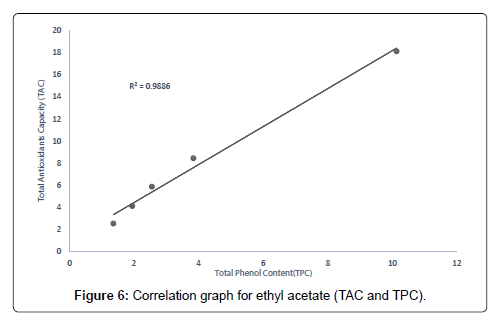
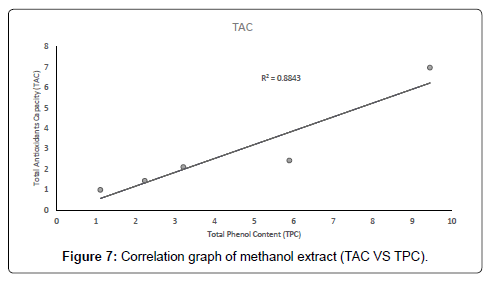
The correlation between the total antioxidant capacity and the total phenol content of the extracts (methanolic, ethyl acetate and petroleum ether) was calculated by establishing the correlation coefficient (r) of the scatter plots as shown in Figures 4-7 respectively. These were 0.9943, 0.9938, and 0.9484 for methanolic, ethyl acetate and petroleum ether extracts respectively. From the correlations coefficient values, it can be inferred that 99.43% of the total antioxidant activities exhibited by the methanolic extract is due to its total phenol content and 99.38% of the total antioxidants activities exhibited by the ethyl acetate extract is due to its total phenol content and also, 94.84% of total antioxidants activities exhibited by the petroleum ether is due to its total phenol content. The total phenol content of the extracts is not always directly proportional to the total antioxidants activities of the extracts because different compounds possess various degrees of antioxidant activities. This corresponds with the findings [37], that Total phenol of an extract is a crude measure of the total phenolic compounds present in the extract and not specific total polyphenol, but many interfering compounds that also react with the reagent giving elevated apparent phenolic concentrations [37].
In-vivo anti-inflammatory activities
The mean ±SEM and the significance of variations among the various concentrations of the extracts (methanolic, ethyl acetate and petroleum ether) and the standard drug were established using oneway ANOVA and Dunnet’s multiple comparism test analysis. From the statistical analysis the methanolic extract showed more in-vivo antiinflammatory activity, followed by ethyl acetate extract and the least activity was observed in petroleum ether extract as shown in Table 8.
The P-values of the methanolic extract at concentration of 300 mg/ kg gave a P˂0.001, and concentration of 100 mg/kg gave P˂0.01 and for the concentration of 30 mg/kg gave P˂0.01, while the percentage inhibitions of paw size were 59.12%, 54.62%, and 51.55% for 300 mg/ kg, 100 mg/kg and 30 mg/kg respectively as shown in Figure 9.
The therapeutic activities of the ethyl acetate extract gave a P-value of P˂0.01 for a concentration of 300 mg/kg and P˂0.05 for 100 mg/ kg and 30 mg/kg with percentage inhibitions of 52.38%, 46.92%, and 42.34% for 300 mg/kg, 100 mg/kg and 30 mg/kg respectively as shown in Figure 10.
The therapeutic activities from the petroleum ether gave a P-value of P˂0.05 for 300 mg/kg but did not show any significant differences for the concentrations of 100 mg/kg and 30 mg/kg as compared to the negative control with the percentage inhibitions of 42.85%, 36.23% and 20.89% for the concentrations of 300 mg/kg, 100 mg/kg and 30 mg/kg respectively as shown in Figure 11.
The standard drug (Diclofenac sodium) at the concentrations of 10 mg/kg, 30 mg/kg and 100 mg/kg gave the P-values of P˂0.001, P˂0.001 and P˂0.001 respectively and the percentage inhibitions of 83.67%, 78.34% and 74.54% for 100 mg/kg, 30 mg/kg and 10 mg/kg respectively. From the results presented, it can be inferred that the anti-inflammatory activities of the extracts are directly related to the concentrations of the extracts, thus, the percentage of inhibitions increases when the concentration of the extracts increase.
The methanolic extract gave more in-vivo anti-inflammatory activities as compared to that of ethyl acetate and petroleum ether extracts. The percentage of inhibitions shown by the methanolic and ethyl acetate extracts may be due to the presence of flavonoids, tannins, anthraquinones, glycosides and saponins while the percentage inhibitions exhibited by the petroleum ether might be due to the absence of flavonoids, tannins, anthraquinones, glycosides and saponins which are known to possess some anti-inflammatory activity have shown that, most of the constituents in herbal preparations have some form of activity against inflammations reported that, some groups of phenolic compounds such as flavonoids and tannins have the capacity of inhibiting some mediators of inflammation which help in contributing to the fight against inflammations of body tissues [38-40]. The results obtained from the anti-inflammatory activities of the stem bark of Heisteria parvifolia corresponds with the findings of the anti-inflammatory activities of flavonoids on the proliferative and the exudative stages of inflammations in laboratory rats, especially when the flavonoids contain gallotannins and quercitin or quercitin-like structures [40]. The anti-inflammatory activity of phenolic compounds can also be confirmed by the work of that, phenolic compounds such as gallotannins, condensed tannins and flavonoids respond to inflammation by blocking cyclooxygenases, cytokines, nuclear factorkappa B and metalloproteinases using different mechanisms of action.
From the results of the anti-inflammatory screening, it can be observed that at concentrations of 30 mg/kg, 100 mg/kg and 300 mg/kg, the methanolic extract showed significant activity against inflammation and the ethyl acetate at concentration of 100 mg/kg and 300 mg/kg showed significant anti-inflammatory activities but the petroleum ether at a concentration of 300 mg/kg shows only some activity against inflammations.
Author’s Contributions
The concept of this research was initiated by Sylvenus Aguree and made possible through his hard work. All laboratory analysis was conducted by same including the drafting of this manuscript. Peter Jagri Onilimor provided critical review of the manuscript and approved the manuscript for submission
Conflicts of Interest
No conflict of interest exists.
References
- Rousseaux CG, Schachter H (2003) Regulatory issues concerning the safety, efficacy and quality of herbal remedies. Birth Defects Research. Part B, Develop Reprod Toxicol 68: 505-510.
- Tang JL, Liu BY, Ma KW (2008) Traditional Chinese medicine. Lancet 372: 1938-1940.
- Zamble AM, Kandoussi A, Petrault OF, Martin-Nizard FJ (2006) Cardiovasc Pharmacology Laboratoire de Pharmacognosie. Faculté de Pharmacie 2: 143-159.
- Evans WC (2002) Trease and Evans Pharmacognosy, 15th edition. WB Sauders Company Ltd, London, pp: 137-139.
- Hanson JA (2003) Natural products; the secondary metabolites. The Royal Society of Chemistry, Cambridge, pp: 34-38.
- Namdeo AG (2007) Plant cell elucidation for production of secondary metabolites: Pharmacognosy. Review 1: 69-79.
- Oskman-Caldentelyl KM, Inze D (2004) Plant cell factories in the post-genomic Era; new ways to produce Designer secondary metabolites. Trends plant science 9: 433-440.
- Bent S, Ko R (2004) Commonly used herbal medicines in the United States: a review. Am J Med 116: 478-485.
- Ashafa AOT, Sunmonu TO, Afolayan AJ (2010) Toxicological evaluation of aqueous leaf and berry extracts of Phytolacca dioica L. in male Wistar rats. Food Chem Toxicol 48: 1886-1889.
- Keay RWJ (1958) Olacaceae in Flora of West Tropical Africa. Crown Agents for Oversea Governments and Administrations, London, United Kingdom 1: 644-649.
- Hall JB, Swaine MD (1981) Distribution and ecology of vascular plants in a tropical rain forest: forest vegetation of Ghana. W Junk Publishers, the Hague, Netherlands, p: 383.
- Lucas GLL (1968) Olacaceae in Flora of Tropical East Africa. Crown Agents for Oversea Governments and Administrations, London, United Kingdom, p: 16.
- Hawthorne WD (1995) Ecological profiles of Ghanaian forest trees. Tropical Forestry Papers, Oxford Forestry Institute, Department of Plant Sciences, University of Oxford, United Kingdom 29: 345.
- Irvine FR (1961) Woody plants of Ghana, with special reference to their uses. Oxford University Press, London, United Kingdom, p: 868.
- Louis J, Léonard J (1948) Olacaceae In Flore du Congo belge et du Ruanda-Urundi. Spermatophytes. Institute National pour l’Étude Agronomique du Congo belge, Brussels, Belgium. Asian Journal of Plant Science and Research 1: 249-278.
- Malaisse F, N’Gasse G, Lognay G (2004) Heisteria parvifolia (Olacaceae), an underestimated shrub or small tree with oil-producing seeds. Systematics and Geography of Plants 74: 17-25.
- Ayensu I, Quartey AK (2014) Phytochemical Screening and In-Vitro Antioxidant Properties of the Stem Bark of Trichilia Tessemannii. World Journal of Pharmacy and Pharmaceutical Sciences 4: 76-90.
- Giorgio M, Migliaccio E, Orsini F (2005) Electron transfer between cytochrome-C and reactive oxygen species that trigger mitochondrial apoptosis. Cell 122: 221-233.
- Cole GM, Lim GP, Yang F, Teter B, Begum A, et al. (2005) Prevention of Alzheimer's disease: Omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol Aging 26: 133-136.
- Hall JB, Swaine MD (1981) Distribution and ecology of vascular plants in a tropical rain forest: forest vegetation of Ghana. W Junk Publishers, Hague, Netherlands, p: 383.
- Lucas GLL (1968) Olacaceae in Flora of Tropical East Africa. Crown Agents for Oversea Governments and Administrations, London, United Kingdom, p: 16.
- Ragab EA, Hosny M, Kadry HA, Ammar HA (2010) Flavanone glycosides from Gleditsia caspia. J Nat Prod 3: 35-46.
- Sies H (1997) Oxidative Stress: Oxidants and Antioxidants. Exeritnental Physiology 82: 291-295.
- Hasegawa T, Tanaka A, Hosoda A, Takano F, Ohta T (2008) Antioxidant C-glycosyl flavones from the leaves of Sasa. kurilensis var gigantean, Phytochemistry 6: 1419-1424.
- Boudet A (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68: 2722-2735.
- Roach JT, Sufka KJ (2003) Characterization of the chick carrageenan response. Brain research 994: 216-225.
- Stickel F, Patsenker E, Schuppan D (2005) Herbal hepatotoxicity. J Hepatol 43: 901-910.
- Siddique NA, Mujeeb M, Najmi AK, Akram M (2010) Evaluation of antioxidant activity, quantitative estimation of phenols and flavonoids in different parts of Aegle marmelos. Afr J Plant Sci 4: 1-5.
- Hurrell R (2003) Influence of vegetable protein sources on trace element and mineral bioavailability. Asian pacific journal 133: 25-31.
- Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123: 1173-1183.
- Mensor TA, Mateus N, Machado M (2001) Estimation of antiradical properties of antioxidants using DPPH assay: Critical review and results. Food Chem 79: 61-67.
- Firenzuoli F, Gori L (2007) Herbal medicine today: Clinical and research issues. Evid Based Complement, Alternat Med 4: 37-40.
- Sies H, Stahl W (2003) Carotenoids and UV protection. Photochem Photobiol Sci 3: 749-752.
- Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical biochemistry 269: 337-341.
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N (2006) Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr Journal Biotech 5: 1142-1145.
- Adyemi O, Okpo SO, Ogunti O (2002) Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea a mericana mill (Lauraceae). Fitoterapia 73: 375-380.
- Bagul MS, Srinivasa H, Kanaki NS, Rajani M (2005) Anti-inflammatory activity of two Ayurvedic formulations containing guggul. Indian J Pharmacol 37: 399-400.
- Fawole OA, Ndhlala AR, Amoo SO, Finnie JF, Staden JV (2009) Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. Ethno pharmacology 123: 237-243.
- Guabiraba R, Lemos VS, Mauro M, Souza ALS, Lugnier C, et al. (2010) The flavonoid dioclein reduces the production of pro-inflammatory mediators in vitro by inhibiting PDE4 activity and scavenging reactive oxygen species. European Journal of Pharmacology 633: 85-92.
Citation: Aguree S, Onilimor PJ (2020) Assessing the Antioxidants and the Anti- Inflammatory Activities of Methanolic, Ethyl Acetate and Petroleum Ether Extracts of the Stem Bark of Heisteria parvifolia. Clin Pharmacol Biopharm 9: 191. DOI: 10.4172/2167-065X.1000191
Copyright: © 2020 Aguree S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2328
- [From(publication date): 0-2020 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1596
- PDF downloads: 732