Assessing Pito Contamination of Heavy Metals (Pb, Cu And Zn) from Local Aluminium Pots in Navrongo
Received: 07-Apr-2021 / Accepted Date: 14-Apr-2021 / Published Date: 21-Apr-2021
Abstract
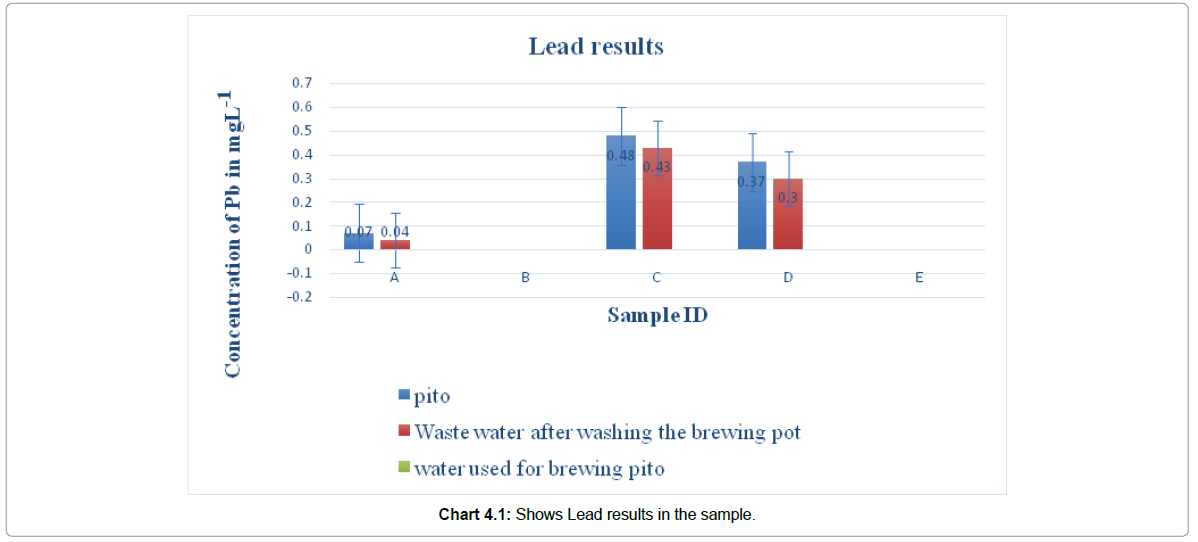
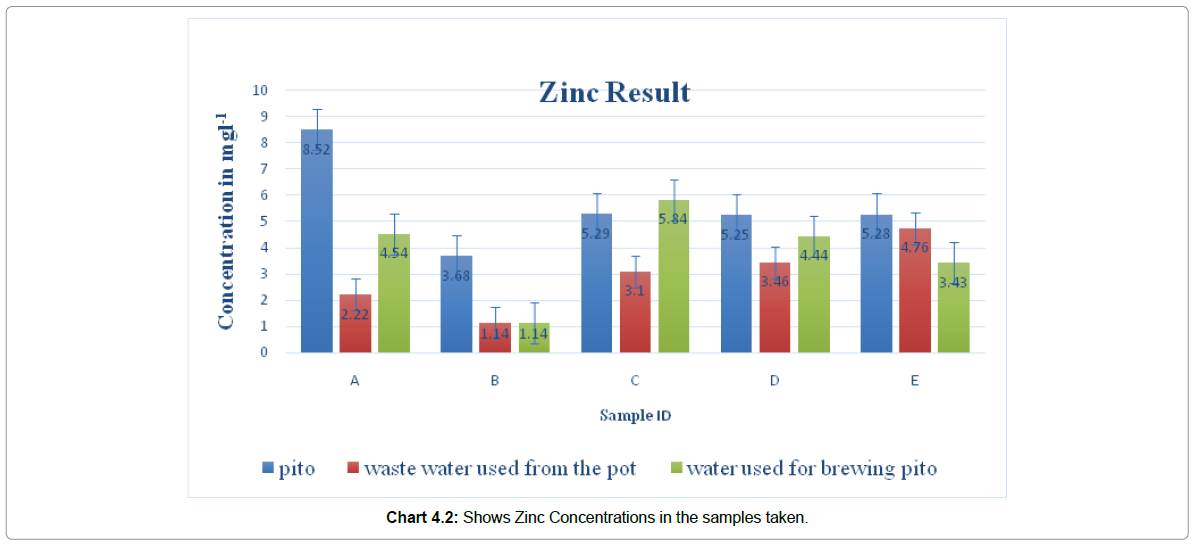
A heavy metal is any metallic element that has relatively high density, some of which are toxic or poisonous even at low concentrations. Example Pb, Cr, Hg, etc. Exposure to heavy metals can lead to a high proportion of mortality caused by Kidney and Liver morbidity, pains in bones, mutagenic, carcinogenic and teratogenic effects (Ndiokwere, 2004)The study was aimed at assessing pito contamination of heavy metals (Pb, Cu, and Zn) from local Aluminum pots used for brewing pito in Navrongo. Fivepito brewers were randomly selected and their pito samples, waste water after washing the brewing pot, and water used for brewing and for washing the pots were obtained. These were digested using aqua regia and analyses for Pb, Zn, and Cu using AAS. The mean concentrations of the metals in mg/L ranged from not detected to 0.48 for Pb ; not detected for Cu; and 3.68 to 8.52 for Zn in pito, 1.14 to 4.76 for Zn in waste water and 1.14 to 5.84 for Zn in water used for brewing pito. In conclusion the result show that Zinc and Copper contamination may not be due to the local Aluminum pots used for brewing pito but Lead contamination may be due to the local Aluminum pots used for brewing pito.
Keywords: Pito, Atomic absorption Spectrometer, Aqua regia, Local Aluminium pots, Heavy metals, AOAC, Deionized water
Introduction
A heavy metal can be defined as any metallic element that has a relatively high density and is toxic or poisonous even at low concentration. (Cobbina et al., 2015). Contamination of heavy metals and its accumulation in the environment is a serious problem around the world due to the potential threat to food safety and its detrimental effects on human health (Nriagu, 1990). This is a source of worry as high levels of heavy metals in human system possess a risk to human health. Contamination of foods and drinks can occur during agricultural production of crops (Lar, 2014) or during preparation of these agricultural products into palatable food or drinks (Anderson, 1992). The heavy metal contaminants can come from the ingredients, water and or food wares used when they contain high levels of heavy metals. The different conditions such pH, type of food or high temperature reached during cooking can cause heavy metals to migrate (leach) from the material into food or beverages if the material contains high levels of heavy metals (Hermogene et al., 2012). In humans, metallic toxicants accumulate and biomagnify attacking proteins notable the enzymes (Ademoroti, 1990). Heavy metals have been implicated in upsurge of liver and kidney morbidity, pains in bones, mutagenic, carcinogenic and teratogenic effects (fischer, 1987) [1-8]. The local blacksmiths do not have Pure Aluminium but extract used canisters and any material that is suspected to contain Aluminium by melting them at high temperatures. It is alleged that Lead from car batteries are often added and alloyed to Aluminium to reduce the melting temperature. Pito is a type of beer made from fermented millet or sorghum in northern Ghana, parts of Nigeria and other parts of West Africa. It is made by small (household level) producers and typically serve in a calabash outside the producer’s home. It can be served warm or cold. Aside serving as an inebriating drink, pito is important in fulfilling social obligation such as marriages, naming and funeral ceremonies, parties and other social gatherings (Aaron et al., 2014). The research seeks to asses pito contamination of heavy metals (Cu, Pb and Zn) from local Aluminium pots used for brewing pito in Navrongo and to initiate an investigation into a possible source of contamination that may been revealed in human body scans in Navrongo to have contained some heavy metals [9-12].
Materials and Methods
Materials
Apparatus: The following apparatus were used in order to perform the project work. 250 ml conical flasks, measuring cylinders, 10 ml pipettes, 100 ml volumetric flasks, funnel, stopwatch, plastic bottles, pipette and pipette sucker, hot plate, filter papers.
Chemicals/reagents used: Conc. HCL from Department of Applied Chemistry and Biochemistry Laboratory chemical store (UDS Navrongo Campus).
Conc. HNO3 from Department of Applied Chemistry and Biochemistry Laboratory chemical store (UDS Navrongo Campus).
Deionized water from Navrongo Senior High School (NAVASCO) laboratory
Methods
Sample taking and preparation
The pito brewers were randomly selected in Navrongo (5 women) Five (5) replicates of pito were taken from every woman Five (5) replicate of the waste water after washing the Aluminium pot was taken and three (3) replicates of the water for washing the pots and for brewing were sampled into HCl rinsed and further rinsing in distilled water polyethylene bottles with capacity 200 ml. The samples were sent to laboratory and put in a fridge at 2.0oC pending digestion and analyses.
Sample digestion
Samples were digested according to the method described by the Association of Official Analytical Chemists (AOAC, 2006) modified. The samples were pipette (10 ml) into a100 ml conical flask and 10 ml aqua regia added and then put on a hot plate at 100ºC and boiled for 30 minutes. The sample were cooled transferred into 100 ml standard flasks and topped up to the mark. These samples were analysed for Pb, Cu and Zn on an AAS.
Sample analyses
In a laboratory-based work or experiment, heavy metal content (Pb, Cu and Zn) in pito and waste water after washing the local Aluminium pot used for brewing pitowere analyzed using AAS (Agilent Technologies Australia 200 series AA).
Results and Discussions
Results
Key
Replicate 1 = water used for brewing pito
Replicates 2 & 3 =washings from the pots used for brewing pito
Discussions
Concentration of Copper (Cu) in pito and water used for brewing in Navrongo recorded in this study was below detectable range by 200 Series AA of Agilent Technologies. Of the twenty-five (25) samples from five (5) selected different women in Navrongo, no appreciable Copper concentration was recorded. This result is comparable to levels found in literature. For example, Chukwujindu et al. (2014) reported Copper concentrations in the range of not detected to 0.08 mg/L in pito samples. The concentrations of Zinc (Zn) in the water used for brewing pito obtained from five different brewers in Navrongo were: 4.54 mgL-1, 1.14 mgL-1, 5.84 mgL-1, 4.44 mgL-1, and 0.43 mgL-1 for samples A, B, C, D, and E respectively.However, the mean concentrations of Zinc (Zn) from the washings of the brewing pots were: 2.22 mgL-1, 1.04 mgL-1, 2.00 mgL-1, 2.46 mgL-1, and 4.35 mgL-1 for samples A, B, C, D, and E respectively. From the above figures, it can be observed that apart from the sample E, whose Zn concentration in the washings from the pot is higher than that of water used for brewing, all other samples have their Zn concentrations in the washings from the pots lower than the concentrations in the water used for brewing pito. Could it be that, there is probably something in the burnt malt under the pots that is absorbing some of the Zinc? This could also be that some of the Zinc precipitate in the process of washing the pot. The decrease could also be attributed to the stacking of Zinc onto the Aluminum pot. The concentrations of Zn in these pito samples from five different brewers, were in the range of 3.64-9.87 mg/L. The highest mean concentration of Zn was observed in sample A while the lowest was observed in sample B as shown in chart 4.1 & 4.2. From one-way ANOVA analysis the is a significant difference in zinc concentration in the five different samples taking The distribution pattern of Zn in these five different pito samples followed the order: A ˃ E˃ D ˃ C ˃ B. The different concentrations of Zinc of the same species of cereal crops used in the brewing can partially be explained by the different sources of water used for the brewing as the concentrations in the Water varies from woman to woman as showed in Table 4.5. Also, this difference could also result from the different soils on which these cereal crops were cultivated, pollution, industrial region, and use of pesticides or fertilizers as reported by Serfor-Armah et al. (2003).
| Sample ID | Replicate1 (Cu Conc.mgL-1 |
Replicate2 (Cu conc. mgL-1 |
WHO Guidelines mgL-1 |
|---|---|---|---|
| A | ≤0.02 | ≤0.02 | 2 |
| B | ≤0.02 | ≤0.02 | 2 |
| C | ≤0.02 | ≤0.02 | 2 |
| D | ≤0.02 | ≤0.02 | 2 |
| E | ≤0.02 | ≤0.02 | 2 |
Table 4.1: Copper concentration in pito brewed in Navrongo.
| Sample ID | Replicate1 (Cu Conc.mgL-1 |
Replicate2 (Cu Conc.mgL-1 |
Replicate3 (Cu Conc.mgL-1 |
WHO Guidelines mgL-1 |
|---|---|---|---|---|
| A | ≤0.02 | ≤0.02 | ≤0.02 | 2 |
| B | ≤0.02 | ≤0.02 | ≤0.02 | 2 |
| C | ≤0.02 | ≤0.02 | ≤0.02 | 2 |
| D | ≤0.02 | ≤0.02 | ≤0.02 | 2 |
| E | ≤0.02 | ≤0.02 | ≤0.02 |
Table 4.2: Copper concentration in the water used for brewing pito in Navrongo.
Conclusion
The results of the present study indicated that Copper (Cu) occurred in pito at concentrations below WHO limits permissible in alcoholic beverages.However, of the five different pito sampled, four (4) representing 80% have Zinc concentrations higher than the WHO Guidelines of 5 ml/L in beverages. This implied that consumers of pito in Navrongo are likely to suffer from Zn poisoning. Zinc poisoning results in abdominal pain, nausea, vomiting, anaemia, dizziness, and Zinc induced Copper deficiency.The low Zn concentration from the washings from the pots as compare to the concentration in the water used for brewing pito suggest that Zn contamination of pito is not from the local Aluminium pots used for brewing Pito in Navrongo. Also, the relatively high levels of Zinc, ranging from 1.14 mgL-1 to 4.54 mgL-1 in the water used for brewing may contribute to the high levels of zinc found in pito
References
- Aaron N A, Apori N, Samuel AB (2014) Analysis of essential elements in pito-a cereal food drink and its brands by the single- comparator method of neutron activation analysis. Food Sci Nutr 2(3): 230-233.
- Ademoroti C M (1996) Environmental Chemistry and Toxicology 2nd ed, Foludex Press Ltd, Ibadan, pp 412-415
- Agren MS (1990) Percutaneous absorption of zinc from zinc oxide applied topically to intact skin in man. Dermatologica 180: 36-39
- Agren MS, Krusell M, Franzen L (1991) Release and absorption of zinc from zinc oxide and zinc sulfate in open wounds. Acta Dermato-Venereol 71(4): 330-333.
- Anderson RA, Bryden NA, Polansky MM (1992) Dietary Chromium intake Freely chosen diets, institutional diet and individual foods. Biol Trace Elem Res 31: 117-121
- AOAC (2006) Official Methods of Analysis 18th Edition, Association of Official Analytical Chemists, Gaithersburgs, MD.
- Chukwujindu MA, Anwuli LO, Francisca IB (2014)A survey of metal profiles in some traditional alcoholic beverages in Nigeria. Food Sci Nutr 2(6): 725-727
- Cobbina SJ, Abudu BD, Reginald Q, Samue O, Noel B (2015) Comparative Assessment of Heavy Metals in Drinking Water Sources in Two Small-Scale Mining Communities in Northern Ghana. Int J Environ Res Public Health 12(2): 10620-10625
- Fischer A B (1987) Mutagenic effects of Cd alone and in combination with antimutagenic selenite. Proceedings of 6th International Conference on Heavy Metals in the Environment 2 pp 112-114
- Hermogene N, Munyentwali A, Muhayimana P, Muhizi T (2012) Assessment of heavy metals leachability from traditionalclay pots“inkonoâ€and“ibibindi†used as food contact materials. Life Natural Science 15(8): 2-3
- Lar UC (2014) Locally made utensils as potential sources of heavy metals contamination of water: A case study of some pots made in Nigeria. American J Environ Protection 3: 35-40
- Nriagu JO (1996) A history of global metal pollution. J Environ Sci 23(5): 223-224
Citation: Njorfuni C (2021) Assessing Pito Contamination of Heavy Metals (Pb, Cu And Zn) from Local Aluminium Pots in Navrongo. J Nutr Sci Res 6: 140.
Copyright: © 2021 Njorfuni C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 3434
- [From(publication date): 0-2021 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 2554
- PDF downloads: 880