Research Article Open Access
Arsenic Distribution in Shoots of the Halophyte Plant Species Atriplex atacamensis Growing in an Extreme Arid Mining Area from Northern Chile
| Delphine Vromman1, Bérengère Paternostre1, Margarita Briceño2, Carolina Teixeira-Cardoso1, Alejandra Flores-Bavestrello2, Nicolas Vanhecke1, Mahendra Kumar3, Juan-Pablo Martínez4 and Stanley Lutts1* | |
| 1Groupe de Recherche en Physiologie végétale, Earth and Life Institute-Agronomy, Université catholique de Louvain, Louvain-la-Neuve, Belgium | |
| 2Facultad de Ciencias de la Salud, Universidad Arturo Prat, Av. Arturo Prat 2120 Iquique, Chile | |
| 3Science Research, Av. Costanera 4976, Iquique, Chile | |
| 4Instituto de Investigaciones Ageopecuarias (INIA – La Cruz), Chorillos n°86, La Cruz, Chile | |
| *Corresponding Author : | Stanley Lutts Groupe de Recherche en Physiologie végétale Earth and Life Institute-Agronomy, Université catholique de Louvain Louvain-la-Neuve, Belgium E-mail: Stanley.lutts@uclouvain.be |
| Received March 30, 2016; Accepted April 19, 2016; Published April 25, 2016 | |
| Citation: Vromman D, Paternostre B, Briceño M, Teixeira-Cardoso C, Flores-Bavestrello A, et al. (2016) Arsenic Distribution in Shoots of the Halophyte Plant Species Atriplex atacamensis Growing in an Extreme Arid Mining Area from Northern Chile. J Bioremed Biodeg 7: 349. doi:10.4172/2155-6199.1000349 | |
| Copyright: © 2016 Vromman D, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The oasis of Quillagua is an As contaminated area located in the North part of Chile along the Rio Loa. This study was undertaken in order to quantify As accumulation in water, soil and plants of the xerohalophyte species Atriplex atacamensis growing along the river. The water samples from the Rio Loa contained up to 2.5 mg L-1 arsenic and were highly saline (EC 14.1 mS cm-1). High concentration of B was associated to near-neutral pH values. The soil was typically corase – textured with a high proportion of sand. A mean As concentration of 220 mg Kg-1 was recorded in the soil and an important proportion was associated to iron oxide. Mature plants of Atriplex atacamensis were able to grow on this contaminated area and accumulated moderate amounts of As in the shoots. Arsenic concentration was higher in the leaves tan in the stems, but consistent amounts of As accumulated in the mature seeds and in surrounding bracts. The vegetative plant organs also accumulated high amounts of Na, confirming the halophytic nature of the plant species. Mature shoots are characterized by low amounts of P, probably as a consequence of As competition for P transporters, and by high amounts of S, probably resulting from phytochelatins and glutathione over synthesis.
| Keywords |
| Atriplex atacamensis; Groundwater; Phytoremediation; Arsenic |
| Introduction |
| In Northern Chile, the region of Antafogasta is a major producer of copper ore [1]. This mining area is extremely arid with a mean annual rainfall lower than 5 mm year-1. One single river issued from Andean mountains is present in this zone: the Rio Loa has 440 km long and constitutes the main water resource for 420,000 inhabitants as well as for the mining industry [2]. The quality of water in the whole basin is rather poor, because of high salinity but also because of the presence of arsenic that is worsened by extremely arid conditions. The soil of this region is characterized by the absence of surface horizons and very low organic matter [3]. |
| The level of As concentration in water of Rio Loa was quantified between 100 μg l-1 to 1000 μg l-1 with an average of 440 μg l-1 [4]. It comes from natural sources such as intense volcanic activity (eruption, geysers, etc.). The Rio Loa is As contaminated by the geothermal springs of El Tatio (up to 27 mg l-1 in the water) located in the Andes and erosion of volcanic rocks [2]. Arsenic may also be spread in the environment as a consequence of anthropogenic activities mainly in relation to mining industry. Mining activity in the Loa basin takes place in the intensively mineralized porphyry-Cu belt with developments at large Cu deposits and release of SO2 and As2O3 into air [5]. |
| At the beginning of the 1960s the first dermatological problems caused by arsenic were noted in northern Chile especially amongst children [6]. Smith et al. [7] estimated that an exposure to As in drinking water to concentrations in the order of 500 μg l-1 of As was responsible for around 7% of all deaths occurring between 1989 and 1993 in Antofagasta. Arsenic causes a variety of adverse health effects to humans such as skin-pigmentation changes, keratosis, skin cancer, cardiovascular problems and respiratory disease. More recently, chronic arsenic ingestion has been linked to lung and bladder cancer [6]. Today, the urban population of major cities in Northern Chile uses treated water and desalted seawater. However, rural communities still largely rely on untreated water supplies which contain high As concentration [8]. |
| The Loa River is a fragile environment but constitutes a habitat for endemic animal and plant species. These species show adaptation to this extremely arid and contaminated region. The vegetation coverage offers a unique opportunity to study the putative link between local geomorphological factors and As accumulation in living organisms [9]. Arsenic bioavailability for plant uptake is directly influenced by edaphic properties such as pH, redox potential, structure and texture of soil, organic matter and the presence of iron oxide [10,11]. The saltbush Atriplex atacamensis is endemic of this region and can survive in this extremely hostile environment. This highly branched dioecious shrub, measures up to 3 m of height and is able to produce high amount of biomass. The leaves of 10 to 25 mm in length and 8-15 mm in width are alternate with small and axillary leaves [12]. On the surface of leaves, trichomes shelters salt crystals that give a white or gray color to the leaf. The seed (1 to 3 mm of diameter) possesses a brown seed coat, protected by beige and oval bracts welded to their basis and broader than long. Like other perennial Amaranthaceae, A. atacamensis presents a deep and well-developed root system that is capable of taking large amounts of groundwater [13]. |
| Atriplex ssp. appears as suitable candidates to rehabilitate arid areas [14]. The use of Atriplex species has also been recommended in heavymetal polluted environments [15,16]. Some studies about Atriplex atacamensis in a controlled environment have already suggested that A. atacamensis copes well with arsenic toxicity, but only few studies were devoted to A. atacamensis behavior in its natural environment [3,17]. The possibility to use this species as a bioindicator of As pollution and/ or as a promising material for phytoremediation purposes requires to analyze the ability of the plant to accumulate As in its natural environment, to determine As distribution in the different plant parts, and to analyze the relationship between As content in the plant and As content and bioavailability in the soil. |
| Materials and Methods |
| Site description, soil, water and plant sampling |
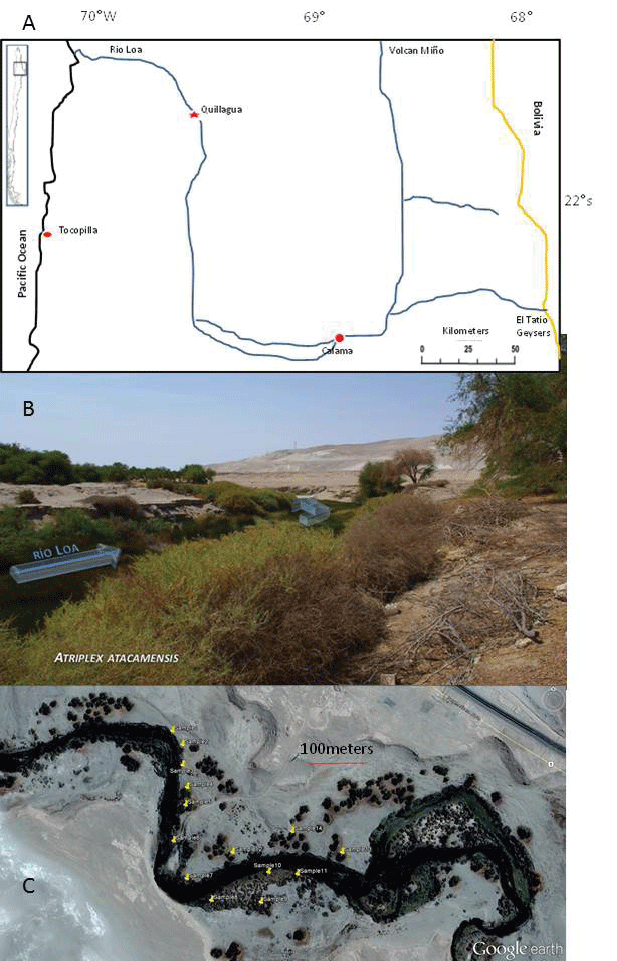
| Samples were collected in North of Chile, near the city of Quillagua (Latitude S of 21°37’; Longitude W 69°33’ (Figure 1) along the Rio Loa. The site is located at the border between the first and second Region of Chile. In the environment of Atriplex atacamensis, trees such as Prosopis sp., Geoffroea decorticans and aquatic plant as Scirpus americanus, Ruppia maritime and Stygeoclonium sp. were also observed. |
| Fourteen plants of A. atacamensis were identified (10 female and 4 male plants) on a surface area of 0.25 ha. The soil samples (approximately 500 g each) were taken to a depth of 0 to 40 cm (4 samples at the base of each plant were pooled). Five samples of water (200 mL each) were also taken from the Rio Loa, immediately filtered (chromafil 45 μm) and stored at low temperature. For each considered plant, stems and leaves were randomly harvested from the middle part of the plant. Fruits were also collected from 4-5 branches of female plants. Those fruits, still present on the plant, were produced during the growing season before the sample collection and comprise the seed enclosed in the bracts. Male flowers were similarly harvested on the male plants: at the time of harvest (February), male flowers were just mature and were at the full anthesis stage. The collected samples were incubated in an oven at 70°C during 48 h and then conserved under dry conditions until further analysis. |
| Soil analysis |
| For each soil sample, 10 g of soil were weighted and then heated at 105°C during 24 h. The humidity was calculated by difference between fresh weight and dry weight. Soil electrical conductivity (E.C., mS cm-1) was determined on another set of samples using conductimeter (Inolab Merck, Multi-Level 1) in a 1:5 (soil: water) solution. pH was measured in water with a 1:2.5 soil/water ratio using a glass electrode (WTW 82362 Weilheim). Organic carbon contents were measured by dichromate oxidation followed by the titration of excess dichromate with Fe(II) [18]. The organic matter content (OM) was estimated by multiplying Corg by 1.72. |
| The measures of available phosphorus, chloride, SO42-, NO3- and B were performed according to Carter [18]. Chloride was quantified on the water extract used for C.E. measurements by titration with AgNO3 0.01M in the presence of K2CrO4 5%. S-SO4 extraction using a Ca(H2PO4)2 0.01 M solution was followed by a turbidimetric measurement of sulphate as BaSO4. Nitrate was extracted with 50 ml of 2M KCl on 5 g of soil; the mix was shaked for 30 min and filtered on Whatman n°42 filter paper. The absorbance was then measured at 220 nm using a Spectronic 20 Genesys spectrophotometer and quantification was performed on the basis of a calibration curve with 0, 0.2, 0.5, 1, 3 and 7 ppm of nitrate added as NH4NO3. Available P was extracted with a solution of 0.5M NaHCO3 at pH 8.5 for 30 min. Determination of available P was performed using a spectrophotometer, (Spectronic 20 Genesys) with the addition of 10 ml molybdate blue solution to 10 ml of the extractant solution at 880 nm wavelength [19,20]. |
| The determination of available boron was performed with azometina-H in extract from 20 g of soil and 40 ml of 0.01M of CaCl2. Solution was heated until boiling during 10 min. Four ml of solution were then added to 4 ml of azeometina buffer-H and incubated during 40 min at room temperature. The absorbance was read at 420 nm and quantification was established on the basis of a standard curve with 0, 0.2, 0.4, 1 and 2 ppm of B (added as Na2B4O7.10H2O). |
| Concentrations of other elements (including As) were obtained after acid attack of soil samples (c.a. 4 g) which were placed in a Teflon beaker in the presence of 6 ml HNO3 68% (v/v) and 10 ml HF 48% (v/v). The mixture was heated on a hot plate until completely dry. The residue was redissolved with HClO4 [70-72%] and evaporated to dryness. This residue was redissolved again with aqua regia, a mix of HCl 37%-HNO3 68% (3:1) and filtered with filter paper Whatman 41. Samples were analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES; Thermo Jarrell Ash, ITRIS Advantage). Dissolution technique used for estimating complexed iron and various oxide iron fractions (crystalline and no crystalline phase) was DCB method (Dithionite-Citrate- Bicarbonate) [21]. For each soil sample (1 g), 0.3 M of sodium dithionite sodium citrate and sodium bicarbonate was added and mixed at 75°C during 20 min. Iron concentration was quantified by ICP-AES. |
| Plant samples analysis |
| Plant samples were briefly rinsed with deionized water to remove the dust particle present at the surface. Seeds were separated from bracts. All samples were incubated in an oven for 48 h to 70°C. For ion quantification, 50 mg of dry matter were digested in 35% HNO3 and evaporated to dryness on a sand bath (Gerhardt, Königswinter, Germany) at 80°C. Minerals were then dissolved in 0.1 N HCl and ion concentration was determined by atomic absorption spectrophotometry (Varian SpectrAA-300, Palo Alto, CA, USA) or by inductively coupled plasma – atomic emission spectrometry (ICP-AES; Thermo Jarrell Ash, ITRIS Advantage). All plant samples were analyzed in triplicates. |
| Statistical treatment of the data |
| Ion concentration in plant tissues data were subjected to analysis of variance (ANOVA 1 and 2) using SAS software, the Student-Newman- Keuls test at 5% level was used in order to state the statistical significance of the results. Pearson’s correlations between ions were calculated with SAS software. The multiple factor analysis (MFA, a subset of principal component analysis) was done with R commander software via package FactoMineR with 12 parameters’ (As, B, Ca, Cu, Fe, K, Mg, Na, P, S, Zn content for each organs and As content in soil). The number of dimension used is five but only the first and second were showed. |
| Results and Discussion |
| Water and soil analysis |
| Parameters and elements in water from the Rio Loa are given in Table 1. The water contains a high As concentration which is up to 100 times higher than the maximal concentration allowed by the WHO for drinking water. The water is also highly saline, with a mean EC value higher than 14 mS cm-1 in relation to a high concentration of Na and Cl. Similar As values in the Rio Loawere reported by Bugueño et al. [9] and Romero et al. [2]. According to these authors, the As concentration in water of Rio Loa is quite lower in the upper section and progressively increased from the Andes mountain to the pacific ocean, as a consequence of extreme desert climate favoring high evaporation rates and the lack of tributaries or fresh groundwater contribution. Such As-enrichment thus occurs as a natural process, although some anthropogenic activities related to mining ore processing may also be involved. Beside As, water also contains high amounts of B, and a near-neutral to alkaline pH. Those pH values, high salinity and high As concentrations do not favour the adsorption of As(V) species [22]. Considering the mean redox potential, both HAsO4- and H3AsO3 may be present in the water [23]. At pH<7 in oxidizing conditions, As may be immobilized by co-precipitation/sorption with Fe and Mn oxy-hydroxides [24]. Despite a high total dissolved solid concentration (TDS), Fe and Mn are poorly represented in the water of Rio Loa. Similarly, under acidic sulfate reducing conditions, As can be accumulated as stable sulfur minerals [25] which probably does not occur at the pH recorded in the samples. For Ca/As molar ratio higher than 1 and low dissolved Fe and Mn concentration, As may be associated with calcite and kaolinite minerals [26] and the presence of this complex in suspension could not be ruled out under the recorded conditions. |
| Data issued from bulk soil analysis are given in Table 2. Soils of Quillagua were slightly alkaline and highly saline, containing only a small amount of organic matter. They were typically coarse-textured, dominated by medium and fine sand. The total concentration of As was high (220 mg Kg-1), which is by far higher than the world average concentration reported for this matrix [27]. Higher values (up to 1500 mg Kg-1) in Quillagua were reported by Romero et al. [2] and Bugueño et al. [9] but these authors studied sediments present in the river while our samples were taken on dry soil at least 30 m away from the river flow. De Gregori et al. [28] analyzed soil sample from Quillagua and reported values similar to those quantified in this study. According to these authors, Quillagua is the most As contaminated site among 27 analyzed sites in Regions I, II and V in Chile. The recorded values confirmed that the Quillagua oasis is indeed heavily contaminated by As and that this element easily spread from the river to the surrounding area. Iron oxides were estimated at 5.57 g Kg-1 and constitute 20.3% of the total amount of iron. DCB extraction showed that 50 ± 11 μg of As g-1 of soil were linked with iron oxide and that 22% of total As were not bioavailable. Despite the proximity of copper mining industry in this area, the recorded Cu concentration remained low and did not exceed the world average concentration (30 mg Kg-1) [28]. De Gregori et al. [28] reported high concentration of antimony in Quillagua but this element was not analyzed in the present study. A high concentration of B was also recorded in our soil samples (more than 500 ppm), confirming that Quillagua area is polluted by several elements. According to Nable et al. [29], total B concentration remains a poor indicator of plant available B. The hot water soluble B estimated in our samples was 12.8 mg Kg-1, which is higher than the maximal permissible level in mine soil [30]. |
| Plant analysis |
| The mean arsenic concentration in leaves and stems of adult plants of Atriplex atacamensis (Table 3) were 8 and 4 μg g-1 DW for plants growing on the contaminated soil of Quillagua. A similar As accumulation has been reported for natural As-resistant vegetation colonizing As-contaminated Technosols from a former mining area [31]. In Scirpus americanus harvested in Quillagua, Bugueño et al. [9] reported an As concentration of 21 μg g-1 DW but this species is an aquatic plant growing directly in the river and which is in close and permanent contact with contaminated water. Terrestrial plants growing on uncontaminated soils exhibit a mean shoot As concentration in the range of 0.009 to 0.1 μg g-1 DW [32]. Atriplex atacamensis thus appears able to accumulate a moderate amount of As, though it should not be regarded as a hyperaccumulating plant species and As concentration in the leaves remained 25 fold lower than in the soil. The recorded As concentration in leaves and stems of A. atacamensis in Quillagua were however higher than the values reported by Tapia et al. [17] for the same species growing on another chilian As-contaminated are of ChiuChiu. It has to be mentioned that Quillagua is more contaminated tan ChiuChiu and that we harvested samples from mature plants exposed since a long time to As contamination while Tapia et al. [17] performed pot experiments with young plants exposed for only 90 days to As pollution. In the close-related species Atriplex halimus growing on a site contaminated with 300 mg Kg-1 DW As, Rabier et al. [33] did not detect significant As accumulation in the leaf since the resulting As concentration remained below the detection level. It may thus be assumed that A. atacamensis is able to cope with a moderate As accumulation but displays exclusion mechanisms allowing the plant to limit As translocation from the roots to the shoots. Such a hypothesis is supported by the fact that a significant negative correlation was found between the soil and the shoot As concentration (r=-059; P<0.01) which suggest that above a threshold level, the stressed plants clearly adopted an exclusion strategy. This conclusion, however, should be mitigated by the fact that high As concentration were recorded in fruits and male flowers (Table 3). In both cases, As concentration was higher than 20 μg g-1 DW. This is a surprising result since it is commonly considered that plants exposed to ion toxicities protect their reproductive structures from pollutant accumulation, even if the presence of salt may somewhat modify heavy metal or metalloids distribution [34]. A hypothesis may be that those structures harvested in fields were contaminated by atmospheric pollution occurring as dust particles. However, our samples were thoroughly washed before analysis and there is no reason to claim that dust contamination was higher for flowers and fruits than for leaves. Moreover, the seeds of plants from the Atriplex genus develop in enclosing bracts which, as transpirating tissues, may accumulate ions and must commonly be released to allow an efficient germination [35]. In our samples, however, As concentration in the bracts and in the seeds of A. atacmensis were in the same order of magnitude (Table 3), thus suggesting that As may be translocated to the ovary and seeds issued from its maturation. |
| A high B content was also recorded in all plant parts and the maximal value (up to 649 μg g-1 DW) was recorded in the seeds. Plants from the genus Atriplex were already reported to be able to cope with high endogenous B concentrations [36] and A. atacamenis thus appears as a promising material for phytomanagement of soils simultaneously contaminated by both As and available B. Copper and Zn accumulated to high concentrations in the bracts (Table 3). In Atriplex halimus, some heavy metals may accumulate in the stems and it is considered that lignified tissues may sequester toxic ions through cell wall fixation to avoid their translocation to the leaves [15]. Our data suggest that this process does not occur in A. atacamensis where stems growing on the contaminated site of Quillagua displayed the lowest concentration for As, B, Cu and Zn (Table 3). |
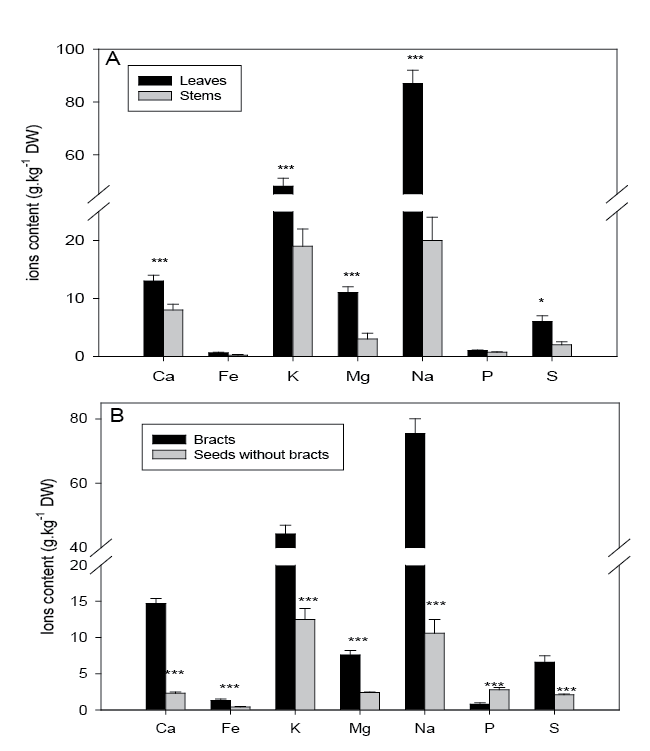
| The concentration of other elements in plant organs are given in Figure 2. The ion concentrations were always higher in the leaves than in the stems. This may be explained by the fact that transpiration stream is transporting mineral elements which then accumulated in leaf mesophyll close to the sub-stomatal cavity. The vegetative plant tissues are characterized by high Na concentration, which confirms the halophyte nature of A. atacamensis. The high EC value recorded in the soil samples of Quillagua, correlated with the high Na accumulation in the leaves confirm that A. atacamensis is able to cope with high rates of Na accumulated within photosynthetic tissues and thus displays salttolerance mechanisms. In the genus Atriplex, an important proportion of Na is excreted in the trichomes present at the leaf surface and which remove toxic ions from metabolic-active sites. It has been demonstrated that high endogenous concentration of the plant hormone abscisic acid, which is over-produced under drought conditions, may increase salt excretion in trichomes [37]. It is thus possible that A. atacamensis growing under extremely arid conditions could efficiently remove salt from the leaf. Other studies suggested that heavy metals may also accumulate in trichomes but that salinity may reduce this excretion process [16]. To the best of our knowledge, however, no data are available regarding As behaviour in this respect. |
| The vegetative organs of A. atacamensis presented rather low P concentration. Because arsenate and phosphate have similar size and charge, arsenate is known to penetrate in the plants through phosphate translocators [38]. It may therefore be hypothesized that the low P concentration could be linked to As contamination present in Quillagua. Nevertheless, A. atacamensis remains able to efficiently translocate P to the seeds since it was the only element that accumulated to higher extent in the seeds than in the bracts; while others major elements were more accumulated in bracts than in seeds (Figure 2B). It has also to be noticed that sulfur was present at high concentration in the harvested samples (Figure 2). Sulfur is an important component of the endogenous antioxidant glutathione, which acts as a precursor of phytochelatins. These thiol-rich peptides protect plant tissues from the deleterious impact of heavy metals and As [10,11,29]. Moreover, S was recently showed to decrease the expression of genes coding for transporters putatively involved in As absorption [39]. |
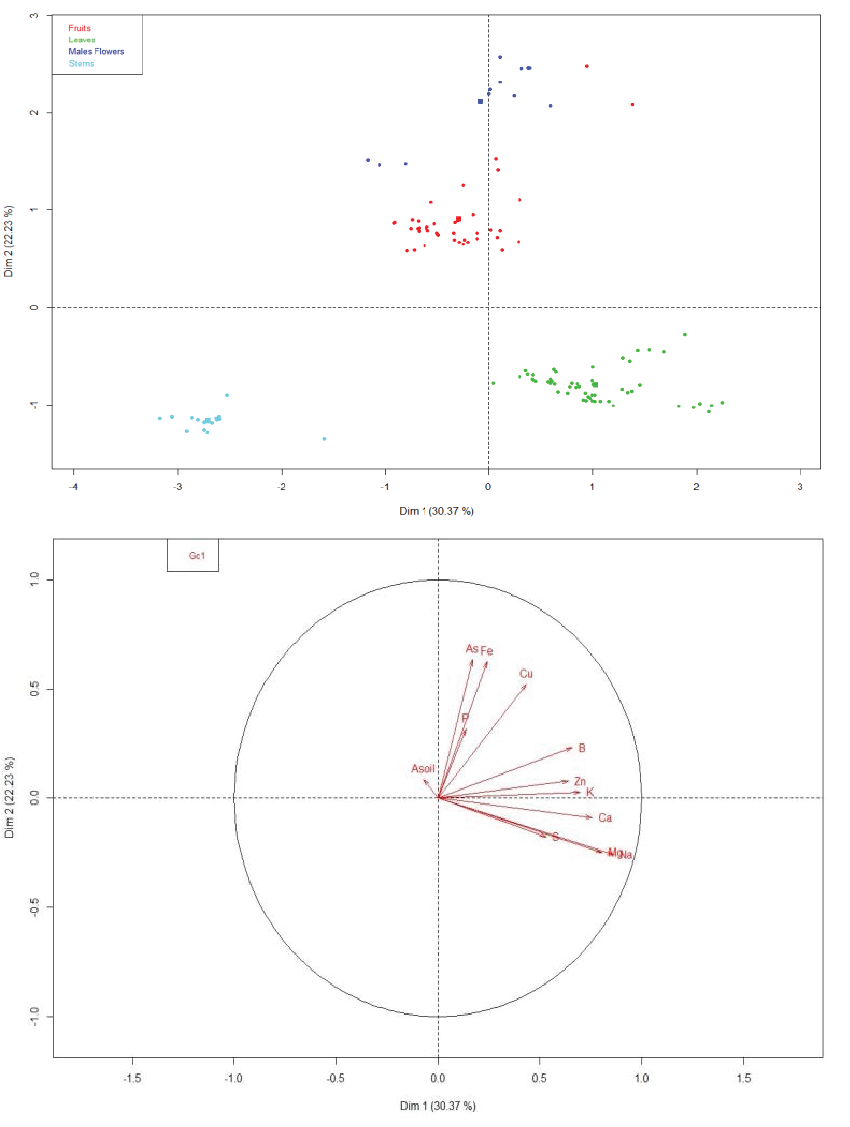
| The ACP analysis (Figure 3A) allowed us to clearly discriminate between the four types of organs (stems, leaves, male flowers and fruits). Magnesium and Na were highly correlated and strongly contribute to the first dimension while As and Fe mainly contribute to the second one (Figure 3B). The first dimension explained more than 30% of the variation and discriminate between leaves and stems. The second dimension, which explained 22% of the variability, allowed to discriminate between the vegetative and the reproductive organs and, to a lower extent, between male flowers and fruits. These data confirm that mineral nutrition in A. atacamensis, and ion distribution between organs, is highly regulated at the whole plant level and suggest that complementary experiments are required to identify the physiological and molecular properties regulating this complex process in plants growing on As-contaminated area. |
| Conclusions |
| It is concluded that Atriplex atacamensis is able to survive and grow under harsh environmental conditions characterized not only by high As concentration in the soil, but also by high B availability, high salinity, high temperature and very higher vapour pressure deficit. This species thus appears resistant to as a promising candidate for the phytomanagement of contaminated soils under arid conditions. |
| References |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 10393
- [From(publication date):
May-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 9502
- PDF downloads : 891
