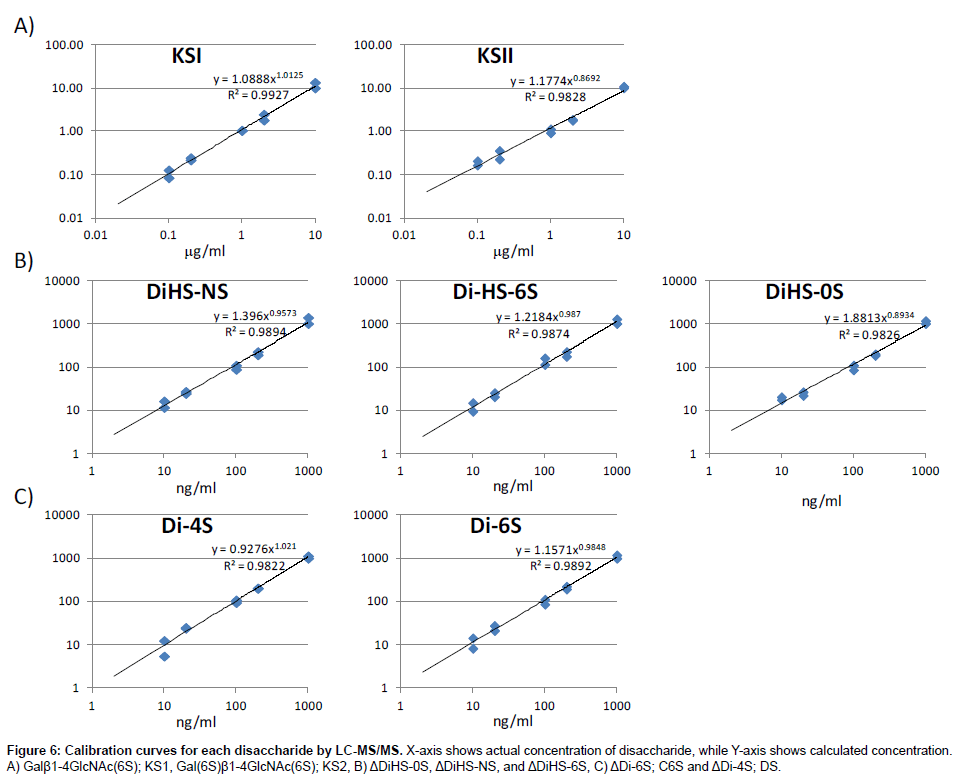Research Article Open Access
Assay for Glycosaminoglycans by Tandem Mass Spectrometry and its Applications
Shunji Tomatsu1*, Tsutomu Shimada1, Robert W Mason1, Joan Kelly2, William A LaMarr2, Eriko Yasuda1, Yuniko Shibata3, Hideyuki Futatsumori3, Adriana M Montaño4, Seiji Yamaguchi5, Yasuyuki Suzuki6 and TadaoOrii7
1 Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE, USA
2 Agilent Technologies, Inc., Wakefield, MA, USA
3 Central Research Lab., R&D Div. Seikagaku Co. Tokyo, Japan
4 Department of Pediatrics, Saint Louis University, St. Louis, Missouri, USA
5 Department of Pediatrics, Shimane University, Izumo, Japan
6 Medical Education Development Center, Gifu University, Japan
7 Department of Pediatrics, Gifu University, Gifu, Japan
- *Corresponding Author:
- Prof. Shunji Tomatsu, M.D., Ph.D.
Director, Skeletal Dysplasia Center
Nemours Biomedical Research
Nemours/Alfred I. duPont Hospital for Children
1600 Rockland Rd., Wilmington, DE. 19899-0269, USA
Tel: 302-298-7336
Fax: 302-651-6888
E-mail: stomatsu@nemours.org
Received date: December 16, 2013; Accepted date: January 30, 2014; Published date: February 01, 2014
Citation: Tomatsu S, Shimada T, Mason RW, Kelly J, LaMarr WA, et al. (2014) Assay for Glycosaminoglycans by Tandem Mass Spectrometry and its Applications. J Anal Bioanal Tech S2:006. doi: 10.4172/2155-9872.S2-006
Copyright: © 2014 Tomatsu S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Glycosaminoglycans (GAGs) are distributed in the whole body and play a variety of important physiological roles associated with inflammation, growth, coagulation, fibrinolysis, lipolysis, and cell-matrix biology. Accumulation of undegraded GAGs in lysosomes gives rise to a distinct clinical syndrome, mucopolysaccharidoses. Measurement of each specific GAG in a variety of specimens is urgently required to understand GAG interaction with other molecules, physiological status of patients, and prognosis and pathogenesis of the disease.
We established a highly sensitive and accurate tandem mass spectrometry (LC-MS/MS) method for measurements of disaccharides derived from four specific GAGs [dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), and chondroitin sulfate (CS)]. Disaccharides were produced by specific enzyme digestion of each GAG, and quantified by negative ion mode of multiple reaction monitoring. Subclasses of HS and GAGs with identical molecular weights can be separated using a Hypercarbcolumn (2.0 mm×50 mm, 5 μm) with an aectonitrile gradient in ammonium acetate (pH 11.0).
We also developed a GAG assay by RapidFire with tandem mass spectrometry (RF-MS/MS). The RF system
consists of an integrated solid phase extraction robot that binds and de-salts samples from assay plates and directly injects them into a MS/MS detector, reducing sample processing time to ten seconds. RF-MS/MS consequently yields much faster throughput than conventional LC-MS/MS-based methods.
However, the RF system does not have a chromatographic step, and therefore, cannot distinguish GAGs that
have identical molecular weights. Both methods can be applied to analysis of dried blood spots, blood, and urine specimens.
In this article, we compare the assay methods for GAGs and describe their potential applications.
Keywords
Tandem mass spectrometry; Glycosaminoglycans; Mucopolysaccharidoses; Chromatogram; Newborn screening
Abbreviations
CS: Chondroitin Sulfate; CNS: Central Nervous System; DBS: Dried Blood Spot; DM: Diabetes Mellitus; DMB: Dimethyl Methylene Blue; DS: Dermatan Sulfate; GAG: Glycosaminoglycans; HS: Heparan Sulfate; KS: Keratan Sulfate; KS1: Galβ1-4GlcNAc(6S); KS2: Gal(6S)β1-4GlcNAc(6S); LC-MS/MS: High Performance Liquid Chromatography Tandem Mass Spectrometry; MPS: Mucopolysaccharidoses; MRM: Multiple Reaction Monitoring; OA: Osteoarthritis (OA); NBS: Newborn Screening; RF-MS/MS: RapidFire Tandem Mass Spectrometry
Introduction
Glycosaminoglycans (GAGs) comprise chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), and hyaluronan. These GAGs are found in various tissues and are major components of the extracellular matrix (ECM). GAGs (except for hyaluronan) are sulfated polysaccharides made of repeating disaccharides, consisting of uronic acid (or galactose) and hexosamines. Polymeric GAGs are covalently bound through a linkage region to core proteins to yield proteoglycans (PGs), which have various biological functions. PGs play important roles in control of growth and differentiation. Particular sulfation patterns in the GAG chains allow interactions, normally of ionic nature, with growth factors. The PG protein cores are not just scaffolds for GAGs: they contain domains that have particular biological activities [1]. Many PGs are thus multifunctional molecules that engage in several different specific interactions simultaneously.
Mucopolysaccharidoses (MPS) are a group of lysosomal storage diseases caused by deficiency of the lysosomal enzymes required for degradation of GAGs. There are 11 known enzyme deficiencies, resulting in seven distinct forms of MPS with a collective incidence of approximately 1 in 25,000 live births. GAG accumulation causes a progressive damage of multiple tissues including brain, lung, heart, liver, kidney, joint, and bone. Most clinical signs and symptoms for MPS patients do not appear immediately after birth but onset of clinical signs and symptoms progresses with age. Although the symptoms and severity of MPS vary with each individual patient and its subtype of MPS, the average life span in most patients is one to two decades if untreated.
Currently, enzyme replacement therapy (ERT), hematopoietic stem cell transplantation, substrate reduction therapy and gene therapy are in clinical use or being investigated in clinical trials for patients with some types of MPS. Starting these treatments at birth or at very early stages has the greatest positive impact on the clinical course of the disease [2]. To provide a better efficacy and quality of life of the patients, early diagnosis and early treatment as well as accurate prognosis and monitoring are essential.
Newborn Screening (NBS) is recognized as an essential, preventive public health program for early identification of diseases in newborn that can affect their long-term health. Tandem mass spectrometry (MS/MS) has evolved as a powerful tool to detect small compounds because detection is based on the mass of the parent compound and a specific fragment(s) of the compound. Other sensitive detection methods, such as amperometric electrochemical detection, do not detect specific compounds and consequently quantitation will be compromised if other compounds co-elute with target compound after chromatographic separation. This is a particular concern in our studies of multiple disaccharides that have similar chromatographic and molecular properties. We developed a method that can assay DS, HS, and KS simultaneously in blood and/or urine samples using high performance liquid chromatography tandem mass spectrometry (LCMS/ MS) for MPS [3-10]. The LC-MS/MS method could not only show sensitivity and specificity for detecting all subtypes of MPS but is also able to monitor therapeutic efficacy in MPS patients and animal models [2,6,10-12]. We show here that CS can be also measured simultaneously using a modification of this technique.
The main drawback of the LC step is that the process is time consuming, limiting its utility for the screening of large numbers of NBS samples. The use of the RapidFire (RF) high-throughput mass spectrometry system removes the bottleneck of throughput, while maintaining the quality and reliability of standard MS/MS read-outs. Samples are absorbed to a matrix to concentrate and desalt, and then eluted directly into the MS/MS without chromatographic separation. Each sample is processed in less than ten seconds, yielding much faster throughput than conventional LC-MS/MS based methods. A single 384 well plate can be read in ~45 min, indicating that this RF-MS/ MS system can analyze over one million samples annually. Moreover, the RF-MS/MS has been shown to provide sensitivity and specificity equivalent to a scintillation proximity assay [13], and LC-MS/MS system in some applications. [14]. The speed and efficiency of the RF can allow for experiments that would otherwise be deemed “untenable” under normal circumstances. The RF system has been validated as suitable for many drug discoveries [13,15-21], and ADME (Absorption, Distribution, Metabolism and Excretion) based applications [14].
Overall, establishment of GAG assays by tandem mass spectrometry are urgently required to apply to not only clinical indications but basic research. In this article, we describe GAGs assay methods by using LCMS/ MS and RF-MS/MS, indicating their application in clinical and basic research.
Procedures of Tandem Mass Spectrometry for Assay of GAGs
Standards and enzymes
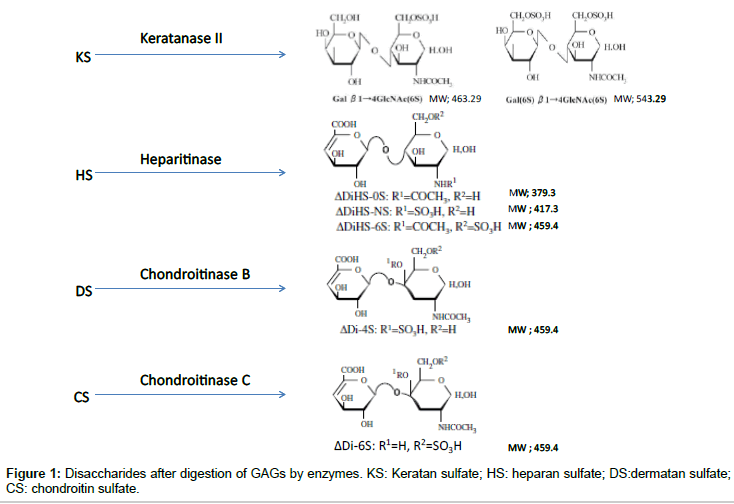
To digest “polymer” CS, DS, HS, and KS to disaccharides, the enzymes of chondroitinase C, chondroitinase B, heparitinase, and keratanase II, respectively, were provided from Sigma-Aldrich (St. Louis, MO) and Seikagaku Co. (Tokyo, Japan). Chondrosine (Seikagaku Co.) was used as an internal standard (IS), while unsaturated disaccharides, [ΔDiHS-0S, 2-acetamido-2-deoxy-4-O-(4-deoxy-a-L-threo-hex- 4-enopyranosyluronic acid)-D-glucose; ΔDiHS-NS, 2-deoxy-2- sulfamino-4-O-(4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-D-glucose; ΔDi-4S/6S (DS or CS), 2-acetamido-2-deoxy-4-O- (4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-4 or 6-O-sulfo- D-glucose] were used for making standard curves. Stock solutions of ΔDi-6S (250 μg/ml), ΔDi-4S (250 μg/ml), ΔDiHS-0S (100 μg/ml), ΔDiHS-NS (100 μg/ml), ΔDiHS-6S (100 μg/ml), and IS (5 mg/ml) were prepared separately in ddH2O. Mono-sulfated KS [KS1; Galβ1- 4GlcNAc(6S)] and di-sulfated KS [KS2; Gal(6S)β1-4GlcNAc(6S)] were made by digestion of bovine cornea KS with keratanase II, since these disaccharides were not available. Sixty percent of KS-I was digested with keratanase II and that the ratio of Gal(6S)β1-4GlcNAc(6S) (KS1) toGalβ1-4GlcNAc(6S)(KS2) was 42:58 [22]. A stock solution of digested KS (1000 μg/ml KS1+KS2) in ddH2O was prepared.
For standard curves, dilutions of the stock solutions were prepared as follows: ΔDi-6S, ΔDi-4S, ΔDiHS-0S, ΔDiHS-NS, and ΔDiHS-6S (1, 5, 10, 50, 100, 500, and 1000 ng/ml), and digested KS (0.01, 0.05, 0.1, 0.5, 1, 5, and 10 μg/ml), each containing IS solution (50 ng/ml).
ΔDi-4S is composed of DS, and ΔDi-C6S derived from CS, while ΔDiHS-0S, ΔDiHS-NS, and ΔDiHS-6S are subclasses of HS.KS comprises KS1 and KS2 (Figure 1). Note that ΔDi-4S, ΔDiHS-6S, and ΔDi-C6S have identical molecular weights.
Sample preparation
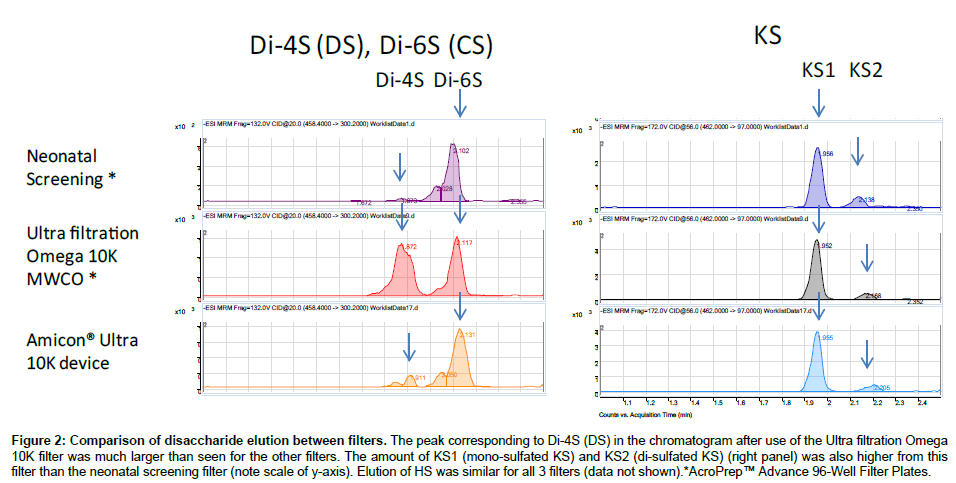
Purified polymer CS (shark cartilage), DS (pig skin), HS (bovine kidney), and KS (bovine cornea) were digested by a mixture of chondroitinase C (Sigma-Aldrich Co, St. Louis MO), chondroitinase B, heparitinase, and keratanase II (all other reagents except chondroitinase C provided by Seikagaku Co). Samples to be measured were centrifuged to remove insoluble material. Ten μL of the supernatant was mixed with 90 μL of 50 mM Tris-HCl Buffer (pH 7.0), and then added to a filter well. This was centrifuged at 2,500 g for 15 min to deplete low molecular compounds that could interfere with the assay. The filters were transferred to fresh plates and 20 μL of internal standard (500 ng/mL chondrosine), 20 μL of 50 mM Tris-HCl buffer and 10 μL of chondroitinase C, chondroitinase B, heparitinase, and keratanase II, respectively, (each 2 mU/10 μL of 50 mM Tris-HCl buffer) were added onto each filter. Samples were incubated with shaking at 37°C for 15 hr to digest the GAGs. Filter plates were then centrifuged at 14,000 g for 15 min. Twenty μL of ddH2O was added to each flow-through sample, and mixed by vortexing for 10 sec. Samples were stored at -20°C until injection to LC-MS/MS or RF-MS/MS. In a preliminary study we tested three different filter systems for efficiency of extraction and recovery: AcroPrepTM Advance 96-Well Filter Plates, with Ultra filtration Omega 10K(PALL Co., NY, USA); Neonatal Screening, AcroPreptTM Advance 96-Well Filter Plates (PALL Co.); and Amicon Ultra 10K device (Milipore Co.) and monitored recovery of Di4S and KS (Figure 2). The optimized Di-4S assay also detects Di-6S because these disaccharides have the same molecular mass and yield similar fragments. The KS assay detects both mono and disulfated KS because the fragmentor can remove a sulfate from the disulfated form before it enters the mass spectrometer. The filter with the ultrafiltration Omega 10K filters gave the highest yield of disaccharides, especially for Di-4S.
Figure 2: Comparison of disaccharide elution between filters. The peak corresponding to Di-4S (DS) in the chromatogram after use of the Ultra filtration Omega 10K filter was much larger than seen for the other filters. The amount of KS1 (mono-sulfated KS) and KS2 (di-sulfated KS) (right panel) was also higher from this filter than the neonatal screening filter (note scale of y-axis). Elution of HS was similar for all 3 filters (data not shown).*AcroPrep™ Advance 96-Well Filter Plates.
Equipment
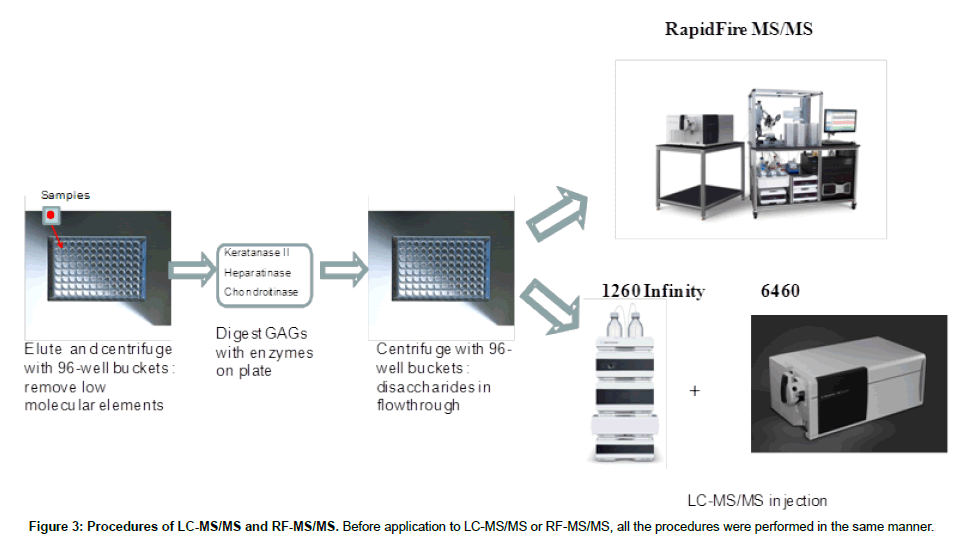
Liquid chromatography tandem mass spectrometry (LC-MS/ MS): The instrument used for high performance liquid chromatography tandem mass spectrometry (LC-MS/MS) was a 1260 infinity LC /6460 Triple Quad (Agilent Technologies, Palo Alto, CA, USA). Samples were removed from the -20°C and were allowed to thaw at an ambient temperature. Sample tubes were quickly spun by using a VWR Galaxy Ministar centrifuge to collect all samples to the bottom of the tube. Twenty-five μL of each sample was transferred to a 96 well polypropylene plate, and diluted with 25 μL of 50 mMTris buffer (pH 7.5). The plate was centrifuged at 3,500 g for 5 min prior to analysis with the 1260 infinity LC /6460 Triple Quad (Figure 3).
RapidFire tandem mass spectrometry (RF-MS/MS): The samples purified by the filters as described above for LC-MS/MS were also analyzed using the RapidFire high-throughput mass spectrometry platform (Agilent Technologies, Inc: Santa Clara, CA). Individual AUC (areas under the curve) for each analyte in each sample was determined using the RapidFire Integrator software. Each sample was prepared and analyzed in duplicate on separate days. All GAG levels were measured and averaged.
LC-MS/MS: Determination of levels of disaccharides derived from GAGs
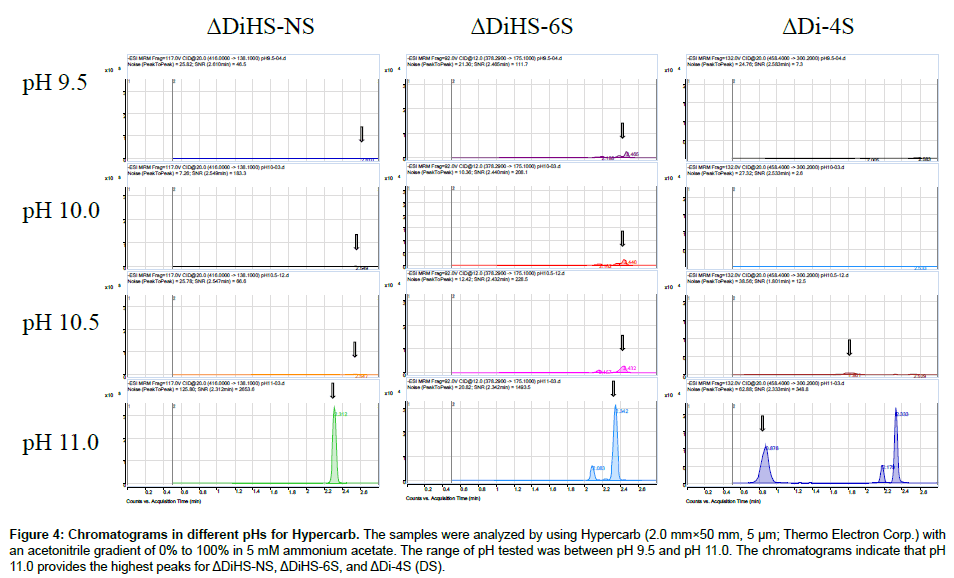
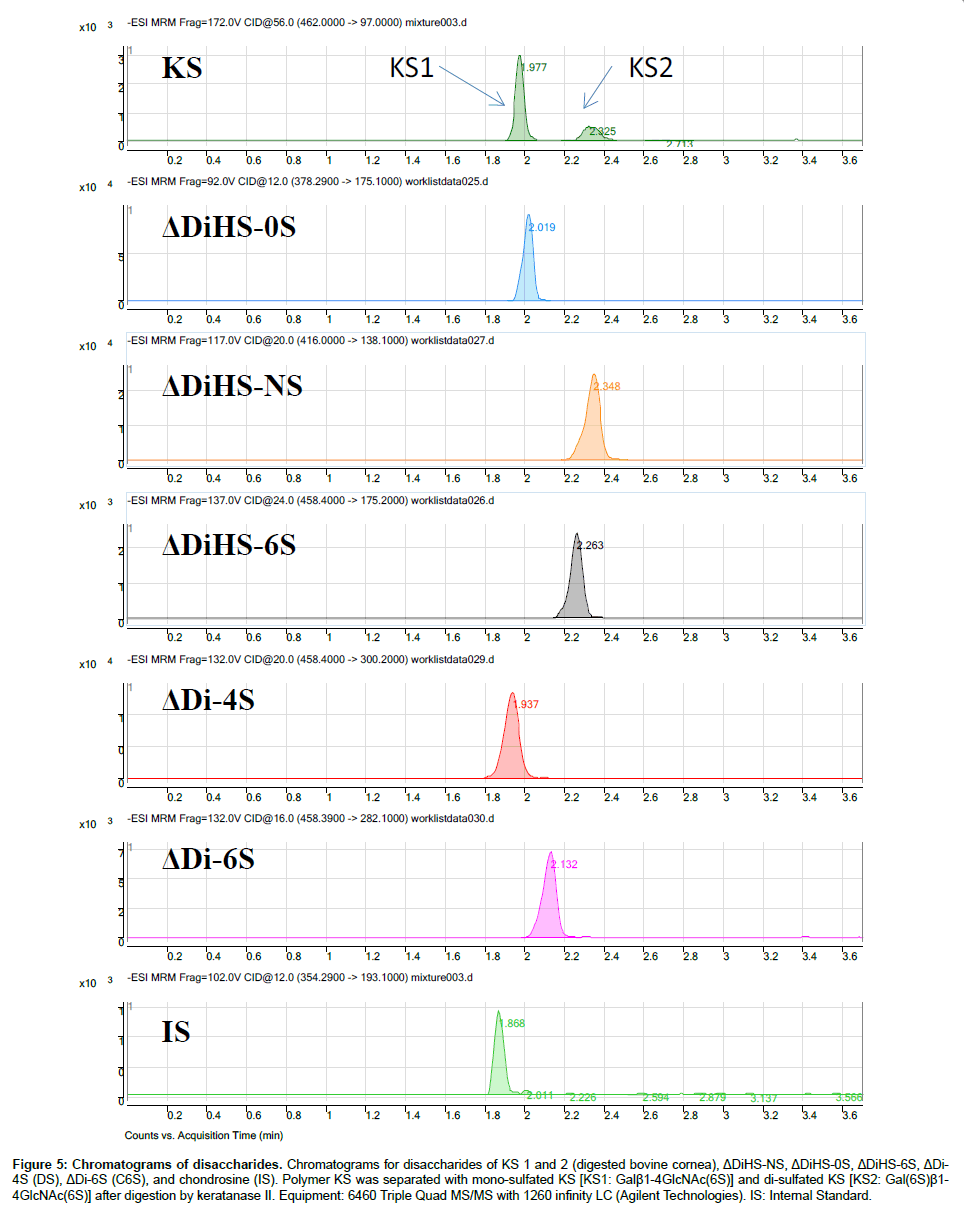
Chromatography and selectivity: Disaccharides were separated on a Hypercarb (2.0 mm×50 mm, 5 μm; Thermo Electron Corp.) column and eluted with an acetonitrile gradient of 0% to 100% in 5 mM ammonium acetate. Signals for ΔDiHS-6S, ΔDi-4S (DS), and ΔDi-6S (C6S) were very low at pH values below 11 (Figure 4), probably due to poor ionization of the sugars.Using purified preparations ofΔDiHS- 0S, ΔDiHS-NS, ΔDiHS-6S, ΔDi-4S, and ΔDi-6S, we optimized MRMs for each individual sugar and obtained single peaks corresponding to the pure compound (Figure 5). All disaccharides are eluted in less than 3 min (Figure 5). As discussed earlier, although ΔDiHS-6S, ΔDi-4S (DS), and ΔDi-6S (C6S) have the same molecular weights, they elute at different times from the column, so they can be quantiifed separately. Furthermore, the elution position of Di-4S varies dependent upon the sample preparation, and elutes at 0.9 min after extraction from some samples (e.g. Figure 4). All other disaccharides consistently eluted as seen for the standards.As discussed earlier, the detection method for mono-sulfated Galβ1-4GlcNAc(6S) (KS1) also detected some Gal(6S) β1-4GlcNAc(6S) (KS2), due to loss of a sulfate, but the two forms were clearly separated by chromatography.
Figure 4: Chromatograms in different pHs for Hypercarb. The samples were analyzed by using Hypercarb (2.0 mm×50 mm, 5 μm; Thermo Electron Corp.) with an acetonitrile gradient of 0% to 100% in 5 mM ammonium acetate. The range of pH tested was between pH 9.5 and pH 11.0. The chromatograms indicate that pH 11.0 provides the highest peaks for ΔDiHS-NS, ΔDiHS-6S, and ΔDi-4S (DS).
Figure 5: Chromatograms of disaccharides. Chromatograms for disaccharides of KS 1 and 2 (digested bovine cornea), ΔDiHS-NS, ΔDiHS-0S, ΔDiHS-6S, ΔDi- 4S (DS), ΔDi-6S (C6S), and chondrosine (IS). Polymer KS was separated with mono-sulfated KS [KS1: Galβ1-4GlcNAc(6S)] and di-sulfated KS [KS2: Gal(6S)β1- 4GlcNAc(6S)] after digestion by keratanase II. Equipment: 6460 Triple Quad MS/MS with 1260 infinity LC (Agilent Technologies). IS: Internal Standard.
Calibration curves: Calibration parameters of disaccharides derived from CS, DS, HS, and KS were assessed. Calibration curves for Galβ1-4GlcNAc(6S) (KS1), Gal(6S)β1-4GlcNAc(6S) (KS2), ΔDiHS-0S (H0S), ΔDiHS-NS (HNS), and ΔDiHS-6S (H6S), ΔDi-6S (C6S), and ΔDi-4S (DS) are linear over the concentration ranges of 0.1to 10 μg/ml, 0.1 to 10 μg/ml, 10 to 1,000 ng/ml, 10 to 1,000 ng/ml, 10 to 1,000 ng/ml, 10 to 1,000 ng/ml, and 10 to 1,000 ng/ml, respectively. The correlation coefficients of determination (r) were not less than 0.98 (Figure 6).
Precision: The results of intra- and inter-assay precision for ΔDiHS- 0S, ΔDiHS-NS, and ΔDiHS-6S, ΔDi-6S, and ΔDi-4S in control serum are as follows. The intra-assay precision values/coefficient of variation (CV) determined from analysis of ΔDiHS-0S, ΔDiHS-NS, ΔDiHS-6S, ΔDi-6S, and ΔDi-4S for control serum are less than 8.9, 1.7, 11.7, 11.9, and 12.1%, respectively. The inter-assay precision values/CVs for these disaccharides in control serum are less than 7.0, 0.6, 12.2, 12.2, and 12.4%, respectively. The intra-assay precision values/CVs for Galβ1- 4GlcNAc(6S) and Gal(6S)β1-4GlcNAc(6S) in control serum were less than 6.8 and 14.6% andinter-assay precision values/CVs were less than 6.5 and 14.3% (Table 1). These results demonstrate the reproducibility and accuracy of the method.
| Intra-assay precision (n=3) CV% | Inter-assay precision (n=5) CV% | |
| ΔDiHS-OS | 8.9 | 7.0 |
| ΔDiHS-NS | 1.7 | 0.6 |
| DiHS-6S | 11.7 | 12.2 |
| ΔDi-6S | 12.1 | 12.2 |
| ΔDi-4S | 11.9 | 12.4 |
| Galβ1-4GlcNAc(6S) | 6.8 | 6.5 |
| Gal(6S)β1-4GlcNAc(6S) | 14.6 | 14.3 |
CV=coefficient of variation
Table 1: Intra- and inter-day precision of the method for determination of disaccharides in human control serum (LC-MS/MS).
RF-MS/MS: Disaccharide determination derived from GAGs
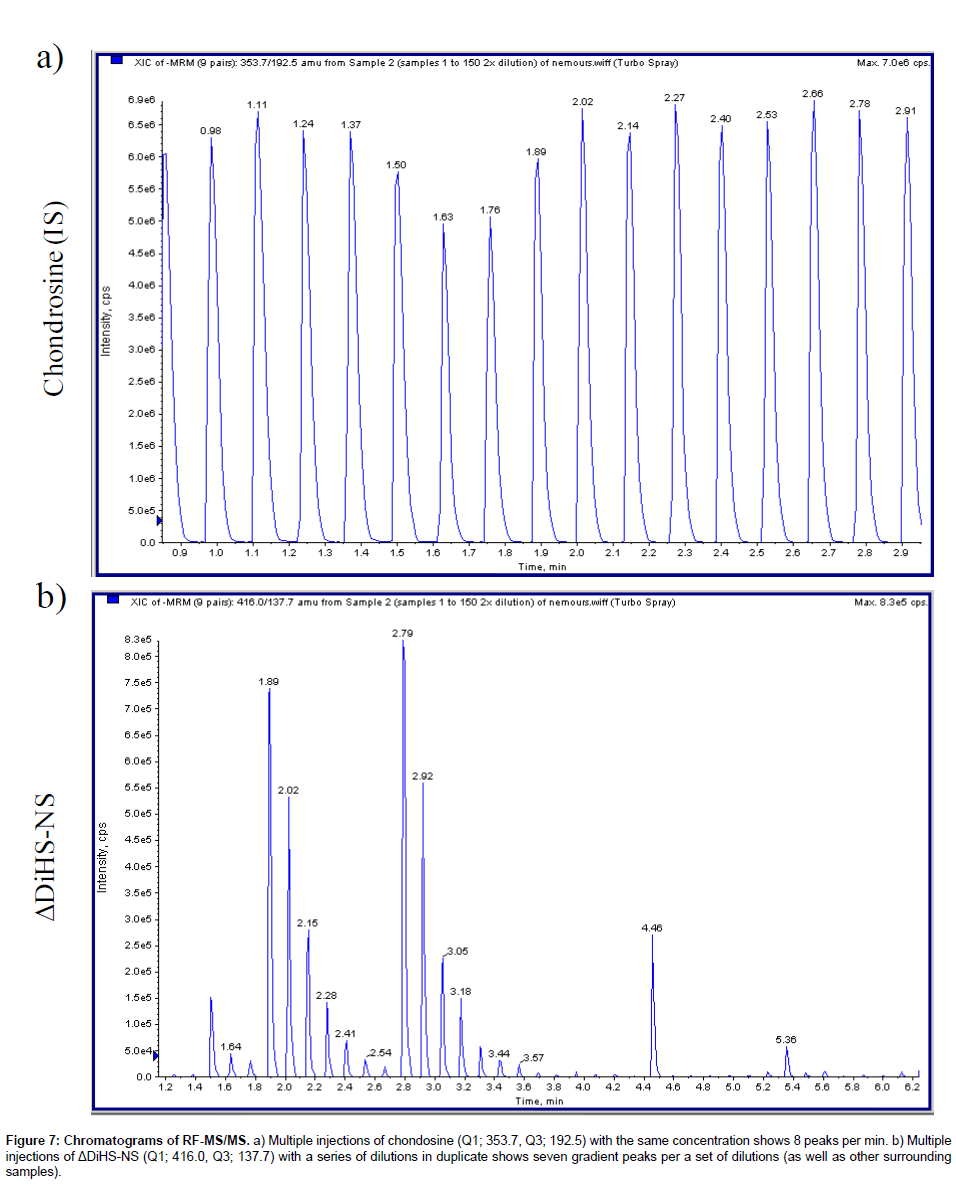
Chromatography: The RF system does not have a chromatographic separation step but rather samples are first bound to solid phase matrix for desalting and concentration and then eluted directly into an MS/ MS system. Consequently, ΔDiHS-6S, ΔDi-4S, and ΔDi-6S cannot be distingished because they have identical molecular weights and give similar mrms. However the MS/MS can distinguish and quantify combinations of different disaccharides (for example see chondrosine and ΔDiHS-NS in Figure 7).
Figure 7: Chromatograms of RF-MS/MS. a) Multiple injections of chondosine (Q1; 353.7, Q3; 192.5) with the same concentration shows 8 peaks per min. b) Multiple injections of ΔDiHS-NS (Q1; 416.0, Q3; 137.7) with a series of dilutions in duplicate shows seven gradient peaks per a set of dilutions (as well as other surrounding samples).
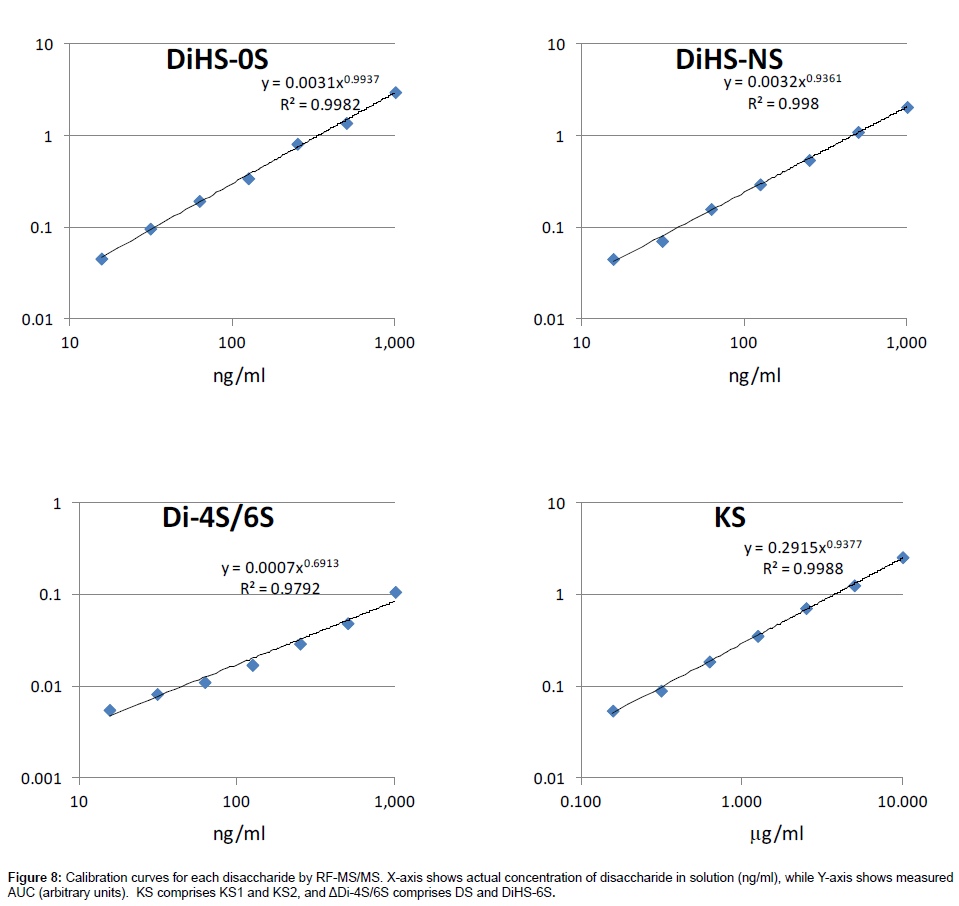
Calibration curves: Calibration parameters of disaccharides derived from DS, HS, and KS were assessed. Calibration curves for KS, ΔDiHS-0S, ΔDiHS-NS, and ΔDi-4S/6S are linear over the concentration ranges of 0.156 to 10.0 μg/ml, 16 to 1,000 ng/ml, 16 to 1,000 ng/ml, and 16 to 1,000 ng/ml, respectively. The correlation coefficients of determination (r) are 0.98 or more (Figure 8).
Precision: The results of intra- and inter-assay precision for ΔDiHS-0S, ΔDiHS-NS, and ΔDi-4S/6S in control serum are as follows. The disaccharide (ΔDi-4S) is detected by the same ions (m/z 462/97) as those of ΔDiHS-6S. Thus, the total concentration of disaccharides expressed as ΔDi-4S includes both ΔDi-4S and ΔDiHS-6S. The intraassay precision values/coefficient of variation (CV) determined from analysis of ΔDiHS-0S, ΔDiHS-NS, and ΔDi-4S for control serum are less than 6.7, 7.9, and 15.8%, respectively. The inter-assay precision values/CVs for these disaccharides in control serum are less than 5.2, 6.8, and 14.8%, respectively (Table 2). The intra-assay precision values/CVs for Galβ1-4GlcNAc(6S) and Gal(6S)β1-4GlcNAc(6S) in control serum were less than 8.2 and 5.3% andinter-assay precision values/CVs were less than 6.6 and 5.7%. These results demonstrate the reproducibility and accuracy of the method.
| Intra-assay precision (n=3) CV% | Inter-assay precision (n=5) CV% | |
| ΔDiHS-OS | 6.7 | 5.2 |
| ΔDiHS-NS | 7.9 | 6.8 |
| ΔDi-4S | 15.8 | 14.8 |
| KS | 9.6 | 9.5 |
CV=coefficient of variation
Table 2: Intra- and inter-day precision of the method for determination of disaccharides in human control serum (RF-MS/MS).
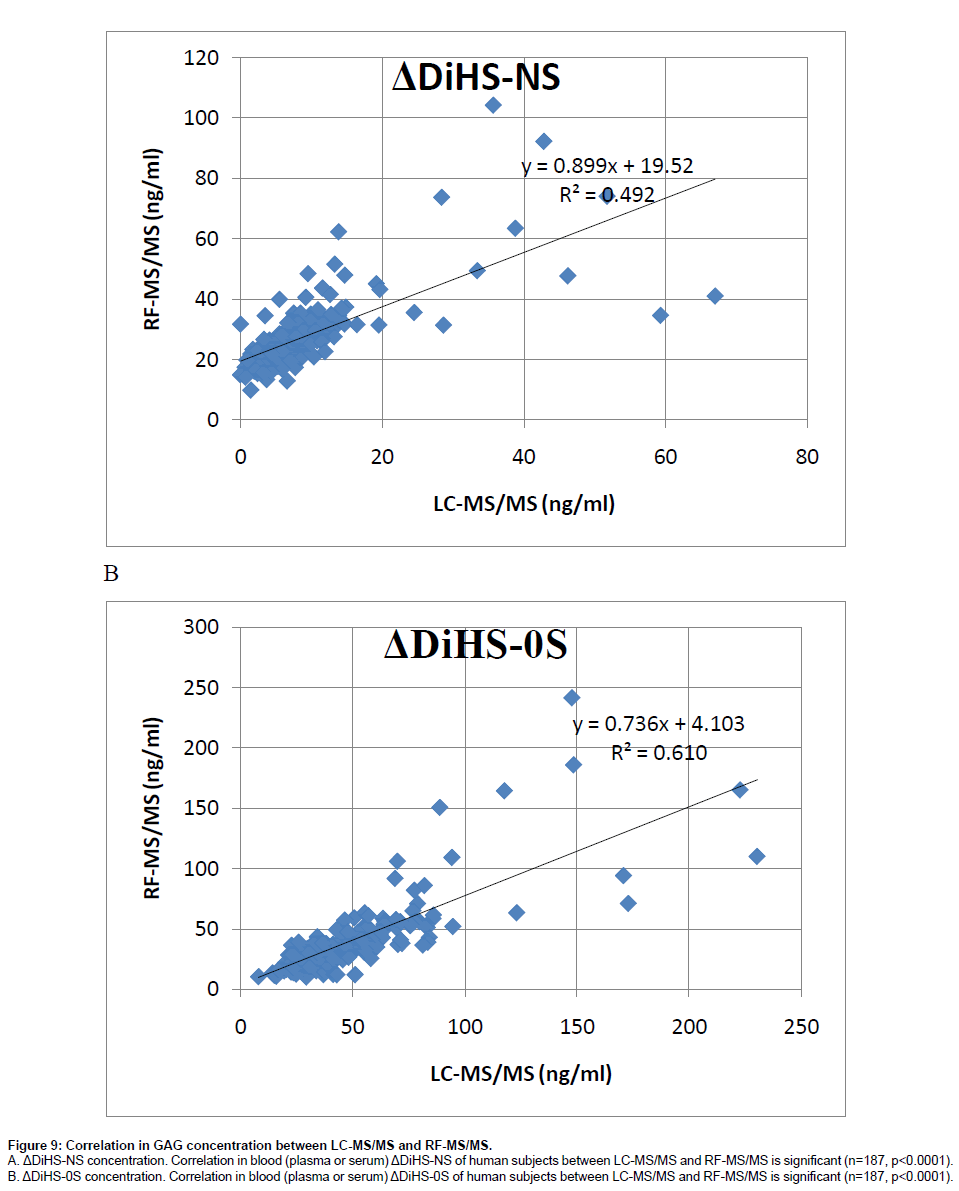
Correlation of GAG levels between LC-MS/MS and RF-MS/ MS: We analyzed 59 different plasma samples for DiHS-NS, DiHS-0S, Di-4S, and KS by both LC-MS/MS and RF-MS/MS. The correlation between LC-MS/MS and RF-MS/MS determinations was tested by a simple linear regression analysis. We interpreted correlation strength as outlined by Johnston [23]. Briefly, correlations are interpreted based on r values as follows: 0.0 to 0.2, very weak to negligible correlation; 0.2 to 0.4, weak correlation; 0.4 to 0.7, moderate correlation; 0.7 to 0.9, strong correlation. Data points that were greater than three standard deviations from the mean for each assay were considered outliers. Analysis was performed using SPSS for Windows (version 17.0, SPSS Inc., Chicago, IL, USA). The moderate correlation in serum ΔDiHS-NS and ΔDiHS-0S measurements of control subjects between LC-MS/MS and RF-MS/MS indicates that results from each assay are comparable (Figure 9). Measurements of KS also showed moderate correlation (data not shown).
Figure 9: Correlation in GAG concentration between LC-MS/MS and RF-MS/MS.
A. ΔDiHS-NS concentration. Correlation in blood (plasma or serum) ΔDiHS-NS of human subjects between LC-MS/MS and RF-MS/MS is significant (n=187, p<0.0001).
B. ΔDiHS-0S concentration. Correlation in blood (plasma or serum) ΔDiHS-0S of human subjects between LC-MS/MS and RF-MS/MS is significant (n=187, p<0.0001).
Applications of GAG assays
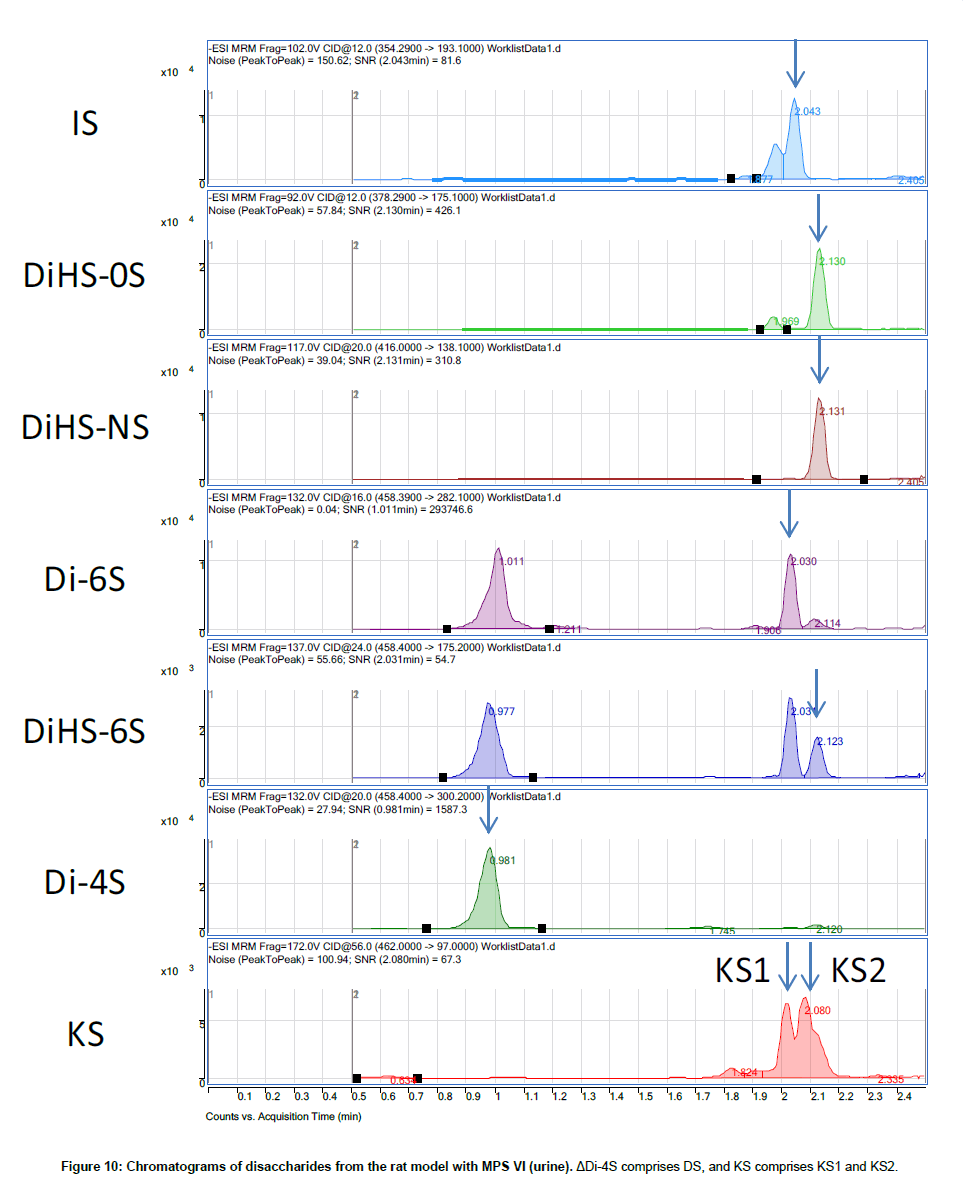
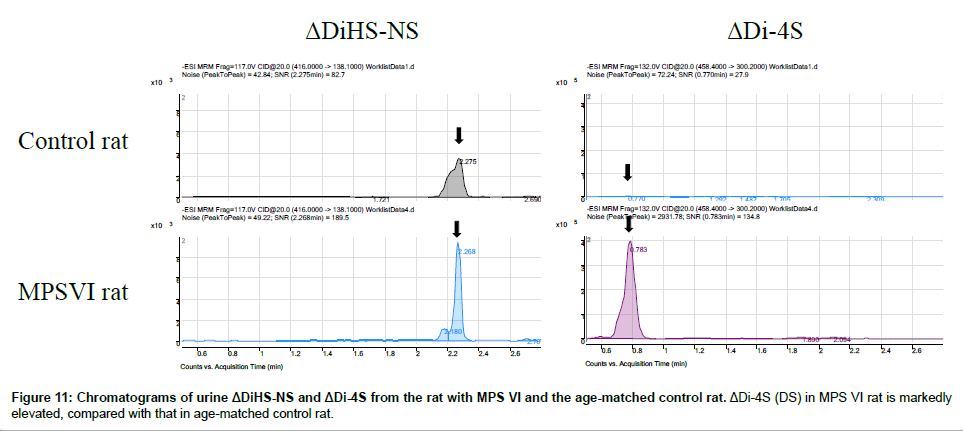
Disaccharide GAGs assayed by LC-MS/MS have been used for diagnosis, screening, prognosis of clinical severity, and assessment of therapeutic efficacy in human patients and animal models with MPS [2,9-12,22,24-27]. In Figure 10, peaks corresponding to expected elution of each disaccharide are marked with arrows. MRM Parameters designed to detect Di-6S and DiHS-6S also detect Di-4S whereas the parameters specifically designed to detect Di-4S do not detect the low levels of Di-6S and DiHS-6S in these samples.A clear difference in Di- 4S level between a rat with MPS VI and a control rat was observed (Figure 11).
Discussion
Our studies have demonstrated that an RF-MS/MS platform is capable of detecting disaccharides of ΔDiHS-0S, ΔDiHS-NS, ΔDi-4S/6S, and KS with similar reproducibility, sensitivity, and specificity as LCMS/ MS [3-10], suggesting that the LC component may not be essential for detection and identification of these disaccharides. The strong correlation between the two methods for detection of disaccharides extracted from plasma indicates that non-specific interactions or signal quenching are not major concerns for the RF system for this application. The filter system used in this study to remove low molecular weight components of serum and then to separate digested disaccharides from larger molecules appears to eliminate the need for further purification prior to MS/MS analysis. RF-MS/MS provides 25-100 fold faster throughput compared to conventional LC-MS/MS, and consequently may be more suitable for mass screening applications such as NBS.
RF-MS/MS may not be useful for all applications or tissue samples. As there is no separation step, interfering compounds will not be distinguishable from the compound under study. We found that extracts of rat urine contained a number of interfering peaks, including one that had the same MRM as the internal standard (Figure 10). The significance of these false signals to this limited study require further clarification, but the data emphasize the need to ensure that samples will not give excessive levels or numbers of false positive signals prior to establishment of a RF technique.
RF-MS/MS cannot distinguish ΔDi-4S, ΔDi-6S, and ΔDiHS-6S that have the same molecular weight, so LC-MS/MS will be required if quantities of specific disaccharides are required.
GAG(s) play an important physiological role as PG and are associated with other common diseases such as osteoarthritis (OA) [28-31], ligament injury or trauma in the knee [32,33], spinal cord injury [34-36], and diabetes mellitus (DM) [37-39]. GAG assay by tandem mass spectrometry should be applied to diagnosis, prognosis, and therapeutic efficacy of these diseases. The LC-MS/MS method is also needed to investigate sulfation levels of HS and KS that can result in functional alterations of proteoglycans. RF-MS/MS will be more valuable for situations where rapid throughput is necessary such as in newborn screening.
GAGs are long, unbranched polysaccharides, and have a high negative charge because of acidic sugar residues and/or modifications by sulfate groups. The acidic sugar alternates with an amino sugar in repeated disaccharide units. The GAGs adopt an extended conformation, attract cations, and bind water. Hydrated GAG gels enable joints and tissues to absorb large pressure changes.
After synthesis, PGs are transported from the Golgi to their destinations; ECM, the cell surface or intracellular organelles. Such determinant transport requires mechanisms for recognition, sorting and delivery. These mechanisms are critical in cells such as epithelial cells and neurons, where the cell membrane comprises separate domains. Recognition and sorting require determinant factors in the GAG chains and/or in the PG protein cores. Thus, the physiological role of GAGs is dependent upon tissue and/or cell type. Sulfation level of GAG(s) also varies with age, tissue, and pathological status. For instance, KS is almost always sulfated on C (6) of GlcNAc, while C (6) of Gal is sulfated to a variable extent, depending on the tissue and age [40]. KS chains of fibromodulin in articular cartilage are highly sulfated compared to that in cornea [41,42], and the levels of sulfation in cornea and cartilage increase during normal aging [43,44]. The mechanism of alteration of their sulfation levels remains unknown. However, corneas in patients with macular corneal dystrophy (MCD) have been reported to synthesize low-sulfated KS, resulting in corneal clouding, and sulfate groups attached to KS play critical roles in maintaining corneal transparency [45]. Investigation of sulfation level in each disease and physiological status should help elucidate the mechanism of roles of GAGs.
Thus, GAGs assayed by tandem mass spectrometry can be used as biomarkers not only for diagnosis, prognosis, and monitoring therapeutic effects for MPS but may also prove valuable for study of other diseases such as OA, DM, and spinal cord injury and physiological roles. The analysis technique should also be applicable to other species.
The mechanism of alteration of GAGs levels and their sulfation levels remains unknown. Investigation of each GAG level and its sulfation level in each disease and physiological status should help elucidate the mechanism of secondary alteration of GAGs and their roles.
Conclusion
In conclusion, both LC-MS/MS and RF-MS/MS methods provide a comparable sensitivity and accuracy for simultaneous measurement of three GAGs (DS, HS, and KS).Advantage of use of LC-MS/MS is that ΔDi-6S (C6S), ΔDi-4S (DS), and DiHS-6S with the same molecular weight can be separated leading to accuracy and specificity of the differential diagnosis although it takes 3 min per sample to be analyzed. The RapidFire platform should provide both high throughput and economic advantages as a screening system for MPS, when compared with the conventional LC-MS/MS methodologies and has the potential of being applied to screening for other inherited metabolic disorders, although this system cannot recognize molecules with the same molecular weight separately.
Acknowledgements
This work was supported by grants from the Austrian MPS Society, International Morquio Organization (Carol Ann Foundation) and Delaware Bioscience Center for Advanced Technology. R.W.M. and S.T. are supported by National Institutes of Health grant 8P20 GM103464-08. S.T. and A.M are supported by National Institutes of Health grant 1R01HD065767-02. The content of the article has not been influenced by the sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
References
- Iozzo RV (1998) Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 67: 609-652.
- Tomatsu S, Montaño AM, Dung VC, Ohashi A, Oikawa H, et al. (2010) Enhancement of drug delivery: enzyme-replacement therapy for murine Morquio A syndrome. Mol Ther 18: 1094-1102.
- Oguma T, Tomatsu S, Montaño AM, Okazaki O (2007a) Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal Biochem 368: 79-86.
- Oguma T, Tomatsu S, Okazaki O (2007b) Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed Chromatogr 21: 356-362.
- Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, et al. (2010) Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol Genet Metab 99: 124-131.
- Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, et al. (2010) Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J Inherit Metab Dis 33: 141-150.
- Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, et al. (2010) Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis 33: S35-S42.
- de Ruijter J, de Ru MH, Wagemans T, Ijlst L, Lund AM, et al. (2012) Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I, II and III. Mol Genet Metab 107: 705-710.
- de Ruijter J, Ijlst L, Kulik W, van Lenthe H, Wagemans T, et al. (2013) Heparan sulfate derived disaccharides in plasma and total urinary excretion of glycosaminoglycans correlate with disease severity in Sanfilippo disease. J Inherit Metab Dis 36: 271-279.
- de Ru MH, van der Tol L, van Vlies N, Bigger BW, Hollak CE, et al. (2013) Plasma and urinary levels of dermatan sulfate and heparan sulfate derived disaccharides after long-term enzyme replacement therapy (ERT) in MPS I: correlation with the timing of ERT and with total urinary excretion of glycosaminoglycans. J Inherit Metab Dis 36: 247-255.
- Montaño AM, Oikawa H, Tomatsu S, Nishioka T, Vogler C, et al. (2008) Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol Genet Metab 94: 178-189.
- Tomatsu S, Montaño AM, Ohashi A, Gutierrez MA, Oikawa H, et al. (2008) Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet 17: 815-824.
- Quercia AK, LaMarr WA, Myung J, Ozbal CC, Landro JA, et al. (2007) High-throughput screening by mass spectrometry: comparison with the scintillation proximity assay with a focused-file screen of AKT1/PKB alpha. J Biomol Screen 12: 473-480.
- Wu X, Wang J, Tan L, Bui J, Gjerstad E, et al. (2012) In vitro ADME profiling using high-throughput rapidfire mass spectrometry: cytochrome p450 inhibition and metabolic stability assays. J Biomol Screen 17: 761-772.
- Leveridge MV, Bardera AI, LaMarr W, Billinton A, Bellenie B, et al. (2012) Lead discovery for microsomal prostaglandin E synthase using a combination of high-throughput fluorescent-based assays and RapidFire mass spectrometry. J Biomol Screen 17: 641-650.
- Hutchinson SE, Leveridge MV, Heathcote ML, Francis P, Williams L, et al. (2012) Enabling lead discovery for histone lysine demethylases by high-throughput RapidFire mass spectrometry. J Biomol Screen 17: 39-48.
- Plant M, Dineen T, Cheng A, Long AM, Chen H, et al. (2011) Screening for lysine-specific demethylase-1 inhibitors using a label-free high-throughput mass spectrometry assay. Anal Biochem 419: 217-227.
- Atkinson KA, Beretta EE, Brown JA, Castrodad M, Chen Y, et al. (2011) N-benzylimidazolecarboxamides as potent, orally active stearoylCoA desaturase-1 inhibitors. Bioorg Med Chem Lett 21: 1621-1625.
- Highkin MK, Yates MP, Nemirovskiy OV, Lamarr WA, Munie GE, et al. (2011) High-throughput screening assay for sphingosine kinase inhibitors in whole blood using RapidFire® mass spectrometry. J Biomol Screen 16: 272-277.
- Langsdorf EF, Malikzay A, Lamarr WA, Daubaras D, Kravec C, et al. (2010) Screening for antibacterial inhibitors of the UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosaminedeacetylase (LpxC) using a high-throughput mass spectrometry assay. J Biomol Screen 15: 52-61.
- Holt TG, Choi BK, Geoghagen NS, Jensen KK, Luo Q, et al. (2009) Label-free high-throughput screening via mass spectrometry: a single cystathionine quantitative method for multiple applications. Assay Drug Dev Technol 7: 495-506.
- Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, et al. (2013) Newborn screening and diagnosis of mucopolysaccharidoses. Mol Genet Metab 110: 42-53.
- Johnston I (2000) I’ll give you a definite maybe: An introductory handbook on probability, statistics, and excel.
- Hintze JP, Tomatsu S, Fujii T, Montaño AM, Yamaguchi S, et al. (2011) Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark Insights 6: 69-78.
- Martell LA, Cunico RL, Ohh J, Fulkerson W, Furneaux R, et al. (2011) Validation of an LC-MS/MS assay for detecting relevant disaccharides from keratan sulfate as a biomarker for Morquio A syndrome. Bioanalysis 3: 1855-1866.
- Auray-Blais C, Lavoie P, Zhang H, Gagnon R, Clarke JT, et al. (2012) An improved method for glycosaminoglycan analysis by LC-MS/MS of urine samples collected on filter paper. Clin Chim Acta 413: 771-778.
- Auray-Blais C, Bhérer P, Gagnon R, Young SP, Zhang HH, et al. (2011) Efficient analysis of urinary glycosaminoglycans by LC-MS/MS in mucopolysaccharidoses type I, II and VI. Mol Genet Metab 102: 49-56.
- Baccarin RY, Machado TS, Lopes-Moraes AP, Vieira FA, Michelacci YM (2012) Urinary glycosaminoglycans in horse osteoarthritis. Effects of chondroitin sulfate and glucosamine. Res Vet Sci 93: 88-96.
- Nakajima A, Nakagawa K, Aoki Y, Sonobe M, Shibata Y, et al. (2013) Changes in synovial fluid biochemical markers following arthroscopic surgery in patients with knee osteoarthritis. Rheumatol Int 33: 209-214.
- Igarashi M, Kaga I, Takamori Y, Sakamoto K, Miyazawa K, et al. (2011) Effects of glucosamine derivatives and uronic acids on the production of glycosaminoglycans by human synovial cells and chondrocytes. Int J Mol Med 27: 821-827.
- Wakitani S, Okabe T, Kawaguchi A, Nawata M, Hashimoto Y (2010) Highly sensitive ELISA for determining serum keratansulphate levels in the diagnosis of OA. Rheumatology (Oxford) 49: 57-62.
- Yoshida H, Kojima T, Kurokouchi K, Takahashi S, Hanamura H, et al. (2013) Relationship between pre-radiographic cartilage damage following anterior cruciate ligament injury and biomarkers of cartilage turnover in clinical practice: a cross-sectional observational study. Osteoarthritis Cartilage 21: 831-838.
- Wakitani S, Nawata M, Kawaguchi A, Okabe T, Takaoka K, et al. (2007) Serum keratan sulfate is a promising marker of early articular cartilage breakdown. Rheumatology (Oxford) 46: 1652-1656.
- Kadomatsu K1, Sakamoto K2 (2014) Sulfated glycans in network rewiring and plasticity after neuronal injuries. Neurosci Res 78: 50-54.
- Ito Z, Sakamoto K, Imagama S, Matsuyama Y, Zhang H, et al. (2010) N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J Neurosci 30: 5937-5947.
- Hilton BJ, Lang BT, Cregg JM (2012) Keratan sulfate proteoglycans in plasticity and recovery after spinal cord injury. J Neurosci 32: 4331-4333.
- Zhao T, Lu X, Davies NM, Gong Y, Guo J, et al. (2013) Diabetes results in structural alteration of chondroitin sulfate in the urine. J Pharm Pharm Sci 16: 486-493.
- Nishiguchi KM, Ushida H, Tomida D, Kachi S, Kondo M, et al. (2013) Age-dependent alteration of intraocular soluble heparan sulfate levels and its implications for proliferative diabetic retinopathy. Mol Vis 19: 1125-1131.
- Reine TM, Grøndahl F, Jenssen TG, Hadler-Olsen E, Prydz K, et al. (2013) Reduced sulfation of chondroitin sulfate but not heparan sulfate in kidneys of diabetic db/db mice. J Histochem Cytochem 61: 606-616.
- Bhavanandan VP, Meyer K (1968) Studies on keratosulfates. Methylation, desulfation, and acid hydrolysis studies on old human rib cartilage keratosulfate. J Biol Chem 243: 1052-1059.
- Lauder RM, Huckerby TN, Nieduszynski IA (1997) The structure of the keratansulphate chains attached to fibromodulin from human articular cartilage. Glycoconj J 14: 651-660.
- Nieduszynski IA, Huckerby TN, Dickenson JM, Brown GM, Tai GH, et al. (1990) There are two major types of skeletal keratansulphates. Biochem J 271: 243-245.
- Liles M, Palka BP, Harris A, Kerr B, Hughes C, et al. (2010) Differential relative sulfation of Keratan sulfate glycosaminoglycan in the chick cornea during embryonic development. Invest Ophthalmol Vis Sci 51: 1365-1372.
- Brown GM, Huckerby TN, Bayliss MT, Nieduszynski IA (1998) Human aggrecankeratan sulfate undergoes structural changes during adolescent development. J Biol Chem 273: 26408-26414.
- Hasegawa N, Torii T, Kato T, Miyajima H, Furuhata A, et al. (2000) Decreased GlcNAc 6-O-sulfotransferase activity in the cornea with macular corneal dystrophy. Invest Ophthalmol Vis Sci 41: 3670-3677.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16245
- [From(publication date):
specialissue-2014 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 11564
- PDF downloads : 4681