Aspirin plus Vitamin C Provides Better Relief than Placebo in Managing the Symptoms of the Common Cold
Received: 28-Nov-2017 / Accepted Date: 15-Dec-2017 / Published Date: 22-Dec-2017
Abstract
Background and objective: Acetylsalicylic acid (ASA) has demonstrated efficacy in reducing fever and sore throat pain in patients with common cold symptoms caused by acute upper respiratory tract infection. The purpose of the present study was to evaluate the efficacy and tolerability of a formulation of ASA plus vitamin C in the treatment of common cold symptoms using the Wisconsin Upper Respiratory Symptom Survey (WURSS) questionnaire, a validated measure for evaluating common cold symptom severity and quality of life.
Methods: We compared ASA 800 mg plus vitamin C 480 mg (2 tablets of ASA 400 mg plus vitamin C 240 mg) versus placebo in a randomized, double-blind, multicenter study of adult patients whose onset of cold symptoms occurred ≤ 48 h prior to screening. The primary endpoint was the change in Cold Symptoms (WURSS domain 2) from baseline (0 min, directly before the first dose of study drug on Day 1) to the 2 h assessment on Day 1.
Results and discussion: A total of 388 patients were randomized, 189 to ASA plus C and 199 to placebo. Prior to randomization, the median cold duration was 1 day in both treatment groups and both groups had mild to moderate symptom severity at baseline. Two hours after treatment on Day 1, there was significantly greater improvement in Cold Symptoms in the ASA plus C group compared with placebo (score change (LS means) -19.6 vs. -15.2, P=0.012). At the evening of Day 1, a significant difference in Cold Symptoms remained between the groups (P=0.016). Also by the evening of Day 1, there was a significantly (P=0.015) greater overall relief in the severity, symptoms and functional impairments as measured by the Common Cold Profile (sum of WURSS domains 1-3) associated with the common cold in the ASA plus C group compared with placebo.
Conclusion: ASA plus C was shown to be more effective than placebo in providing early relief of the symptoms of the common cold.
Keywords: Aspirin, Acetylsalicylic acid, Vitamin C, Ascorbic acid, Common cold, Sore throat, Pharyngitis, Wisconsin upper respiratory symptom survey, Surveys and questionaires
Introduction
Throughout the world, the common cold is one of the most frequently occurring illness in people of all ages [1]. On average, adults experience 2 to 4 common cold episodes per year [2,3], although some estimates are as high as 4-6 per year [4]. Though virtually never fatal and rarely complicated, common colds are responsible for major discomforts in quality of life and considerable economic burden [2,5]. While usually a self-limiting illness, with a mean duration of 7-10 days, in approximately 25% of cases common cold symptoms last longer [2,6]. Symptoms generally begin 10 to 16 h following intranasal exposure, peak on days 2 to 3 of infection and then decrease rapidly thereafter [2,6]. During this peak period of illness, symptomatic relief is fundamental to improving a patient’s quality of life.
Acetylsalicylic acid (ASA), well-known for its analgesic, anti-pyretic and anti-inflammatory actions, has demonstrated efficacy in reducing fever, headache, muscle aches and sore throat pain in patients with common cold symptoms [7,8] or tonsillo-pharyngitis [9]. However, patients enrolled in these studies were characterized by a relatively wide variability regarding the onset of symptoms (enrollment occurred for patients whose symptoms started up to 5 days earlier). This in turn leads to variability in the symptoms patients present, since acute URTI typically last 7-10 days, during which the different symptoms (e.g sneezing, cough, rhinorrhea) start and stop with characteristic patterns [10]. As a consequence, previous studies generally focused on a main symptom, like fever or sore throat, in order to address the efficacy of ASA. Therefore, the purpose of the present study was to obtain data on the efficacy and tolerability of a formulation of ASA plus vitamin C compared with placebo in patients with a more uniform pattern of symptoms (e.g. symptoms of more recent onset) using the Wisconsin Upper Respiratory Symptom Survey (WURSS) questionnaire [11,12], which is a validated measure for comprehensively evaluating common cold symptom severity and quality of life [11,12].
ASA plus vitamin C formulations are widely available in several countries and this combination is frequently used by patients to treat symptoms of common cold. ASA addresses pain, fever and inflammation, whereas vitamin C provides immune system support. The aim of the current study is to provide evidence that this combination as a whole is effective in improving quality of life.
Methods
Study design and patients
This was a randomized, double-blind, placebo-controlled, multicenter study. All participating physicians were trained during an investigation meeting about study procedures. Males and females between 18 and 65 years of age with onset of cold symptoms ≤ 48 h prior to screening and baseline Physical Features of Upper Respiratory Tract Infection Scale (PFURTIS) score ≥ 11 points and WURSS domain 2 score ≥ 18 points were eligible for study enrollment. The PFURTIS questionnaire, which is used to diagnose the common cold and document the severity of cold symptoms, consists of a general features scale (6 items: Tenderness over frontal sinus, tenderness over maxillary sinus, wateriness of eyes, coughing, sneezing, muscle soreness), a nasal evaluation scale (3 items: Degree of swelling of turbinates, amount of nasal mucus, color of nasal mucus) and a tonsillo-pharyngitis scale (6 items: Tympanic temperature, oro-pharyngeal color, size of tonsils, number of oro-pharyngeal enanthems, presence/amount of phlegm in posterior oropharynx, reflex of tympanic membrane). Each item is scored on a 0–3 rating scale, where 0=no symptom/normal and 3=severe/severely impaired. Total score (maximum possible=45 points) is determined by summing all responses. The WURSS questionnaire consists of 44 cold-specific items in 4 domains (Table 1): Domain 1 global cold severity (1 item), domain 2 cold symptoms (32 items), domain 3 cold-specific functional impairments (10 items), domain 4 change in global cold severity (1 item) [11,12]. Specifically for domain 2, each of the 32 cold symptoms is rated from 0 (no symptom) to 7 (symptom is severe), with a maximum possible score of 224 points. The PFURTIS and WURSS questionnaires were translated to Italian for use in this study. Authorization for translation was given by the University of Wisconsin.
| Domain 1 | Domain 2 | Domain 3 | Domain 4 | |
|---|---|---|---|---|
| Description | Global Cold Severity | Cold Symptoms | Cold-specific Functional Impairments | Change in Global Cold Severity |
| Item | How sick do you feel today? | cough, coughing stuff up, cough interfering with sleep, sore throat, scratchy throat, hoarseness, runny nose, plugged nose, sneezing, headache, body aches, feeling run down, sweats, chills, feeling feverish, feeling dizzy, feeling tired, irritability, sinus pain, sinus pressure, sinus drainage, swollen glands, lugged ears, ear discomfort, watery eyes, eye discomfort, head congestion, chest congestion, chest tightness, heaviness in chest, lack of energy, loss of appetite | think clearly, speak clearly; sleep well; breathe easily; walk, climb stairs, exercise; accomplish daily; activities; work outside the home; work inside the home; interact with others; live your personal life | Compared to yesterday, I feel that my cold is |
| Measure | 0 [not sick] to 7 [severely] | 0 [no] to 7 [severe] | 0 [no] to 7 [severe] | 0 [very much better] to 6 [very much worse] |
| Maximum Score | 7 | 224 | 7 | 6 |
Table 1: Symptoms and Functional Impairments Evaluated by WURSS
Key exclusion criteria were active peptic ulcer or history of chronic or recurrent ulcer disease or history of gastrointestinal or non- gastrointestinal bleeding, hypersensitivity to any study medications as well as any salicylates, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) or other anti-inflammatory or antirheumatic drugs, known hereditary glucose-6-phosphate-dehydrogenase deficiency, impaired hepatic or renal function and intake of any medication considered by the clinical investigator as a reason for exclusion within the 30 days before screening.
Patients were also not permitted to have taken any menthol- containing product within 4 h before baseline, any local or systemic short-acting cough and cold preparation (e.g., decongestant or antihistaminic drugs) within 6 h before baseline, any local or systemic long-acting cough and cold preparation (e.g., decongestant or antihistaminic drugs) or analgesic agent within 12 h before baseline or any prescription medication for treatment of the current acute common cold. The prior use of methotrexate, anticoagulants, thrombolytics or other antiplatelet agents (e.g., ticlopidine) and NSAIDs (other than ASA plus C) was allowed provided that 10 times their respective half- lives had passed before baseline. All of the above listed medications were prohibited during the study.
Paracetamol was permitted if no medication had been taken within the 12 h prior to baseline and was allowed during the study as rescue medication. During the first 2 h of the study, until the second WURSS questionnaire was completed, intake of rescue and inhalation medications was strongly discouraged.
The protocol was developed by Bayer Consumer Health, Basel, Switzerland. The study was conducted according to the principles of Good Clinical Practice (GCP). Each patient provided written informed consent prior to any study procedure (including screening). The coordinating investigator of the study group had unrestricted access to all study data.
Treatments
Each patient received Aspirina™ C (acetylsalicylic acid 400 mg+ascorbic acid 240 mg, Bayer HealthCare AG) or matching placebo, as effervescent tablets. Albeit AspirinaTM C is the most common formulation of aspirin used in many countries, like Italy and Germany, for the symptomatic treatment of URTI, its efficacy has never been tested in a randomized, controlled clinical trial. Therefore, this specific formulation was chosen as test drug in order to assess the effect of ASA on URTI symptoms. Paracetamol (500 mg tablets) was available to patients as rescue medication throughout the in-patient and follow-up portions of the study.
On Day 1, at the study site, all subjects received 2 tablets of ASA plus C or placebo and were followed for 2 h. All remaining treatments through Day 5 (study end) were out-patient. Six hours after the initial dose, further study treatment was allowed as required, but was limited to 1 to 2 tablets every 4 to 6 h, maximum of 6 tablets (2400 mg) per 24 h period. The dose of paracetamol was limited 1 to 2 tablets every 4 to 8 h, maximum of 6 tablets (3000 mg) per 24 h period.
Blinding
Patients were assigned to one of two treatment groups using a computer-generated randomization list. After having completed the baseline WURSS assessment and eligibility established, each patient was assigned to the next available number in ascending order. Active study drug and placebo tablets were identical in appearance. The solutions generated by dissolving each tablet in water were identical in taste.
Study assessments
Patient assessments were conducted on Day 1 before study treatment was administered (baseline), 2 h after treatment administration on Day 1 and each evening of Days 1–5, inclusive. With exception of the 2 h assessment point, patients had to provide responses to questions in all 4 WURSS domains at each study assessment. The 2 h assessment was made for WURSS domains 1, 2 and 4 only. The domain 3 was omitted. For questions in domains 1, 2 and 3, patient responses were provided as 8-point Likert scales with the following assigned values: 0=no symptom/not sick; 1=very mild; 2=somewhat mild; 3=mild; 4=somewhat moderate; 5=moderate; 6=somewhat severe; 7=severe/ severely sick. Domain 4 responses were provided as a 7-point Likert scale with the following point values: 0=very much better; 1=somewhat better; 2=a little better; 3=the same; 4=a little worse; 5=somewhat worse; 6=very much worse. The Common Cold Profile score was determined by summing domains 1–3; maximum score was 301 points and high scores indicate poor quality of life.
Primary endpoint
The primary endpoint was the change in Cold Symptoms (WURSS domain 2) from baseline (0 min, directly before the first dose of study drug on Day 1) to the 2 h assessment on Day 1.
Secondary endpoints
Secondary endpoints were the changes from baseline in Common Cold Profile (sum of WURSS domains 1–3), Global Cold Severity (WURSS domain 1), Cold Symptoms (WURSS domain 2), Cold- specific Functional Impairments (WURSS domain 3) and Global Cold Severity (WURSS domain 4) to each of 5 evenings during the follow- up period (or directly prior to the first intake of rescue medication or disallowed medication during the follow-up period).
Statistical analysis
All data were summarized and listed by treatment group and time point. The primary analysis was performed on the population consisting of the full analysis set, defined all randomized patients who took at least 1 dose of study drug and provided valid data for the 2 h in-patient period (≤ 3 missing paired items in the assessment of cold symptoms at baseline (0 min) and 2 h). Differences between treatment groups were analyzed using an analysis of covariance model with treatment and study site as factors and baseline value as a covariate. All hypothesis tests were one-sided, with a 2.5% level of significance. All analyses were performed using SAS version 8.12 (SAS Institute, Inc., Cary, NC, USA).
Determination of sample size
The sample size was based upon general assumptions, since for the treatment of the common cold no WURSS data were available. The assumptions included a Type I error with α=2.5 %, a one-sided, Type II error with ß=20%, a common standard deviation of =18 and in the WURSS domain 2, only approximately 5 of the 32 items would be positive per patient. A 5-point improvement difference in the primary endpoint: (ASA plus C (2 h–0 min)) – (placebo (2 h–0 min)) could only be demonstrated with valid data from approximately 200 patients per treatment group, i.e., a total of 400 patients. The drop-out rate was expected to be low, since the study duration was only 5 days.
Results
Patients
A total of 388 patients from 32 investigational sites in Italy were randomized, 189 to ASA plus C and 199 to placebo (Table 2). The first patient enrolled in October 2005 and the last patient completed in June 2007. Of the 388 randomized patients, 385 constituted the primary evaluable population; 3 patients from the ASA plus C group were excluded due to significantly incomplete documentation of the 2 h in-patient WURSS (domain 1 missing, >3 paired items missing for domain 2 or >1 item missing for domain 3). A total of 377 patients competed the study and 11 discontinued early (7 due to adverse events, 3 due to lack of efficacy and 1 voluntary withdrawal). In general, the groups had similar characteristics at baseline (Table 3). The majority of patients were Caucasian, with a mean age of 37 years. The placebo group had approximately equal numbers of men and women enrolled, but more women (59%) than men (41%) were in the ASA plus C group. Prior to randomization, the median cold duration was 1 day in both treatment groups. The mean WURSS domain 2 score of was 82.5 for patients in the ASA plus C group and 79.7 for those in the placebo group. Both groups had a mean PFURITIS score of 18.1, indicating mild to moderate symptom severity at baseline.
| Patient Disposition | ASA+C | Placebo |
|---|---|---|
| Randomized | 189 | 199 |
| Evaluable | 186 | 199 |
| Completed | 184 | 193 |
| Discontinued Reasons for Discontinuation Adverse Events Lack of efficacy Voluntary Withdrawal |
5 5 0 0 |
6 2 3 1 |
Table 2: Patient Disposition
| Characteristic | ASA+C [n=189] | Placebo [n=199] |
|---|---|---|
| Age [yrs], mean [SD] | 37.2 [12.8] | 36.8 [11.8] |
| Sex, n [%] Male Female |
78 [41.3] 111 [58.7] |
99 [49.7] 100 [50.3] |
| Race Caucasian Hispanic Other |
185 [97.9] 3 [1.6] 1 [0.5] |
195 [98.0] 2 [1.0] 2 [1.0] |
| Height [cm], mean [SD] | 167.9 [8.9] | 169.3 [8.6] |
| Weight [kg], mean [SD] | 68.8 [14.1] | 69.0 [14.7] |
| BMI [kg/m2], mean [SD] | 24.4 [4.3] | 24.0 [4.2] |
| WURSS domain 2, mean [SD] | 82.5 [36.8] | 79.7 [35.8] |
| PFURTIS score, mean [SD] Range |
18.1 [3.96] 12-32 |
18.1 [4.15] 11-35 |
* All randomized patients who received at least 1 dose of study treatment. Bmi=body mass index; Pfurtis=physical features of upper respiratory tract infection scale; Wurss=wisconsin upper respiratory symptom survey.
Table 3: Baseline Demographics and Characteristics*
Primary endpoint
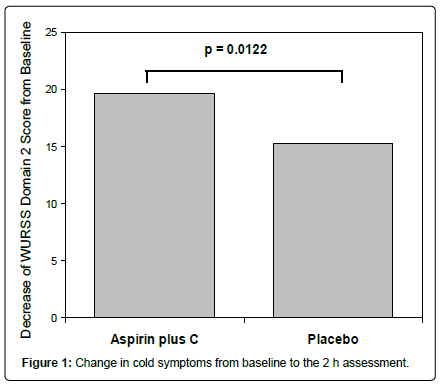
As shown in Figure 1, ASA plus C was significantly more effective than placebo in treating common cold symptoms 2 h after drug intake. The least squares means (LS means) WURSS domain 2 score decreased 19.6 points in the ASA plus C group vs. 15.2 points in placebo group. The difference between treatments (-4.4, 95% CI (-8.2, -0.57)) was statistically significant (P=0.012).
Secondary endpoints
With regard to the Change in Cold Symptoms (WURSS domain 2) from baseline to evening of Day 1, ASA plus C was significantly better than placebo — 20.6 point (LS means) decrease among ASA plus C patients vs. 15.6 point decrease (LS means) for the placebo group (LS means difference: -5.0, 95% CI (-9.6, -0.4); P=0.016) (Table 4). This trend continued for the evenings of Day 2 (30.4 for the ASA plus C group vs. 27.2 for the placebo-treated group) and Day 3 (43.2 for the ASA plus C group vs. 40.8 for the placebo group), but the LS means differences between groups at these timepoints were not statistically significant. For the evening of Day 4 and the evening of Day 5, no relevant differences between treatments were observed.
| Domain | ASA+C | Placebo | P-value |
|---|---|---|---|
| Domain 1 [Global Cold Severity] “How sick do you feel today?” | -0.1 [0.09] | 0.1 [-0.2] | 0.048 |
| Domain 2 [Cold Symptoms] “Do you have this symptom?” | -20.6 [1.77] | -15.6 [1.73] | 0.016 |
| Domain 1-3 [Common Cold Profile] | -26.0 [2.27] | -19.5 [2.22] | 0.015 |
| Domain 3 [Cold-specific Functional Impairments] “Over the last 24 h, how much has your cold interfered with your ability to…” | -5.1 [0.81] | -4.0 [0.77] | 0.138 |
* Values are least squares [LS] means [standard error of LS means].
Table 4: Selected Secondary Endpoints, Evening of Day 1 minus Baseline*
As shown in Table 4, for relief from their cold symptoms as measured by the change in Common Cold Profile (sum of WURSS domains 1–3) by the evening of Day 1, ASA plus C was better than placebo—26-point LS means decrease in the ASA plus C group vs. a 19.5-point LS means decrease in the placebo group, P=0.015 for the difference between groups (LS means difference=-6.5, 95% CI (-12.4, -0-7)). Patients in both treatment groups continued to show improvement when evaluated on the evenings of Days 2-5; however, the differences between treatments at these time points were not statistically significant.
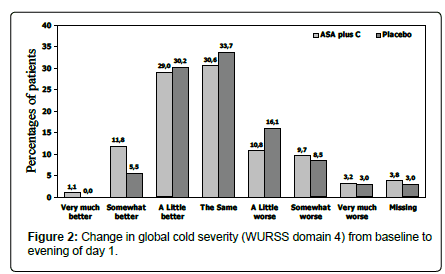
With regard to Global Cold Severity (WURSS domain 1), patients in both treatment groups had reduced scores on the evening of Day 1 compared to baseline (Table 4) and on all subsequent evenings (Days 2–5) of the treatment period, but differences between treatments were not significant. For domain 3 Cold-specific Functional Impairment there were no significant differences between treatment groups after Day 1 or at any other time point evaluated. At baseline, when asked “compared to yesterday, I feel that my cold is…” most patients (approximately 37% in each group) reported “the same” (WURSS domain 4, Change in Global Cold Severity). On the evening of day 1, 12.9% of patients in the ASA plus C group, but only 5.5% of patients in the placebo group reported that their cold was “somewhat better” or “very much better” (Figure 2). Differences were also seen on the evenings of day 2, 3 and 4, but the differences were diminishing.
Safety
Adverse events were reported by 19.0% of patients in the ASA plus C group and 14.6% of patients in the placebo group. Adverse events considered to be causally related to study treatment occurred in 11.6% of patients in the ASA plus C group and 7.0% of patients in the placebo group. The most commonly reported events were gastrointestinalrelated (for all events: 11.6% of ASA plus C patients and 9.5% of placebo patients; for events considered to be causally related: 9% for ASA plus C and 6% for placebo), with dyspepsia, upper abdominal pain and nausea occurring most frequently (Table 5). Adverse events leading to study discontinuation occurred in 5 ASA plus C patients (pyrexia, nausea/ vomiting, dizziness, acute bronchitis, upper abdominal pain) and 2 placebo patients (vasovagal syncope, nausea/eructation); all events resolved (Table 5). Most adverse events were mild or moderate (51% and 41%, respectively for ASA plus C and 51% and 40%, respectively for placebo). In the ASA plus C group, 6.8% of all AEs were severe and in the placebo group, 9.0% of allAEs were severe. There was 1 serious adverse event (severe hematemesis) and this occurred in a placebotreated patient. There were no deaths reported.
| Adverse Event [AE] | ASA+C [n=189] | Placebo [n=199] |
|---|---|---|
| Number of patients [%] | ||
| All reported | ||
| Patients with at least 1 AE | 36 [19.0] | 29 [14.6] |
| All gastrointestinal [GI] AEs | 22 [11.6] | 19 [9.5] |
| Most Common GI AEs* Nausea Dyspepsia Abdominal pain |
7 [3.7] 6 [3.2] 6 [3.2] |
5 [2.5] 1 [1.5] 2 [1.0] |
| Treatment-Related AEs | ||
| Any treatment-related AE | 22 [11.6] | 14 [7.0] |
| All treatment-related GI AEs | 17 [9.0] | 12 [6.0] |
| Most common treatment-related GI AEs* Dyspepsia Abdominal pain |
6 [3.2] 5 [2.6] |
3 [1.5] 2 [1.0] |
* Events occurring in >2% of patients in the ASA+C group.
Table 5: Adverse Events
Discussion
Self-medication is a frequent practice for the treatment of symptoms associated with the common cold or acute viral URTI. One key reason for self-medication is the expectation of rapid symptom relief. In our study of patients suffering from the common cold, we found that within 2 h following intake, ASA plus C provided significantly greater symptomatic relief than placebo.
Our trial is the first to evaluate ASA plus C in the treatment of common cold symptoms not only in a randomized, double-blind setting, but also using a comprehensive quality-of-life measurement of the negative effects of the common cold [11,12]. Furthermore, patients were enrolled in medical practices that best resembled real-life setting. This setting allowed the enrolment of patients with symptoms of recent onset (within 48 h). As a consequence, a at study entry, the population enrolled in our trial had a median cold duration of one day and thus were enrolled at a time of peak symptom severity. There was also more uniformity in the symptoms they presented, since URTI symptoms are well known to change as the disease progress to resolution [10]. On Day 1 of the study, we found that ASA plus C in a single-tablet formulation demonstrated superiority to placebo in treatment of common cold symptoms 2 h after drug intake as well as when evaluated on the evening of Day 1 (WURSS Domain 2). Also by the evening of Day 1, patients treated with ASA plus C had significantly greater improvement compared with placebo in the severity, symptoms and functional impairments of the common cold as measured by the Common Cold Profile (WURSS sum of domains 1–3) (P=0.015). While there was continued improvement on the evenings of Days 2-5, the differences compared with placebo were not significant. These results are not surprising, considering both the self-limiting nature of the common cold as well as our particular study population of patients with only mild to moderate symptom severity at baseline (as indicated by a mean PFURITIS score of 18 out of a possible 45 and WURSS Domain 2 score of 80-82 out of 224).
With regard to safety and tolerability, we found that overall incidence of adverse events as well as adverse events judged to be treatment-related were within the range of that commonly observed in trials with ASA up to 1,000 mg per dose [13-16]. The most common events in both treatment groups were gastrointestinal-related. The majority of adverse events were of mild or moderate intensity and only a few severe events occurred. No serious adverse events occurred.
The results of our study add to the small field of controlled, clinical trial-evidence on the use of ASA for the treatment of common cold symptoms. In early trials, ASA 800 mg and ASA 1300 mg were shown to be effective in treating sore throat pain due to tonsillo-pharyngitis [9,17]. In one study, however, no formal assessment on the efficacy of ASA vs. either placebo or paracetamol was made, since the study was designed to validate new scales on sore throat [17]; whereas in the other one, only the effects on sore throat–throat pain, pain relief, difficulty in swallowing, degree of swollen throat and on body temperature (in the subset of patients, approximately 50%, that showed a raise in temperature above 37°) were considered [9]. Several years later, Eccles and colleagues compared 2 effervescent tablets of ASA 400 mg plus 240 mg ascorbic acid [7,18] with placebo for the treatment of sore throat pain and other pain symptoms of the common cold. These investigators found that ASA 800 mg plus 480 mg ascorbic acid was significantly more effective than placebo in relieving sore throat pain as well as headache and muscle aches and pain [7]. A study focused on the efficacy of ASA 500-1000 mg on fever from URTI (mean baseline temperature 38.96°) showing comparable efficacy vs. paracetamol at the same doses [8]. Analysis from secondary endpoints showed efficacy also on headache, achiness and feverish discomfort; besides other secondary endpoints were not met, like reduction of sore throat intensity, it must be noted that the study was stopped after the second interim analysis since all the predefined null-hypothesis were rejected, therefore secondary analysis was conducted on a lower-than-planned sample size. In another study, ASA 500-1000 mg daily plus pseudoephedrine was found to be more effective than placebo in providing symptomatic relief of sore throat pain associated with the common cold [19]. Likewise, in a study of the evaluation of nasal congestion associated with the common cold, ASA plus pseudoephedrine was found to be more effective than placebo in relieving muscle aches associated with the common cold. Conversely, our study is the first to address URTI symptoms in their totality, by means of a structured questionnaire that not only considers pain, but also, among others, cough, ear, eyes and chest symptoms, lack of energy and loss of appetite. Moreover, one domain of the WURSS questionnaire specifically addresses the effects of URTI on daily activities. This is the first study to show that ASA, in combination with vitamin C, is effective in relieving the wide array of symptoms of the common cold. It showed also a trend, albeit no- significant, towards an improvement of functions that are specifically impaired by cold.
In conclusion, we found ASA plus C to be more effective than placebo in providing early relief of the symptoms of the common cold and was well tolerated. Providing symptomatic relief during the first 24-48 h of common cold onset [peak symptomatic period] improves quality-of-life and provides important benefits to the patients carrying out normal daily activities.
References
- Monto AS, Monto AS (2002) Epidemiology of viral respiratory infections. Am J Med 112: 4-12.
- Taverner D, Latte J (2007) Nasal decongestants for the common cold. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD001953.
- Fendrick AM, Monto AS, Nightengale B (2003) The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med 163: 487-494.
- Gwaltney JM (2002) Clinical significance and pathogenesis of viral respiratory infections. Am J Med 112: 13-18.
- Eccles R, Loose I, Jawad M (2003) Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Med 4: 118-124.
- Bachert C, Chuchalin AG, Eisebitt R (2005) Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: A multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6 h dose-ranging study. Clin Ther 27: 993-1003.
- Schachtel BP, Fillingim JM, Lane AC (1991) Caffeine as an analgesic adjuvant. A double-blind study comparing aspirin with caffeine to aspirin and placebo in patients with sore throat. Arch Intern Med 151: 733-377.
- Eccles R (2005) Understanding the symptoms of the common cold and influenza. Lancet Infect Dis 11: 718-725.
- Barrett B, Locken K, Maberry R (2002) The Wisconsin Upper Respiratory Symptom Survey (WURSS). A new research instrument for assessing the common cold. J Fam Prac 51: 249-257.
- Barrett B, Brown R, Mundt M (2005) The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable and valid. J Clin Epidemiol 58: 609-617.
- Edwards JE, Oldman A, Smith L (1994) Single dose oral aspirin for acute pain. The Cochrane Database of Systematic Reviews 18: CD002067.
- Edwards JE, Oldman AD, Smith LA (1999) Oral aspirin in postoperative pain: A quantitative systematic review. Pain 81: 289-297.
- Voelker M (2004) Safety and tolerability of aspirin in randomised controlled clinical trials. Drug Saf 27: 968.
- Steiner T, Voelker M (2008) Gastrointestinal tolerability of aspirin and the choice of over-the-counter analgesia for primary headache disorders. Poster presented at the European Headache and Migraine Trust International Congress 2008, September 4-7, London, UK.
- Schachtel BP, Fillingim JM, Beiter DJ, Lane AC, Schwartz LA (1984) Rating scales for analgesics in sore throat. Clin Pharmacol Ther 36: 151-156.
- Data on file, Bayer HealthCare AG, Loose I, Report No. PH-32006, 2002-05-06. Loose I, Scanner K, Brown A (2003) Combination of acetylsalicylic acid and pseudoephedrine for the symptomatic treatment of sore throat. Poster presented at the 4th Congress of the European Federation of the International Association for the Study of Pain Chapters. Pain in Europe IV, Prague, Czech Republic, September 2-6, 2003.
- Loose I, Winkel M. (2004) Clinical, double-blind, placebo-controlled study investigating the combination of acetylsalicylic acid and pseudoephedrine for the symptomatic treatment of nasal congestion associated with common cold. Drug Res 54: 513-521.
Citation: Sessa A, Voelker M (2017) Aspirin plus Vitamin C Provides Better Relief than Placebo in Managing the Symptoms of the Common Cold. J Health Care Prev 1: 102.
Copyright: © 2017 Sessa A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 8198
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 7299
- PDF downloads: 899