Aspirin Inhibition of α-Granules Release is Associated with Sirt1/AMPK and PI3K/Akt Pathway in Thrombin-Activated Platelets
Received: 07-Jun-2016 / Accepted Date: 27-Jun-2016 / Published Date: 04-Jul-2016
Abstract
Objectives: To investigated the effect of aspirin on inhibition of platelet α-granules release in thrombin-activated platelets.
Methods: Human platelets were used in the present study. Protein expression and phosphorylation was determined by Western blot. The secreted CD40L and PF4 from platelets were detected by ELISA.
Results: Aspirin reduced CD40L and PF4 expression in thrombin-activated platelets. Sirt1 expression and AMPK phosphorylation was decreased by 24.4% and by 19.2% in thrombin-activated platelets. In contrast, aspirin dosedependently recovered Sirt1 expression and AMPK phosphorylation. EX527 (a Sirt1 inhibitor) treatment resulted in an increase in CD40L and PF4 expression. The combination of EX527 and aspirin weakened the inhibitory effect of aspirin on platelet aggregation, the expression of CD40L and PF4. Moreover, aspirin abolished the phosphorylation of Akt inthrombin-activated platelets. Aspirin has a synergistic action with LY294002 (an Akt inhibitor) on inhibition of thrombin-induced CD40L and PF4 expression.
Conclusions: Our results provide new evidence that the aspirin inhibiting platelet α-granules release not only is associated with suppressing Akt activation but also linked to activating Sirt1/AMPK pathway in thrombin-activated platelets
Keywords: Aspirin; Platelets; Sirt1; CD40 ligand; Platelet Factor 4; Thrombin
78552Introduction
Growing evidence suggests that platelets play a critical role in the onset of vascular inflammation in atherosclerosis [1-3]. Platelets are derived from megakaryocytes that contain abundant α -granules, dense granules, and lysosomes. Once platelets become activated, they release multiple bioactive mediators that are involved in vascular inflammation [4-6]. CD40L and PF4 belong to the chemokine family and are stored in platelet α -granules. The major sCD40L identified is believed to have originated from activated platelets [7]. Interactions of CD40/ CD40L initiate the inflammatory response, including the synthesis of interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF). Clinical studies have shown that serum CD40L level is associated with patient outcome in cardiovascular disease and atherosclerosis [8,9]. PF4 promotes the migration of monocytes to vascular lesions in atherosclerosis [10]. PF4 can enhance the binding of ox-LDL to vascular wall cells, and it also promotes endocytosis and accelerates the formation of foam cells [11]. Our previous studies have shown that serum levels of platelet factor 4 (PF4), chemokine CCL5 (RANTES), and CD40 ligand (CD40L) are increased in ApoE-/- mice [12]. Although these molecules possess their own characteristics, they have a common action of promoting the inflammatory response in atherosclerosis. Sirt1, which is a nicotinamide adenine dinucleotidedependent protein deacetylase, has been studied for its role in caloric restriction, the prevention of aging-related disease and the maintenance of metabolic homeostasis [13-15]. Sirt1 is involved in redox reactions in cell metabolism and increases the lifespan of rodents. The mechanisms that underlie the beneficial effects of Sirt1 include antioxidant effects and the up regulation of endothelial nitric oxide synthase [16,17]. AMP-activated protein kinase (AMPK) is a kinase that has a key role in metabolism and has been shown to regulate Sirt1 activity. AMPK activator can lead to Sirt activation through an increase in the intracellular levels of NAD+ [18]. We have recently reported that Sirt1 expression is unregulated in ginkgolide B-induced endothelial cell protection [19]. However, the role of Sirt1/AMPK pathway in regulation of platelet function remains less understood. A number of studies demonstrated that PI3K/Akt pathway is involved in platelet activation in various agonists including thrombin-stimulated platelets [20]. Akt is a serine/threonine kinase that has been demonstrated to play an important role in survival when cells are exposed to different apoptotic stimuli. Akt signaling pathway is currently attracting considerable attention as new target for effective therapeutic strategies. Aspirin as a classic drug is used for inhibiting platelet function and has been used extensively to prevent and treat cardiovascular disease. Aspirin reduces cardiovascular events by blocking cyclooxygenase-1 (COX-1) and decreasing thromboxane A2 generation in platelets [21,22]. Recently, several studies reported that an aspirin target Sirt1 and AMPK to induce senescence of colorectal carcinoma cell, and aspirin attenuates vinorelbine-induced endothelial inflammation via modulating Sirt1/AMPK axis [23,24]. Therefore, we asked whether aspirin has an influence on Sirt1/AMPK pathway in activated platelets. In the present study, we investigated the effects of aspirin on platelet release and Sirt1/AMPK pathway in thrombin-activated platelets.
Materials and Methods
Ethics statement
Blood was collected from healthy donors, from whom we received written informed consent. Experiments were conducted according to the principles expressed in the Declaration of Helsinki. This study was approved by the Ethics Committee of the Beijing Institute of Geriatrics.
Materials:
Aspirin, phenylmethylsulfonyl fluoride, and leupeptin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-Akt, anti-Sirt1and anti-AMPK antibodies were purchased from Cell Signaling Technologies (Danvers, MA, USA). Anti-PF4 and anti- CD40L antibodies were purchased from Abcam (Cambridge, MA, USA). Human CD40L (ab99991) and PF4 (ab100628) ELISA kits were purchased from Abcam (Cambridge, MA, USA).
Platelet preparation
Citrate anti-coagulated venous blood was obtained from human donors who had not taken any medication for a minimum of two weeks before collection. The blood was centrifuged at 200 g for 15 min to obtain platelet-rich plasma. The platelets were washed twice in Tyrode’s/HEPES buffer with 2 mM ethylene glycol tetraacetic acid (EGDA) and re-suspended in Tyrode’s/HEPES buffer at a concentration of 1108 cells/ml.
Platelet aggregation measurement
Platelet aggregation was measured using a CHRONO-LOG aggregometer. Washed platelet was used in the experiments. Platelet aggregation ratio was calculated by Aggrolink software (Chrono-Log).
Western blotting
The platelets were pretreated by different concentrations of aspirin or EX527 for 10 min, then the sample was stirred at 1200 rpm at 37°C for 1 min. Thrombin (0.5 U/ml) was added into the sample for 5 min to induce platelet activation, then 5 x SDS solution was added into cuvette. Lysate were boiling for 5 min for Western blotting. Platelets lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene fluoride membranes. The membranes were blocked with 1% bovine serum albumin and then incubated with specific antibodies. After three washes in 0.5% Tween 20 phosphate-buffer saline (TPBS), the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies in TPBS. The bands were detected by chemi-luminescence detection reagents. Blot densitometry was then performed, and the bands were analyzed by VILBER LOURMAT (Torcy, Paris, France).
ELISA assay
Washed platelets were incubated with the various concentrations of aspirin for 5 min and the cuvette was move to in CHRONO-LOG aggregometer, thrombin (0.5 U/ml) was added to induce platelet activation. After response for 5min, the samples were centrifuged at 10,000 rpm at 4°C for 30 min. The supernatant was collected as the platelet secreted products. The levels of CD40L and PF4 were assayed using ELISA kits (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions.
Statistical analysis
The data are present as mean ± SEM of at least four experiments. Statistical differences between two groups were analyzed by two-tail unpaired Student’s t-test. All calculations were performed using SPSS 13.0 statistical software (Armonk, NY, USA). A value of p< 0.05 was considered significant.
Results
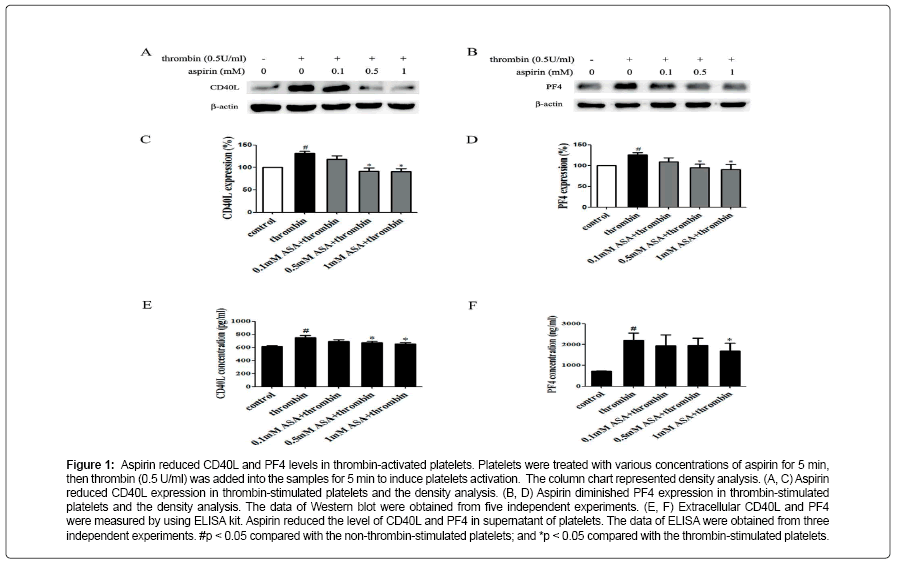
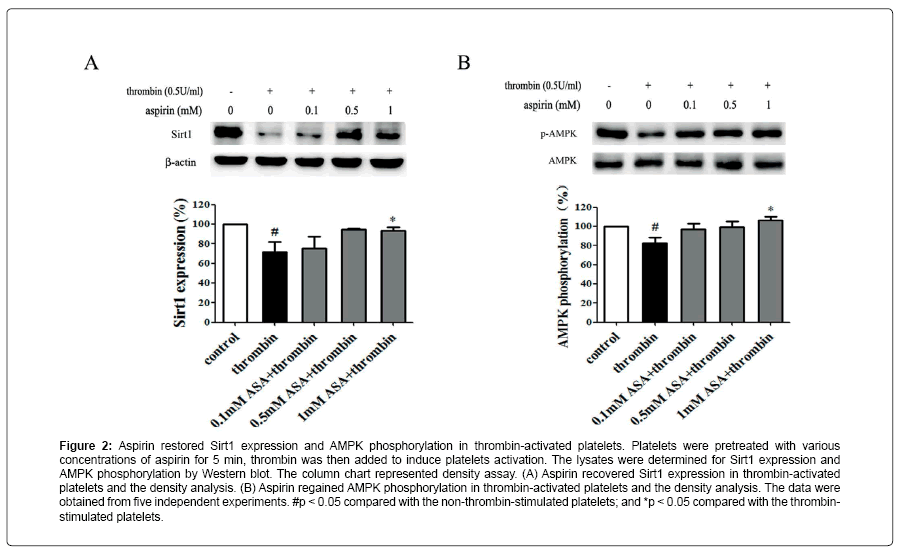
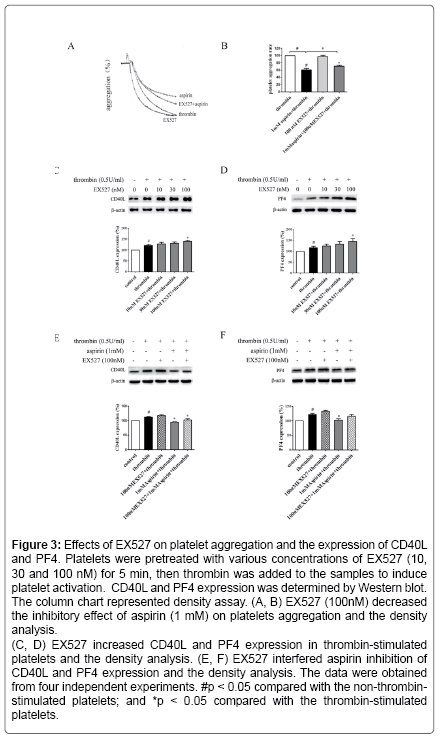
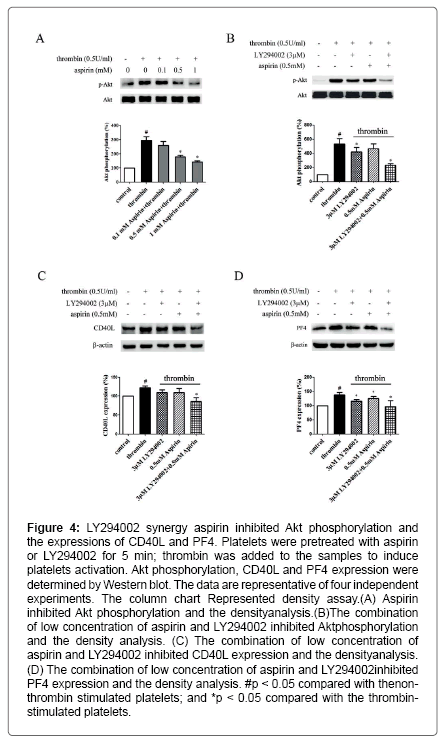
Aspirin decreases the levels of CD40L and PF4 in thrombinactivated platelets. Platelets are involved in inflammation through release various inflammatory mediators from α-granules and densy granules to circulating blood. In the present study, we first determined the effect of aspirin on CD40L and PF4 in thrombin-activated platelets. Platelets were incubated by 0.1, 0.5 and 1 mM aspirin for 10 min, 0.5 U/ml thrombin then was added for 5 min. As shown in Figure 1A-D, CD40L and PF4 expression on protein level was increased by 49.7% and 24.1% in thrombin-stimulated platelets, whereas treatment with aspirin (0.5 and 1 mM) resulted in a significant reduction in CD40L and PF4 expression. Moreover, the extracellular CD40L and PF4 concentrations were measured in the supernatant of platelets as platelets secreted product. As shown in Figure 1E and F, the level of CD40L and PF4 increased by 12.5% and 15.6% in thrombin-stimulated platelets, compared with it, aspirin dose-dependently decreased their secretion. Aspirin recovers Sirt1 expression and AMPK phosphorylation in thrombin-activated platelets Sirt1/AMPK has been described as a protective pathway in vascular cells and adipose tissue. However, the action of Sirt1/AMPK in activated platelets remains unclear. To investigate whether aspirin inhibition of CD40L and PF4 release is associated with Sirt1/AMPK pathway, we detected Sirt1 expression and AMPK phosphorylation by Western blotting. As shown in Figure 2A and B, Sirt1 expression was higher in resting platelets and it was down-regulated by thrombin stimulation, the level of sirt1 expression was decreased by 24.4% in thrombin-stimulated platelets. In contrast, aspirin (0.5 and 1 mM) significantly recovered Sirt1 expression. AMPK is a downstream effector in the Sirt1 signaling [25]. Thrombin stimulation also decreased AMPK phosphorylation by 19.2%, and aspirin recovered this phosphorylation. Blocking Sirt1 interference the effect of aspirin on inhibition of platelet function in thrombin-activated platelets. To determine the effect of Sirt1 inhibition on platelet function, we used EX527 (Sirt1 inhibitor) in the present study. We first evaluated the effect EX527 on platelet aggregation. As shown in Figure 3A and B, EX527 alone had no market impact on platelet aggregation, however, when both of combination EX527 partially reduced the effect of aspirin on inhibiting platelet aggregation induced by thrombin. In addition, we further determined the effect of EX527 on CD40L and PF4 expression. EX527 treatment also let to an increase in CD40L and PF4 expression in thethrombin-activated platelets (Figure 3C and D). Moreover, we investigated whether EX527 has contrary action against aspirin. The result showed that aspirin (1 mM) could completely suppress CD40L and PF4 expression induced by thrombin, whereas the combination with EX527 (100 nM) weakened inhibitory effect of aspirin on PF4 expression (Figure 3E and F). Aspirin synergy LY294002 suppresses Akt phosphorylation, the expression of CD40L and PF4 in thrombinactivated platelets. The involvement of the PI3K/Akt pathway in thrombin-induced platelet activation has been described by previous studies [26]. To investigate whether the aspirin-induced inhibition of CD40L and PF4 is linked to PI3K/Akt pathway, the phosphorylation of Akt was examined. As shown in Figure 4A, Akt phosphorylation was increased by 25.7% in thrombin stimulated platelets, whereas aspirin (1 mM) completely attenuated this phosphorylation. To address the action of aspirin on inhibition of CD40L and PF4 expressions is linked to Akt, we used LY294002 (a PI3K specific inhibitor) in the present study. As shown in Figure 4B-D, the concentration of LY294002 (3 μM) and aspirin (0.5 mM) alone just partly suppressed Akt phosphorylation, but the combination of both completely attenuated Akt phosphorylation. Moreover, LY294002 (3 μM) and aspirin (0.5 mM) alone could not fully inhibit CD40L and PF4 expression, however, the combination of both completely abolished thrombin-induced CD40L and PF4 expression.
Figure 1: Aspirin reduced CD40L and PF4 levels in thrombin-activated platelets. Platelets were treated with various concentrations of aspirin for 5 min, then thrombin (0.5 U/ml) was added into the samples for 5 min to induce platelets activation. The column chart represented density analysis. (A, C) Aspirin reduced CD40L expression in thrombin-stimulated platelets and the density analysis. (B, D) Aspirin diminished PF4 expression in thrombin-stimulated platelets and the density analysis. The data of Western blot were obtained from five independent experiments. (E, F) Extracellular CD40L and PF4 were measured by using ELISA kit. Aspirin reduced the level of CD40L and PF4 in supernatant of platelets. The data of ELISA were obtained from three independent experiments. #p < 0.05 compared with the non-thrombin-stimulated platelets; and *p < 0.05 compared with the thrombin-stimulated platelets.
Figure 2: Aspirin restored Sirt1 expression and AMPK phosphorylation in thrombin-activated platelets. Platelets were pretreated with various concentrations of aspirin for 5 min, thrombin was then added to induce platelets activation. The lysates were determined for Sirt1 expression and AMPK phosphorylation by Western blot. The column chart represented density assay. (A) Aspirin recovered Sirt1 expression in thrombin-activated platelets and the density analysis. (B) Aspirin regained AMPK phosphorylation in thrombin-activated platelets and the density analysis. The data were obtained from five independent experiments. #p < 0.05 compared with the non-thrombin-stimulated platelets; and *p < 0.05 compared with the thrombinstimulated platelets.
Figure 3: Effects of EX527 on platelet aggregation and the expression of CD40L and PF4. Platelets were pretreated with various concentrations of EX527 (10, 30 and 100 nM) for 5 min, then thrombin was added to the samples to induce platelet activation. CD40L and PF4 expression was determined by Western blot. The column chart represented density assay. (A, B) EX527 (100nM) decreased the inhibitory effect of aspirin (1 mM) on platelets aggregation and the density analysis.
(C, D) EX527 increased CD40L and PF4 expression in thrombin-stimulated platelets and the density analysis. (E, F) EX527 interfered aspirin inhibition of CD40L and PF4 expression and the density analysis. The data were obtained from four independent experiments. #p < 0.05 compared with the non-thrombinstimulated platelets; and *p < 0.05 compared with the thrombin-stimulated platelets.
Figure 4: LY294002 synergy aspirin inhibited Akt phosphorylation and the expressions of CD40L and PF4. Platelets were pretreated with aspirin or LY294002 for 5 min; thrombin was added to the samples to induce platelets activation. Akt phosphorylation, CD40L and PF4 expression were determined by Western blot. The data are representative of four independent experiments. The column chart Represented density assay.(A) Aspirin inhibited Akt phosphorylation and the densityanalysis.(B)The combination of low concentration of aspirin and LY294002 inhibited Aktphosphorylation and the density analysis. (C) The combination of low concentration of aspirin and LY294002 inhibited CD40L expression and the densityanalysis. (D) The combination of low concentration of aspirin and LY294002 inhibited PF4 expression and the density analysis. #p < 0.05 compared with thenonthrombin stimulated platelets; and *p < 0.05 compared with the thrombinstimulated platelets.
Discussion
Recent studies have demonstrated that Sirt1 plays a protective role on vascular and atherosclerosis [27]. The endothelial overexpression of Sirt1 has been shown to diminish plaque formation in a mouse model of atherosclerosis [28]. Sirt1 mediates vasodilatation via eNOS-derived nitric oxide (NO) and scavenging reactive oxygen species (ROS) [29,30]. Some studies reported that aspirin enhances Sirt1 expression in endothelial and liver cells [31,32]. Our previous study showed that Sirt1 is involved in the regulation of LOX-1 and ICAM-1 expression in ox-LDL-stimulated human endothelial cells. Therefore, we desired to know whether Sirt1 has an influence for the regulation of platelet function. In the present study, the results showed that thrombin stimulation resulted in a decrease in Sirt1 expression. Since AMPK is upstream molecule of Sirt1 and it can regulate Sirt1 activity, we detected also the phosphorylation of AMPK. Similarly the phosphorylation of AMPK was reduced in thrombin-activated platelets, whereas the treatment with aspirin recovered Sirt1 expression and AMPK phosphorylation. Moreover, blocking Sirt1 activity with EX527 leads to increase in CD40L and PF4 expression and reduced aspirininduced inhibitory effect on CD40L and PF4 expression in thrombinactivated platelets. This suggested that aspirin has impact on Sirt1/ AMPK pathway in thrombin-activated platelets. Previous studies have demonstrated that PI3K/Akt pathway is involved in platelet activation in thrombin-stimulated platelets [33]. Our data further exhibits that aspirin inhibition of platelet release is associated with suppressing Akt phosphorylation. Aspirin has a synergetic effect with LY294002 on inhibiting Akt phosphorylation, and the expression of CD40L and PF4. This suggested that aspirin decreases the level of CD40L and PF4 is linked to suppress Akt phosphorylation. Our previous study in vivo also indicated that aspirin could reduce plasma CD40L and PF4, and plaque area in ApoE gene defective mice [12]. These implied that aspirin inhibition platelet function not only mediates classical cyclooxygenase (COX), but also targeting PI3K/Akt and Sirt1/AMPK pathway. Previous studies have demonstrated that there is an association between Sirt1/ AMPK and PI3K/Akt pathways. Overexpresison of Sirt1 could enhance the phosphorylation of Akt in endothelial progenitor cells (EPCs). LY294002 (a PI3K inhibitor), abolished Ad-SIRT1-induced migration and proliferation of EPCs [24]. Recently another study demonstrated that Sirt 2 is a new player in regulation of platelet function. Sirt2 was present in platelets at the level of mRNA and protein, and its specific inhibition reduced platelet responses. Inhibition of SIRT2 increased theacetylation of Akt kinase, which in turn blocked agonist-induced Akt phosphorylation [34]. However, our result showed that Sirt1 inhibition resulted in an increase in platelet aggregation and release. Inhibition of Akt phosphorylation also led to reduction of platelet release inflammatory mediators. The limitation of the study is lacking determination on the association between Sirt1 and Akt activation in thrombin-activated platelets.
Conclusion
Aspirin can reduce the CD40L and PF4 expression and release induced by thrombin. The mechanism may be associated with recovering Sirt1 expression, AMPK phosphorylation, and suppressing Akt activity. This suggested that aspirin possesses more targets on inhibition of platelet function.
References
- Gawaz M (2006) Platelets in the onset of atherosclerosis. Blood Cells Mol Dis 36: 206-210.
- King SM, McNamee RA, Houng AK, Patel R, Brands M, et al. (2009) Platelet dense-granule secretion plays a critical role in thrombosis and subsequent vascular remodeling in atherosclerotic mice. Circulation 120: 785-791.
- Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, et al. (2009) Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med 15: 97-103.
- Gleissner CA, von Hundelshausen P, Ley K (2008) Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol 28: 1920-1927.
- Enomoto Y, Adachi S, Matsushima-Nishiwaki R, Doi T, Niwa M, et al. (2010) Thromboxane A2 promotes soluble CD40 ligand release from human platelets. Atherosclerosis 209: 415-421.
- Sachais BS, Turrentine T, Dawicki McKenna JM, Rux AH, Rader D, et al. (2007) Elimination of platelet factor 4 (PF4) from platelets reduces atherosclerosis in C57Bl/6 and apoE-/- mice. Thromb Haemost 98: 1108-1113.
- Hausding M, Jurk K, Daub S, Kröller-Schön S, Stein J, et al. (2013) CD40L contributes to angiotensin II-induced pro-thrombotic state, vascular inflammation, oxidative stress and endothelial dysfunction. Basic Res Cardiol 108: 386.
- Yu H, Segers F, Sliedregt-Bol K, Bot I, Woltman AM, et al. (2013) Identification of a novel CD40 ligand for targeted imaging of inflammatory plaques by phage display. FASEB JÂ 27: 4136-4146.
- Wang JH, Zhang YW, Zhang P, Deng BQ, Ding S, et al. (2013) CD40 ligand as a potential biomarker for atherosclerotic instability. Neurol Res 35: 693-700.
- Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, et al. (2000)The beta- thromboglolins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Bio l67: 471-478.
- Nassar T, Sachais BS, Akkawi S, Kowalska MA, Bdeir K, et al. (2003) Platelet factor 4 enhances the binding of oxidized low-density lipoprotein to vascular wall cells. J Biol Chem 278: 6187-6193.
- Liu X, Zhao G, Yan Y, Bao L, Chen B, et al. (2012) Ginkgolide B reduces atherogenesis and vascular inflammation in ApoE-/- mice. PLoS One 7: e36237.
- Wang F, Chen HZ, Lv X, Liu DP (2013) SIRT1 as a novel potential treatment target for vascular aging and age-related vascular diseases. Curr Mol Med 13: 155-164.
- Yao H, Rahman I (2012) Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol 84: 1332-1339.
- Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225-238.
- Hubbard BP, Sinclair DA (2014) Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35: 146-154.
- Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, et al. (2012) Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: protective role of eNOS and SIRT1. PLoS One 7: e29598.
- Nin V, Escande C, Chini CC, GiriS, Pereira JC, et al. (2012) Role of Deleted in Breast Cancer 1 (DBC1) Protein in SIRT1 Deacetylase Activation Induced by Protein Kinase A andAMP-activated Protein Kinase. J Biol Chem 287: 23489-23501.
- Ma L, Liu X, Zhao Y, Chen B, Li X, et al. (2013) Ginkgolide B reduces LOX-1 expression by inhibiting Akt phosphorylation and increasing Sirt1 expression in oxidized LDL-stimulated human umbilical vein endothelial cells. PLoS One 8: e74769.
- Moroi AJ, Watson SP (2015) Impact of the PI3-kinase/Akt pathway on ITAM and hemITAM receptors: haemostasis, platelet activation and antithrombotic therapy. Biochem Pharmacol 94: 186-194.
- Dragani A, Pascale S, Recchiuti A, Mattoscio D, Lattanzio S, et al. (2010) The contribution of cyclooxygenase-1 and -2 to persistent thromboxane biosynthesis in aspirin-treated essential thrombocythemia: implications for antiplatelet therapy. Blood 115: 1054-1061.
- Pulcinelli FM, Biasucci LM, Riondino S, Giubilato S, Leo A, et al. (2009) COX-1 sensitivity and thromboxane A2 production in type 1 and type 2 diabetic patients under chronic aspirin treatment. Eur Heart J 30: 1279-1286.
- Jung YR, Kim EJ, Choi HJ, Park JJ, Kim HS, et al. (2015) Aspirin Targets SIRT1 and AMPK to Induce Senescence of Colorectal Carcinoma Cells. Mol Pharmacol 88: 708-719.
- Tsai KL, Huang PH, Kao CL, Leu HB, Cheng YH, et al. (2014) Aspirin attenuates vinorelbine-induced endothelial inflammation via modulating SIRT1/AMPK axis. Biochem Pharmacol 88: 189-200.
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, et al. (2008) SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 3: 20015-20026.
- Chen J, De S, Damron DS, Chen WS, Hay N, et al. (2004) Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood 104: 1703-1710.
- Stein S, Matter CM (2011) Protective roles of SIRT1 in atherosclerosis. Cell Cycle 10: 640-647.
- Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, et al. (2008) Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 80: 191-199.
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, et al. (2007) SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855-14860.
- Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, et al. (2012) SIRT1 activating compounds reduce oxidative stress and prevent cell death inneuronal cells. Front Cell Neurosci 6: 63.
- Kamble P, Selvarajan K, AlugantiNarasimhulu C, Nandave M, Parthasarathy S, et al. (2013) Aspirin may promote mitochondrial biogenesis via the production of hydrogen peroxide and the induction of Sirtuin1/PGC-1α genes. Eur J Pharmacol 699: 55-61.
- Jones CI, Sage T, Moraes LA, Vaiyapuri S, Hussain U, et al. (2014) Platelet endothelial cell adhesion molecule-1 inhibits plateletresponse to thrombin and von Willebrand factor by regulating the internalization of glycoprotein Ib via AKT/glycogen synthase kinase-3/dynamin and integrin αIIbβ3. Arterioscler Thromb Vasc Biol 34: 1968-1976.
- Li W, Du D, Wang H, Liu Y, Lai X, et al. (2015) Silent information regulator 1 (SIRT1) promotes the migration and proliferation of endothelial progenitor cells through the PI3K/Akt/eNOS signaling pathway. Int J Clin Exp Pathol 8: 2274-2287.
- Moscardó A, Vallés J, Latorre A, Jover R, Santos MT, et al. (2015)The histone deacetylasesirtuin 2 is a new player in the regulation of platelet function. J Thromb Haemost 13: 1335-1344.
Citation: Sun J, Zhang M, Liu X, Zhao X, Jiang Y, et al. (2016) Aspirin Inhibition of α-Granules Release is Associated with Sirt1/AMPK and PI3K/Akt Pathway in Thrombin-Activated Platelets. Cardiovasc Ther 1: 111.
Copyright: © 2016 Sun J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 13017
- [From(publication date): 6-2016 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 12057
- PDF downloads: 960