Are Non-Sexual and Vertical HPV Transmission Increasing the Incidence of Early Onset Cancers in HIV Negative Teenagers in Western Kenya? â A Case Study of Jaramogi Oginga Odinga Teaching and Referral Hospital
Received: 02-Oct-2023 / Manuscript No. ccoa-23-118208 / Editor assigned: 04-Oct-2023 / PreQC No. ccoa-23-118208 (PQ) / Reviewed: 18-Oct-2023 / Revised: 23-Oct-2023 / Manuscript No. ccoa-23-118208 (R) / Accepted Date: 30-Oct-2023 / Published Date: 30-Oct-2023 QI No. / ccoa-23-118208
Abstract
Background: Human Papilloma Viruses is estimated to cause 99% of cervical cancers. It is mostly transmitted sexually, although non-sexual horizontal transmission includes, fomites, fingers, mouth, skin contact (other than sexual), self-inoculation, waterborne transmittal and vertical transmission. The natural history for cervical carcinogenesis is represented by HPV acquisition, HPV persistence, progression to precancerous lesions, then to invasive cancer, which take 15-20 years, but can take 5-10 years in immunosuppressed women, like those with untreated HIV infection. Early onset cancers, are cancers developed at
Objective: This study aimed to explore the increase in incidences of early onset cervical cancer in young HIV Negative women, including teenagers, presenting at the Oncology Clinic of the hospital.
Methodology: A mixed method study was undertaken of purposively recruited HIV negative patients, aged 13-35 years, presenting with Cancer of the Cervix in the 2020-2021 period of study, compared to the previous 2012-2019 period.
Findings: There was a significant increase in Early Onset Cancer of the Cervix in the 2020-2021 period with P-value 0.017 at CI (2.562, 18.938) as compared to the period 2012-2019 with P-value 0.012 at CI (1.921, 11.079), with 12.24% being teenagers with advanced cancer as compared to 0.57% in the retrospective period.
Conclusion: The study concluded that there is a significant increase of Early Onset Cancer of the Cervix amongst teenagers.
Keywords
Non-sexual; Sexual; Horizontal; Vertical; Transmission; Incidence; Early onset; Teenagers; Advanced; Young-women; HIV negative; HPV; Western Kenya
Introduction
Cervical cancer is the fourth most common cancer among women globally, with an estimated 604,000 new cases and 342,000 deaths in 2020 [1]. About 90% of the new cases and deaths worldwide in 2020 occurred in low and middle – income countries [1].
Sub-Saharan Africa (SSA) has the highest burden of cervical cancer in the world. Africa accounted for 21% of total cases and 26% of global deaths from cervical cancer in 2012 [1,2]. In Africa, Cervical cancer is the leading cause of cancer-related deaths in women in Eastern, Western, Middle, and Southern Africa and these women in Sub-Saharan Africa are disproportionately affected with cancer of the cervix, between 2% to 4% having a lifetime risk of the disease [1] In 2018, cervical cancer was the fourth most common cancer among women and the seventh most common cancer overall with 570,000 new cases and 311, 000 deaths reported, (2) 85% of whom were in low-middle-income countries, where vaccination, screening and treatment programs are limited [3] Eighty percent (80%) of the new cases occur in low and middle income countries, where it is the most common cancer in women, accounting for 13 % of all cancers in female patients [1,4] Western countries have experienced dramatic reductions in the incidence of and mortality from invasive cervical cancer, due to interventions that include vaccination against HPV and early diagnosis and treatment of patients with cervical cancer [5].
In Kenya, Cervical cancer contributes approximately 12% of all cancer cases diagnosed, and is the leading cause of all cancer deaths, with over 3,200 deaths reported in 2020 [1]. The uptake of screening is low (approximately 16% in 2015) [6] and only a quarter of 2,927 sampled health facilities offered screening in 2018, [6] despite the fact that Kenya has been implementing a national screening programme for more than a decade [7].
The HIV/AIDS epidemic led to Early Onset incidences of cervical cancer at a global level, with increasing incidence in women below 40 years of age, compared to the previous age - set of women in their 6th- 7th decades of life developing cervical cancer, before the onset of HIV/ AIDS [8,9]. In HIV-infected women, there is an increased risk of HPV infection and squamous intraepithelial lesions {SIL}, the precursor of cervical cancer [10,11].
Early onset cancers, defined as cancers in adults aged 12]. The incidence rate of early-onset cancers increased by 20.5% from1993-2019 in Northern Ireland [12]. The impact of cancer treatment on fertility and fertility preservation treatments is an important consideration, bearing in mind that patients with earlyonset cancers face unique supportive care needs and require holistic care [12]. An increased use of screening programmes has contributed to this phenomenon of early-onset cervical cancer to a certain extent (although the uptake in Kenya is still very low), a genuine increase in the incidence have emerged [12]. Evidence suggests an aetiological role of risk factor exposures in early life and young adulthood [13]. Since the mid-20th century, substantial multigenerational changes in the exposome have occurred, including changes in diet, lifestyle, obesity, environment and the microbiome, all of which might interact with genomic and/or genetic susceptibilities [13]. This may reflect agecohort effects and the emergence of more aggressive histologies with a shorter natural history, possibly the result of Human Papilloma Virus {HPV} infection acquired at a younger age or of increased screening/ awareness resulting in earlier detection of cervical cancer [8,9].
Climate change is also affecting health and health patterns, which need to be studied, to evacuate and mitigate any impacts it may be having in early development of cancers globally.
Human papilloma viruses (HPVs) belong to the Papillomaviridae family and are non-enveloped double-stranded DNA viruses with over 200 types identified [14]. All HPV types are epitheliotropic, infect squamous epithelia (skin and mucosae) [15]. HPV infection, in 2016, was estimated to have a global prevalence of cervical HPV of 11.7% in women with non-cancerous cytology. The most frequent types worldwide are 16 (3.2%), 18 (1.4%), 31 (0.8%) and 58 (0.7%), all oncogenic papillomaviruses [16]. HPV is estimated to be the cause of 99% of cervical cancers [17 ], with HPV type 16 being responsible for 50% of cervical cancer cases, and together with type 18, account for 70% of cases [16]. Most HPV infections are asymptomatic and/or they resolve spontaneously [15]. The clearance time is approximately 6-24 months: Cervical HPV in 9.4 months and genital HPV in men 7.5 months (oncogenic and non-oncogenic types) [15]. Warts develop in approximately 6-10 months after initial infection [18].
The process of cancer development from HPV infection takes approximately 5-10 years minimum and 20-25 years on average [19]. The World Health Organization states that the natural history for cervical carcinogenesis is represented by HPV acquisition, HPV persistence, progression to precancerous lesions, then to invasive cancer, take 15- 20 years, but can take 5-10 years in immunosuppressed women, like those with untreated HIV infection [20]. We observed routinely in the Oncology Clinic and in the wards, that the number of diagnosed Early Onset cancer of the cervix in HIV negative women of ages 13-35 years, had been increasing steadily at JOOTRH as from 2020 as compared to the previous period since inception of the Oncology Clinic in January 2012 to December 2019. This was opposed to the documented age of development of cervical cancer in HIV-negative women, which has been from the 4th to the 7th decade’s overtime, most frequently diagnosed in the U.S between the ages of 35 - 44, with the average age at diagnosis being 50 years old, more than 20% are diagnosed at 65 years of age, according to the American Cancer Society, updated 2023 [7].
In respect to the above, this study’s objective was to investigate the increase in the incidence of Early Onset cancer of the cervix in HIV-VE young women, including teenagers in Western Kenya, coming to the oncology clinic at the Jaramogi Oginga Odinga Teaching and Referral Hospital.
Materials and Methods
Study site
The study was conducted at the Oncology Clinic of the Jaramogi Oginga Odinga Teaching and Referral Hospital {JOOTRH}. Kisumu County, about 6 Kilometers from the Kisumu city business district (CBD), along the Kisumu-Kakamega road next to the Western region’s Blood Transfusion Centre. The Oncology Clinic is a separated from the administration block by a small fishpond and is next to the JOOTRH College’s Director’s office. The Clinic operates 8 hours per day from Monday to Friday and has a staff base made of 1 Gynaecology- Oncologist, 1 Medical-Oncologist, 1 Medical officer, 4 Nurses, 1 Nutritionist, 1 Pharmacist and 1 support staff.
Kisumu City of Kisumu County is the third-largest city in Kenya after the capital, Nairobi, and Mombasa [14]. It is the second-largest city after Kampala in the Lake Victoria Basin. Located at the shores of the world’s second largest freshwater lake, Lake Victoria and at 1,131 m (3,711 ft.), the vibrant third largest city in Kenya, Kisumu City, boasts of a rich history of international trade, tropical climate, good transport network and a vibrant population majorly the Luo ethnic tribe of Kenya. The city has a population of slightly over 600, 000 [14]. The metro region, including Maseno and Ahero has a population of 1,155,574 people (560,942 males, 594,609 females) according to the 2019 Kenya Population and Housing census which was conducted by the Kenya national Bureau of Statistics [14]. Kisumu is the principal city of western Kenya and forms the commercial, industrial and transportation center majorly due to its water and rail connections. Formally the headquarters of the greater Nyanza Province, the town has grown to be the third largest city in Kenya after Nairobi and Mombasa and is now the headquarters of Kisumu County. The main industries in Kisumu are centered on processing of agricultural products, fishing, brewing and textile manufacturing industries. The Luo tribe is the main inhabitants of Kisumu County, but because it is made up of the city and rural areas around the metro zone, it is a melting pot of other tribes like the Luhya, Kisii, Kuria, Somali, Kikuyu, Kamba and others [14]. The JOOTRH also serves the neighboring counties of Kakamega, Siaya, Kisii, Nyamira, Homs Bay, Busia, Bungoma and Migori [14].
JOOTRH is a teaching and referral hospital, where many cancer cases such as gynaecological cancers are treated. It serves as the only oncology referral hospital in the county and for the counties of Siaya, Homa Bay, Migori, Nyamira and Busia. Management of cervical cancer offered at this facility includes surgery and chemotherapy but has no radiotherapy unit. It offers also CT and MRI imaging and a well-equipped Pathology laboratory. The HIV-Clinic screens newly diagnosed patients for cancer of the cervix after Two months of attendance treat pre-cancerous lesions immediately and refer the confirmed cancers to the Oncology Clinic.
Inclusion/exclusion criteria
The study included all the files of patients with early onset cervical cancer of ages 13-35 years, who were both HIV- Negative and HIV - Positive at time of diagnosis and had histological diagnosis, in the period 2012 - 2019. We also purposively recruited all patients with above characteristics in the period of 2020 – 2021.
Sample
In this quantitative and qualitative study, in the 2012-2019 period, patients’ files for all HIV positive and negative cervical cancer patients who were of the ages 13-35 years old were purposively selected. The samples consisted of HIV +VE and HIV-VE patients with Early Onset cervical cancer, and were being treated at the oncology clinic of the JOOTRH, since the inception of the clinic in January 2012-2019 December. In the period of 2020-2021, participants were purposively selected using maximum variation sampling strategy, as they were diagnosed and registered in the Oncology clinic. The patients were drawn from different population categories of ethnicities, socioeconomic statuses, place of residences, level of education and religion.
A total sample size of 52 files was selected, in the period of 2012 – 2019 and a sample of 86 participants was recruited actively in the prospective period of 2020 – 2021.
Procedure and research design
This was a mixed-methods study design, including both quantitative and qualitative components. The quantitative components focused on age sets, HIV statuses, cervical cancer vaccination, screening, diagnosis, histology results, Figo Staging in the period of 2012-2019 and 2020- 2021 data reviews and analysis, with data sources being the patient files in the former period while using clinical research forms and other source documents in the latter period. The qualitative component involved evaluating knowledge about cervical cancer, sourced through review of files and use of clinical research forms and semi-structured interviews in the former and latter periods respectively. The study was based on the JOOTRH’s Oncology Clinic services to patients with early onset cervical cancer in both periods, within the age set of 13-35 years old.
Study period
The review of files was done for the period of 8 years since the inception of the Oncology in January 2012 to December 2019 (2012- 2019), and the period of active recruitment, collection of data with clinical research forms and other source documents was in the period of September 2020 to September 2021 (2020-2021).
Measurement
Data was collected using structured document analysis forms and lists in the period of 2012-2019, while clinical research forms and semi-structured interviews were used for data collection in the period of 2020-2021. The study specifically sought to determine the incidences of early onset cervical cancer cases, HIV-status, the patients’ demographics, knowledge of cancer, vaccinated against HPV, screening, stage of disease and histological results of the cancer tissues.
The primary outcome variable was the incidences of Early Onset Cancer of the cervix in both HIV Positive and Negative women of ages 13-35 years old. This variable was measured through all the reviewed files in the period 2012 - 2019 and of the actively recruited patients in the period 2020 - 2021.
The quantitative data were analyzed using Epi InfoTM 7.0 (US CDC, Atlanta, GA). The qualitative data was thematically tabulated while the quantitative data was summarized in trend series (bar charts and line graphs).
Ethical considerations
All the documents analyzed and patients recruited in this study, were accessed after getting an approval from the JOOTRH’s Ethical Review Committee (I.E.R.C) and express informed consent from the recruited patients. There was no patient who was coerced into joining the study and those who declined were not denied the standard of care for their ailment.
The approval for the review of the hospital records for the period 2012-2019 and active recruitment of patients for the 2020-2021 period, included statements about the rights of the subjects, in terms of their information collected, confidentiality and the publication of this report and any other accompanying information.
Findings
Analysis of the increase in incidence of teenagers with early onset cancer of the Cervix in who was HIV –VE women in the study period 2020 – 2021 (Tables 1-5).
| Characteristics | n = 16 | |
|---|---|---|
| n/median | %/range | |
| Age, years | 27 | 13-35 |
| Residence | ||
| Urban | 6 | 37.50% |
| Rural | 10 | 62.50% |
| Vaccinated against H.P.V | ||
| Yes | 0 | 0% |
| No | 16 | 100% |
| Screened Voluntarily Prior to Symptoms | ||
| Yes | 2 | 12.50% |
| No | 4 | 25% |
| HIV Status | ||
| Negative | 0 | 0% |
| Positive | 16 | 100% |
| On HAART | ||
| Yes | 16 | 100% |
| No | 0 | 0% |
| FIGO 2012/2019 stage | ||
| IIA2 | 12 | 75% |
| IIB | 4 | 25% |
| Tumour size, mm | 48 mm | >40 mm |
| Histology | ||
| Squamous cell carcinoma | 13 | 81.25%% |
| Adenocarcinoma | 2 | 12.50% |
| Adeno - squamous cell carcinoma | 1 | 6.25% |
| Small Cell Neuro-Endocrine carcinoma | 0 | 0% |
| Type of imaging | ||
| CT | 16 | 100% |
| Patient-based nodal status on CT | ||
| Negative | 16 | 100% |
| Inconclusive | 0 | 0% |
| Positive | 0 | 0% |
| Region with positive nodal status on imaging b | ||
| Pelvic | 0 | 0% |
| Common iliac | 0 | 0% |
| Para-aortic | 0 | 0% |
| Patient-based nodal status on pathology | ||
| Negative | 16 | 100% |
| Positive | 0 | 0% |
| Unknown | 0 | 0% |
| Nodal examination | ||
| Absent | 16 | 100% |
| Lymphadenectomy | 0 | 0% |
| Nodal debulking | 0 | 0% |
| Biopsy/fine-needle aspiration | 16 | 100% |
| Sentinel node biopsy only | 0 | 0% |
Table 1: Cancer of Cervix Table in the Period 2012 -2019 for HIV +Ve Cohort.
| Characteristics | n = 22 | |
|---|---|---|
| n/median | %/range | |
| Age, years | 23 | 13-35 |
| Residence | ||
| Urban | 10 | 45.50% |
| Rural | 12 | 54.50% |
| Vaccinated against H.P.V | ||
| Yes | 0 | 0% |
| No | 22 | 100% |
| Screened Voluntarily Prior to Symptoms | ||
| Yes | 7 | 31.80% |
| No | 15 | 68.20% |
| HIV Status | ||
| Negative | 22 | 100% |
| Positive | 0 | 0% |
| On HAART | ||
| Yes | 0 | 0% |
| No | 22 | 100% |
| FIGO 2020/2021 stage | ||
| IIA2 | 2 | 9.10% |
| IIB | 5 | 22.70% |
| IIIB | 5 | 22.70% |
| IIIC1 | 4 | 18.20% |
| IIIC2 | 5 | 22.70% |
| IVB | ||
| Tumour size, mm | 62 | > 40 |
| Histology | ||
| Squamous cell carcinoma | 16 | 72.70% |
| Adenocarcinoma | 2 | 9.10% |
| Adeno - squamous cell carcinoma | 4 | 18.20% |
| Small Cell Neuro-Endocrine carcinoma | 0 | 0% |
| Type of imaging | ||
| CT | 22 | 100% |
| Patient-based nodal status on CT | ||
| Negative | 10 | 45.50% |
| Inconclusive | 3 | 13.60% |
| Positive | 9 | 40.90% |
| Region with positive nodal status on imaging b | ||
| Pelvic | 9 | 40.90% |
| Common iliac | 2 | 9.10% |
| Para-aortic | 5 | 22.70% |
| Patient-based nodal status on pathology | ||
| Negative | 13 | 59% |
| Positive | 9 | 40.90% |
| Unknown | 0 | 0% |
| Nodal examination | ||
| Absent | 3 | 13.60% |
| Lymphadenectomy | 4 | 18.20% |
| Nodal debulking | 0 | 0% |
| Biopsy/fine-needle aspiration | 22 | 100% |
| Sentinel node biopsy only | 0 | 0% |
Table 2: Cancer of Cervix Table in the Period 2012 -2019 for HIV –Ve Cohort.
| Characteristics | n=17 | |
|---|---|---|
| n/median | %/range | |
| Age, years | 31 | 13-35 |
| Residence | ||
| Urban | 14 | 82.40% |
| Rural | 3 | 17.60% |
| Vaccinated against H.P.V | ||
| Yes | 0 | 0% |
| No | 17 | 100% |
| Screened Voluntarily Prior to Symptoms | ||
| Yes | 6 | 35.30% |
| No | 11 | 64.70% |
| HIV Status | ||
| Negative | 0 | 0% |
| Positive | 17 | 100% |
| On HAART | ||
| Yes | 17 | 100% |
| No | 0 | 0% |
| FIGO 2020/2021 stage | ||
| IIA2 | 9 | 52.90% |
| IIB | 3 | 17.60% |
| IIIB | 3 | 17.60% |
| IIIC1 | 1 | 5.90% |
| IIIC2 | 1 | 5.90% |
| IVB | 0 | 0% |
| Tumour size, mm | 75 mm | >40 mm |
| Histology | ||
| Squamous cell carcinoma | 13 | 76.40% |
| Adenocarcinoma | 1 | 5.90% |
| Adeno - squamous cell carcinoma | 3 | 17.60% |
| Small Cell Neuro-Endocrine carcinoma | 0 | 0% |
| Type of imaging | ||
| CT | 17 | 100% |
| Patient-based nodal status on CT | ||
| Negative | 120 | 70.60% |
| Inconclusive | 30 | 17.60% |
| Positive | 2 | 11.80% |
| Region with positive nodal status on imaging b | ||
| Pelvic | 2 | 11.80% |
| Common iliac | 1 | 5.90% |
| Para-aortic | 1 | 5.90% |
| Patient-based nodal status on pathology | ||
| Negative | 15 | 88.20% |
| Positive | 2 | 11.80% |
| Unknown | 0 | 0% |
| Nodal examination | ||
| Absent | 5 | 29.40% |
| Lymphadenectomy | 2 | 11.80% |
| Nodal debulking | 0 | 0% |
| Biopsy/fine-needle aspiration | 17 | 100% |
| Sentinel node biopsy only | 0 | 0% |
Table 3: Cancer of Cervix Table in the Period 2020 -2021 for HIV +Ve Cohort.
| Characteristics | n=49 | |
|---|---|---|
| n/median | %/range | |
| Age, years | 23 | 13-35 |
| Residence | ||
| Urban | 21 | 42.90% |
| Rural | 28 | 57.10% |
| Vaccinated against H.P.V | ||
| Yes | 0 | 0% |
| No | 49 | 100% |
| Screened Voluntarily Prior to Symptoms | ||
| Yes | 10 | 20.40% |
| No | 39 | 79.60% |
| HIV Status | ||
| Negative | 49 | 100% |
| Positive | 0 | 0% |
| On HAART | ||
| Yes | 0 | 0% |
| No | 49 | 100% |
| FIGO 2020/2021 stage | ||
| IIA2 | 4 | 8.20% |
| IIB | 18 | 36.70% |
| IIIB | 10 | 20.40% |
| IIIC1 | 6 | 12.20% |
| IIIC2 | 9 | 18.40% |
| IVB | ||
| Tumour size, mm | 71 | >40 |
| Histology | ||
| Squamous cell carcinoma | 30 | 61.20% |
| Adenocarcinoma | 5 | 10.20% |
| Adeno - squamous cell carcinoma | 10 | 20.40% |
| Small Cell Neuro-Endocrine carcinoma | 4 | 8.20% |
| Type of imaging | ||
| CT | 49 | 100% |
| Patient-based nodal status on CT | ||
| Negative | 30 | 61.20% |
| Inconclusive | 4 | 8.20% |
| Positive | 15 | 30.60% |
| Region with positive nodal status on imaging b | ||
| Pelvic | 15 | 30.60% |
| Common iliac | 4 | 8.20% |
| Para-aortic | 9 | 18.40% |
| Patient-based nodal status on pathology | ||
| Negative | 34 | 69.40% |
| Positive | 15 | 30.60% |
| Unknown | 0 | 0% |
| Nodal examination | ||
| Absent | 10 | 20.40% |
| Lymphadenectomy | 10 | 20.40% |
| Nodal debulking | 0 | 0% |
| Biopsy/fine-needle aspiration | 49 | 100% |
| Sentinel node biopsy only | 0 | 0% |
Table 4: Cancer of Cervix Table in the Period 2020 -2021 for HIV –Ve Cohort.
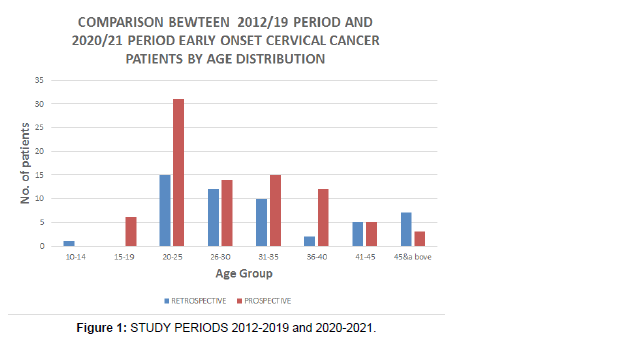
| AGE GROUP | 2012-2019 | 2020-2021 |
|---|---|---|
| Oct-14 | 1 | 0 |
| 15-19 | 0 | 6 |
| 20-25 | 15 | 31 |
| 26-30 | 12 | 14 |
| 31-35 | 10 | 15 |
| 36-40 | 2 | 12 |
| 41-45 | 5 | 5 |
| 45&above | 7 | 3 |
| TOTAL | 52 | 86 |
| P-VLUE | 0.012 | 0.017 |
| MEAN | 6.5 | 10.75 |
| CI (MEAN) | 1.921, 11.079 | 2.562, 18.938 |
| DF | 7 | 7 |
Table 5: SAMPLE.
Total HIV –VE (13-35)=49
Patients aged 13-19 (teenagers)=6
Hence Percentage=(6/49)= 12.24 x 100=12.24% teenagers were diagnosed with early onset cervical cancer in this period.
As compared to the incidence of teenagers with early onset cervical cancer in the Period 2012-2019
Total HIV –VE (13-35)=49
Patients aged 13-19 (teenagers)=1
Hence Percentage= (1/22)=0.0454 x 100=0.454% /8 years (2012- 2019)=0.57% teenagers were diagnosed with early onset cervical cancer in this period.
Interpretation
There was a significant increase in the incidence of early onset cancer of the cervix amongst teenagers in the 2020-2021 study period, at 12.24% as compared to the study period 2012-2019, which only had 0.57% teenagers diagnosed with cancer of the cervix (Table 6).
| Urban | Rural | Total | |
|---|---|---|---|
| HIV+ | 22 | 25 | 47 |
| HIV- | 37 | 55 | 92 |
| Total | 59 | 80 | 139 |
Table 6: Analysis of predisposition of HIV+ women in rural to cervical cancer.

1.3>0.004
The results are statistically significant
Interpretation
• A HIV+ woman living in rural area is 30% more likely to get cervical cancer compared to HIV- in urban area.
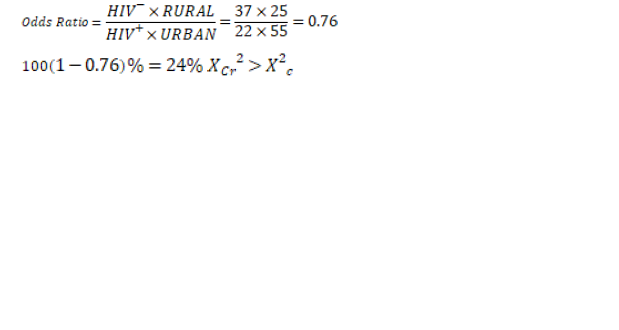
0.76>0.004
The result is statistically significant
Interpretation
• A HIV- woman living in rural area is 24% less likely to get cervical cancer compared to HIV+ woman living in urban area.
The study concluded that there is a significant increase of Early Onset Cancer of the Cervix amongst teenagers (Table 7).
| URBAN | RURAL | Total | |
|---|---|---|---|
| 2012-2019 | 19 | 33 | 52 |
| 2020-2021 | 40 | 47 | 87 |
| Total | 57 | 80 | 139 |
Table 7: The likelihood of getting cervical cancer in 2012-2019 and 2020-2021 periods.
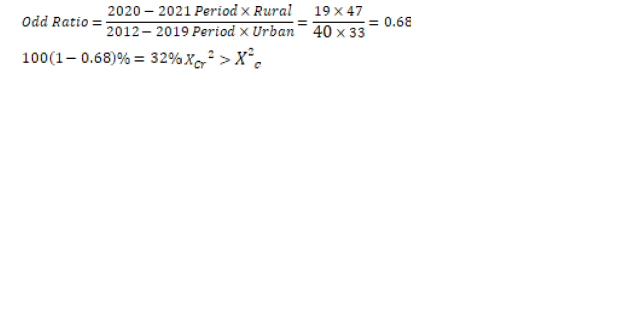
0.68>0.004
The result is statistically significant
Interpretation
• A woman, whether in town or rural area was 32% less likely to get cervical cancer between 2012 and 2019 compared to 2020-2021 period.
Discussions
The study found out that there was a significant increase in the incidence of early onset cancer of the cervix amongst teenagers in the 2020-2021 period, at 12.24% (6 teenagers out of 49 in one year) as compared to the study period 2012-2019, which only had 0.57% (1 out of 22 in a period 8 years) of teenagers diagnosed with cancer of the cervix. This is in agreement to a study that reported that, early onset cancers, defined as cancers in adults aged 12]. It reported also that the incidence rate of early-onset cancers increased by 20.5% from1993-2019 in Northern Ireland [12]. The other study that is in agreement, stated that evidence suggests an aetiological role of risk factor exposures in early life and young adulthood [13 ]. It goes on to that that, since the mid-20th century, substantial multigenerational changes in the exposome have occurred, including changes in diet, lifestyle, obesity, environment and the microbiome, all of which might interact with genomic and/or genetic susceptibilities [13 ]. The additional study that was in agreement, reported that, the findings of early onset cancers, may reflect age-cohort effects and the emergence of more aggressive histologies with a shorter natural history, possibly the result of Human Papilloma Virus {HPV} infection acquired at a younger age or of increased screening/awareness resulting in earlier detection of cervical cancer [21,22].
The findings for the teenagers with invasive and advanced carcinoma is in contrast with the natural history of cancer of the cervix , according to a study that reported that the process of cancer development from HPV sexual infection takes approximately 5-10 years minimum and 20-25 years on average [19]. It is also in contrast with the World Health Organization (WHO), which states that, the natural history for cervical carcinogenesis is represented by sexual HPV acquisition, HPV persistence, progression to precancerous lesions, then to invasive cancer, take 15-20 years, but can take 5-10 years in immunosuppressed women, like those with untreated HIV infection [20]. The number of years for the natural history of cancer of the cervix from sexual HPV infection to invasive cancer, do not conform to the advanced cases of teenage cervical cancers that were diagnosed in this study. If we take 5-10 years, it will mean that the sexual infection happened at 11 years or at 6 or 9 years of age, prior to sexual debut. If we take the 15-20 years as per the WHO then it means, the HPV infection was in infancy or via vertical transmission. A study that was performing a Cancer Intervention and Surveillance Modeling Network (CISNET) comparative simulation models for the natural history of cancer of the cervix, had four models (Harvard, Microsimulation Screening Analysis-Cervix Policy 1-Cervixx and University of Minnesota-HPV Cancer, showing the median time from acquisition to cancer detection ranged from 17.5 to 26.0 years across the four models [23]. Three models projected 50% of unscreened women acquire their causal HPV infection between ages 19 and 23 years, whereas one model projected these infections occurred later (age 34 years) [23]. Consistent with the well-established understanding of cervical carcinogenesis [24], all four models projected that the natural history from acquisition of an HPV infection to clinical cancer generally required 18-26 years [23]. This then leads to the consideration of other horizontal and vertical transmission of HPV in these teenagers. The Horizontal transmission of HPV includes fomites, fingers, and mouth, skin contact (other than sexual) [15]. Self-inoculation is described in some studies as a potential transmission route, and has been detailed in studies of HPV in female virgins [25] and in children with genital warts (low-risk HPV) without a history of sexual abuse [26]. A study on female virgins found 51.1% HPV positive, compared with 69.1% of sexually active females [15], hence supporting the chance of development of invasive cancer in this young group of virgin girls. There was yet another study that concluded that HPV type 16 is the most prevalent in pregnant women, and also in newborns, regardless of the nature of birth, as infection can occur during pregnancy, through the trans-placental route [15]. The finding in this study that HPV 16 was the most prevalent in pregnant women and their newborns, is significant, because studies have shown that HPV 16 is has the highest oncogenic risk, with four Cancer Intervention and Surveillance Modeling Network (CISNET) projection models showing that causal HPV 16 infections occurred between 0.1 and 6.0 years earlier compared with non-HPV 16 infections [23]. In this modeling study, when stratified by HPV genotype, the Harvard model projected a median high-grade pre-cancer dwell time that was 4.4 years shorter for HPV 16-related pre-cancers compare with cancers related to non-HPV 16 infections (i.e., HPV 16-related lesions progressed more quickly) [23]. There was also a study that showed a higher prevalence of HPV DNA in female newborns (17.7%) over males (11.6%) which were observed, without delivery-type relevance [21]. Data also show that there is a correlation between the HPV DNA load of the mother and the ability to transfer it to newborns [14 ]. This could explain the few number of teenagers developing invasive cervical cancer or early onset cancer. A population-based study conducted over 20 years in Denmark found women with warts to have a 200-fold risk of their children developing juvenile laryngeal papillomatosis [14 ]. The possibility of vertical HPV infection was discussed as early as 1950, in a study on infantile anogenital warts, congenital conjunctival papilloma, and juvenile laryngeal papillomatosis [27], hence this transmission route should be investigated further in a longitudinal study to find out if these newborns develop invasive carcinoma of the cervix as teenagers, as these patients in this study did.
Waterborne transmission of HPV has never been proven; still, although HPV DNA has been identified in water habitats [15]. In 2008, a study in the United States concerning the variety of viruses in raw sewage uncovered for the first time 2/12 HPVs in sewage samples, but not in treated waste water [15]. They also discovered oncogenic papillomavirus: 16, 18, 53, suggesting a waterborne route of infection, as was first proposed 40 years ago [13]. This finding of epitheliotropic viruses, as demonstrated by these studies, can be found in sewage [15]. It might be the result of scrubbing the skin and mucous membranes, but it can also be due to urine or feces pollution [16]. The other studies that support this waterborne route, was done in Italy, where Iaconelli et al, in 2015 [28] examined HPVs in two rivers, and identified 56% of the samples as positive. This needs good examining, because 4 of the 6 teenagers, were rural residents, and did not have tap water at home, and would bathe and fetch water from the rivers. Another study by Di Bonito et al, in same 2015 [29] identified 50% of the samples from swimming water as positive for HPV [16]. This finding shows that even swimming pools may be areas of infection for young girls and teenagers, for even oncogenic HPV types.
Hygiene measures are proven to be inefficient in preventing HPV transmission, as the studies which have evaluated samples of HPV on contaminated medical equipment (after conventional disinfection) have found them to still be positive, especially for type 16 [15 ]. One study has been published on HPV type 16 resistance and susceptibility to common disinfectants, and it was found to be resistant to alcohol-based disinfection (ethanol and isopropanol), but sensitive to hypochlorite and high concentrations of per acetic acid-silver-based disinfectant [14,16].
According to the findings of this study, we can argue for other horizontal transmissions of HPVs apart from sexual, for vertical transmission and also waterborne transmission of oncogenic types of HPV’s. A further multi-center and multi-country study needs to be undertaken in low and middle income countries with increasing early onset cervical cancers to explore these possibilities of oncogenic HPV transmission by other horizontal, vertical and waterborne routes other than sexual.
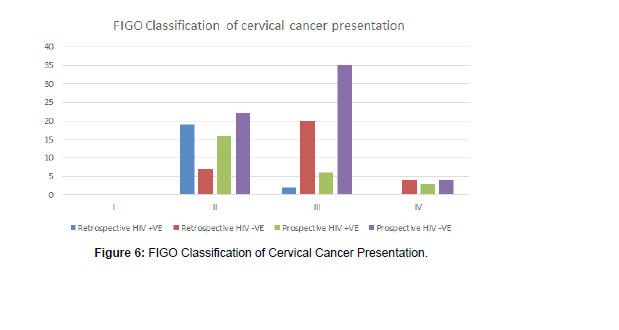
The study found out that most patients presented to the hospital in advanced stages of cancer of the cervix, and 39 (64%) were diagnosed at FIGO Stages III and IV, while just 22 (36%) were diagnosed at Stages I and II in the prospective study, mostly due to presenting themselves for the voluntary screening programme.
This is in agreement to a study that was done in Ghana which revealed that approximately two-thirds, 65.97%, of the cases presented in advanced stages of cervical cancer [30]. It is also in agreement to another study that was done in Tanzania, another East African country, which reported that 63.9% of its study participants presented with advanced stages to the healthcare facility [31]. In yet another East African study that was in agreement to the findings, in a hospital based, cross sectional study in Uganda, 66% of the study participants reported in advanced stages of cervical cancer [32].This is in contrast to the SEER data of the United States of America that shows that their patients are diagnosed at, Stage III at 16%, IVA at 2%, and 6.8% at widely metastatic disease; hence an overall of 24.8% of patients who present with advanced Figo stages [33].
The study showed increasing incidences of Early Onset cancer of the cervix in HIV-VE young women at the JOOTRH. This is in agreement to the recent published data that has seen the changing incidences of cervical cancer at a global level, with increasing incidence in women below 40 years of age [34,35], and a study that reported that eighty percent (80%) of the new cases occur in developing countries, where it is the most common cancer in women, accounting for 13% of all female cancers [36]. This is also in agreement with the findings of a study that stated that in 2020 estimates, cancer of the cervix incidences increased in some countries in Eastern Africa and Eastern Europe [37]. The Global incidence of early-onset cancer increased by 79.1% and the number or early-onset cancer deaths increased by 27.7% between 1990 and 2019 [38]. The results indicated that in low-middle and low Socio-Demographic Index regions, early-onset cancers had a significantly higher impact on women than on men in terms of both mortality and disease burden [38]. This could also be in agreement with climate change contributions, where a study reported that, climate change could lead to disruptions in water supply [39], which impact on the hygiene of women, reducing the frequency of bathing, hence may give micro-organisms like Human Papilloma Virus (HPV) that cause cervical cancer, a conducive environment for growth. The other study in agreement postulates that extremes in weather patterns and temperatures that interfere with the basal body temperatures, may impact on the human immunity to the viruses, making dormant viruses in warm areas become virulent in new cold weather and vice versa [40]. This is in contrast to a report that immune systems of young children and older adults are the ones that seem to be particularly vulnerable to rapid temperature changes [40].
This is in contrast to other previous studies that reported a link between young age at diagnosis of cancer of the cervix with HIV/AIDS, where an association between HIV infection and cervical cancer was noted especially in women aged less than 40 years, which was consistent with the published observations [41 ], although in Romania, which is a leading country in Europe had an incidence of 28.6/100,000 of cervical cancer cases, even though it is not an HIV endemic country, indicating that the increased incidence had not been contributed to by the HIV/AIDS pandemic [42]. A separate study in Kenya, stated that there was no significant change in either age at presentation or severity of cervical cancer between HIV +VE and HIV-VE patients at the Kenyatta National Hospital, although, of the 118 patients who were tested for HIV, 36 (31%) were sero-positive, while the rest were sero-negative and these women according to the study, were 5 years younger at presentation than HIV-VE women [43]. In yet another study in Romania, it reported that HPV prevalence, after age adjustment, was at 51.2% in HIV +VE versus 63.2% in HIV-VE women aged under 25 years of age and 22.2% in HIV+VE versus 47.2% in HIV-VE women aged 25-34 years of age in Romania [44], this showed that overall, HPV prevalence was higher in the HIV-VE women than HIV+VE women, and in the same vein, the HPV was more prevalent in younger patients who are HIV-VE as compared to those who are of the same age group but are HIV+VE in Romania [44] (Figure 1).
• Although the number of observations are different (2020- 2021=86, 2012-2019=52), there is more prevalence of cervical cancer among young (
• Although 2012-2019 study period is longer than prospective, more cases were reported in the latter period of 2020-2021. The incidence rate of early onset cancer of the cervix is increasing with time.s
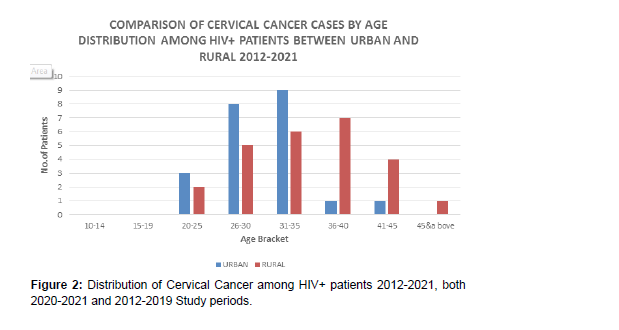
Women in the 20-25 years age bracket exhibit more cases compared to other child-bearing age groups, although with the longer period of eight years (2012-2019) in the , as compared to the shorter period of one year (2020-2021) (Figure 2).
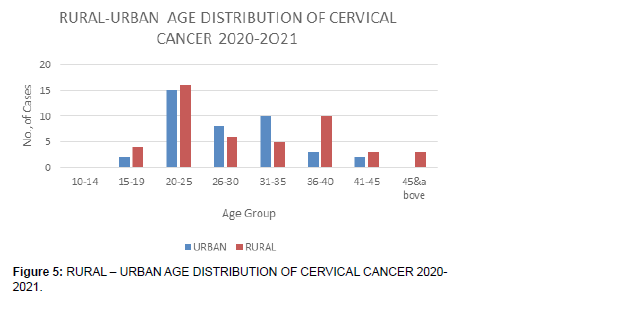
1. Over the period, there is more prevalence in the rural areas.
2. Young women in urban areas experience more incidences compared to similar age group in rural areas, although the first period of study is longer at 8 years (2012-2019), as compared to the shorter period of one year (2020-2021)..
3. Older women in rural areas present more cervical cancer cases compared to those in urban areas.
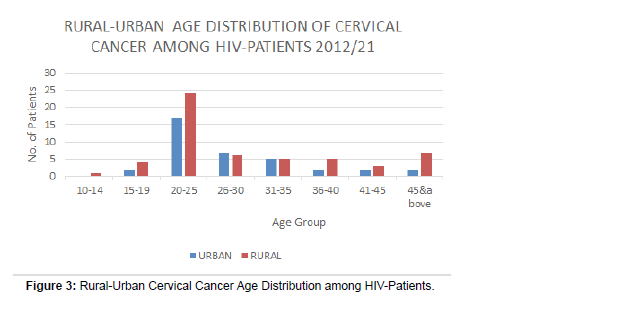
There was a higher percentage of rural residing HIV negative (HIV -VE) young women (Figure 3).
• It is surprisingly 1 patient below 14years in rural area presented with cervical cancer, in the 2012-2019 study periods.
• 4 patients aged between 15-19 in rural presented cervical cancer cases, in the 2020-2021 period of study.
• The prevalent age bracket is 20-25 years old and biased towards rural patients, even though the first period of review is longer at 8 years (2012-2019), while the second period of study is shorter at one year (2020-2021).
• There was a higher percentage of rural residing HIV negative (HIV -VE) young women (
• The first period of study, although is a longer duration of eight years, 2012-2019, the young (
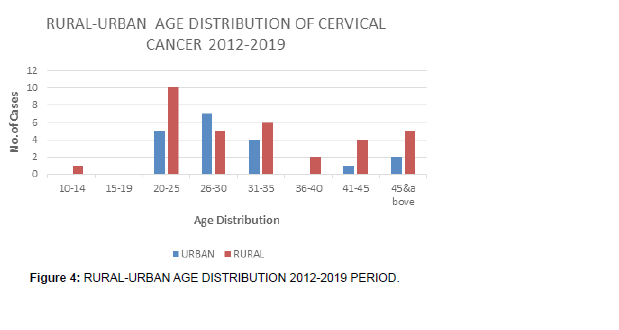
• There is a preponderance of young (Figure 4).
• Although 2012-2019 period is long, only one patient below 20 years old ( 13yrs) presented with cervical cancer, and it is in rural area. No urban case featured in the below 20 years during that period.
• Most incidences in the period was in age group 20-25 mostly in rural areas, but during the longer period of eight years (2012-2019) of study, from year of inception of oncology clinic to end of 2019, the year of review.
• The first period of study, although is a longer duration of eight years, 2012-2019, the young (Figure 5).
• During this period, there were more cervical cancer cases than the previous one.
• There are more cervical cancer cases in very young women in this one year 2020-2021 study period, than in the longer 8 year 2012- 2019 study period, i.e., patients below 19 years than in the previous period.
• There are more cases in rural than urban.
• There most prevalent age group is 20-25 biased in favour of rural residents.
• There was a higher percentage of rural residing HIV negative (HIV -VE) young women (
• There is a preponderance of young (Figure 6).
• The 2012-2019 study period, although is a longer duration of eight years period, the young (
• There is a preponderance of young (
• The FIGO Staging of Cancer of the cervix had a P-value of 0.01906, being statistically significant, meaning diagnosis at advanced stages of III and IV for the young HIV -VE has increased compared to the HIV +VE in this period (Figure 7).
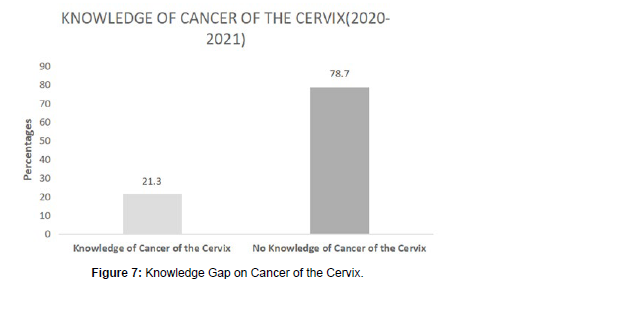
A total of 21% (18 patients) of patients had some prior knowledge of cancer of the cervix, as compared to 79% (68 patients) of patients, who had no idea at all on cancer of the cervix as a disease.
The patients who had some knowledge on cancer of the cervix were mostly the ones who had self-referrals, as compared to those who had no knowledge at all, and thought they had normal vaginal discharge and bleeding post coitus, hence were referred in advanced stages to the oncology clinic.
In the 21% who had some prior knowledge of cancer of the cervix, 22% (4 women) from the rural areas had some knowledge as compared to 78% (14 women) urban residents who had some knowledge.
Amongst the 79% who had no knowledge of cancer of the cervix, 81% (55 women) were residents of the rural communities; whereas 19% (13) were urban dwellers.
Screening of cancer of the cervix
In the first period of study, the voluntary screening programme had 23% HIV -VE patients screened while 77% had not been screened prior in the 2012-2019 period, when the diagnosis of cancer was confirmed. Amongst this 23% group that had been screened, 86% were urban residents, as compared to 14% of rural residents who had been screened in the eight years period of 2012-2019. Amongst the 77% of patients who had never been screened prior to diagnosis of cancer in the eight year study period 2012-2019, 71% were rural residents as compared to 29% who were urban dwellers.
The HIV +VE in the first period of review, 2012-2019, before the policy for routine early screening of cervical cancer in HIV +VE women had been introduced in the HIV Clinics, 10% who had come for the voluntary screening prior before diagnosis of cancer in the eight year period of 2012-2019, while 90% had never been screened before cancer diagnosis in the same eight year period 2012-2019. In this 10% group who had come for voluntary screening, all of them, 100%, were rural residents, none of the urban residents had used the voluntary screening programme before diagnosis of cancer of the cervix. In the 90% group who had never been screened before cancer diagnosis in the eight year period of 2012-2019, 68% were from the rural areas, where as 32% were urban residents.
In the second study period, the voluntary screening programme had 20% HIV -VE patients screened while 80% had not been screened prior in the one year period of 2020-2021, when the diagnosis of cancer was confirmed. Amongst this 20% group that had been screened, 83% were urban residents, as compared to 17% of rural residents who had been screened in the one year period of 2020-2021. In the 80% group that had never been screened prior to diagnosis of cancer, 71% were from the rural areas, where as 29% were urban residents.
The study found out that, in the period 2020-2021, the voluntary routine screening programme had 31.8% HIV -VE patients of ages 13- 35 years screened, while 68.2% had not been screened routinely prior to the diagnosis of cancer of the cervix.
The HIV +VE in the second study period, when the policy for routine early screening of cervical cancer in HIV +VE women had been introduced in the HIV Clinics, had 28% who had come for the voluntary screening prior before diagnosis of cancer in the one year period 2020-2021, while 72% had never been screened before cancer diagnosis in the same one year study period 2020-2021. In this 28% group who had come for voluntary screening, all of them 100%, were urban residents, none of the rural residents had accessed the voluntary screening before diagnosis of cancer of the cervix. Of the 72% who had not been screened prior to diagnosis in the one year period of study, 2020-2021, all of them, 100% were rural residents.
The above findings show a clear increase of 18% of routine early screening of cancer of the cervix in the young HIV +VE patients as compared to their HIV –VE counterparts, from 10% in the 2012-2019 study period to 28% in the 2020-2021 study period, of routine early screening in the young HIV +VE patients as compared to their HIV –VE counterparts
Conclusion
The study concludes that there is a significant increase in the incidence of early onset cancer of the cervix amongst teenagers in Western Kenya, and with the natural history of development of the invasive cancer from HPV sexual infection of between 10-15 years, there is a probability that non-sexual horizontal and vertical transmission may be involved in the cases of these teenagers, even for the high risk HPV type 16.
These patients were also diagnosed in advanced stages (Figo Stages III and IV) of cancer of the cervix, with poor prognosis.
We did not do HPV laboratory investigations in this study; hence we did not identify and isolate the types of the HPV’s that had infected these teenagers.
Recommendations
The study highly recommends a multi-center, multi-country longitudinal cohort study, using electronic health records and/or early-life bio-specimen collection, from ante-natal period of mothers with HPV, until these newborns become sexually active, to find out if some of the participants will develop invasive carcinoma, before they start being infected with HPVs sexually. The types of HPV should also be isolated from the mother and the children. The study also recommends raising awareness of the early-onset cancer of the cervix amongst teenage girls and young women and improving the early-life environment as immediate goals: these are likely to reduce the burden of both early-onset and late onset cancers. The study also recommends raising awareness and knowledge about small cell neuroendocrine cancer of the cervix amongst medics; so that they can intensify the early screening and reduce age at start of screening for cancer, identification of HPVs and their subtypes and initiate aggressive treatment for it. The other recommendation is increasing the general knowledge and awareness of cancer of the cervix, in schools, places of worship, social spaces, funerals and all relevant gatherings that information about cancer of the cervix can be given. The policy of routine HPV vaccination and early screening of cancer of the cervix in all sexually active women should continue and spread all over the country, even to the remotest rural areas to benefit women in those regions. This routine early screening should be done to both the young HIV-VE and HIV +VE women in the entire country.
This study did not involve laboratory investigations of HPV types, hence it needs a follow up research that will involve taking cervical smears to the laboratory to investigate the presence of HPV in the cervix of the respective patients, identify the various types of the HPV, ascertain if there are particular patients who have a combination or mixed presence of two or more HPV types.
Source of Funding
The PhD candidate used his funds, and was helped by the personnel at the hospital, together with volunteering students and the investigators availed their expertise locally to conduct the study. We used the local hospital paper records in the cabinets of the oncology department and the records office. Uzima University School of Medicine supported the study by availing the volunteering students.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 209-49.
- UNAIDS (2022) HIV and Cervical cancer.
- Mbulaiteye SM, Bhatia K, Adebamowo C, Sasco AJ (2011) HIV and cancer in Africa: mutual collaboration between HIV and cancer programs may provide timely research and public health data. J Infectious Agents and Cancer 6: 6-10.
- Taylor RJ, Morrell SL, Wain GV (2001) Effects of screening on cervical cancer incidence and mortality in New South Wales implied by influences of period of diagnosis and birth cohort. J Epidemiol Community Health 55: 782-8.
- Global strategy to accelerate the elimination of cervical cancer as a public health problem.
- Nganga A, Nyangasi M, Nkonge NG (2018) Predictors of cervical cancer screening among Kenyan women: results of a nested case-control study in a nationally representative survey. BMC Public Health 18: 1-10.
- Mwenda V, Mburu W, Bor J, Nyangasi M, Arbyn M, et al. (2022) cervical cancer programme, Kenya, 2011-2020: lessons to guide elimination as a public health problem. Ecancermedicalscience Journal 16: 1442.
- Munoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, et al. (2002) Role of parity and human papillomavirus in cervical cancer: the IARC multicenter case-control study. Lancet 359: 1093-101.
- Bayo S, Bosch FX, Munoz N, Combita AL (2002) Risk factors of invasive cervical cancer in Mali. Int J Epidemiol 31:202-9.
- Smith JS, Green J, Appleby P, Peto J, Plummer M, et al. (2003) Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet 361: 1159-67.
- Kenneth G, Castro MD (1992) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR 41:1-19.
- Hamilton AC, Donnelly DW, Fitzpatrick D, Coleman HG (2022) Early-Onset Cancers in Adults: A Review of Epidemiology, Supportive Care Needs and Future Research Priorities. 14: 4021.
- Ugai T, Sasamoto N, Ando HM, Song M, Tamimi RM, et al. (2022) Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nature Reviews Clinical Oncology 19:656-673.
- Ryndock EJ, Meyers C (2014) A risk for non-sexual transmission of human papillomavirus? Expert Rev. Anti Infect Ther 12: 1165-1170.
- Petca A, Borislavschi A, Zvanca ME, Petca R, Sandru F, et al. (2020) Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp Ther Med 20: 186.
- Rosa La G (2016) Papillomavirus in Global Water Pathogen Project. Rose JB and Jimenez-Cisneros B. UNESCO.
- Castle PE, Maza M (2016) Prophylactic HPV vaccination: Past, present, and future. Epidemiol Infect 144: 449-468.
- Park IU, Introcaso C, Dunne EF (2015) Human papillomavirus and genital warts: A review of the evidence for the 2015 centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 61: 849-855.
- Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, et al. (2018) Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int J Oncol 52: 637-655.
- https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
- You W, Li S, Du r, Zheng J, Shen A (2018) Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp Ther Med 15: 412-418.
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE (2011) the cost-effectiveness of male HPV vaccination in the United States. Vaccine 29: 8443-8450.
- Burger EA, De Kok IMCM, Groene E, Killen J, Canfell K, et al. (2020) Estimating the Natural History of Cervical Carcinogenosis Using Simulation Models: A cisnet Comparative Analysis. J Natl Cancer Inst 112: 955-963.
- Schiffman M, Wentzensen N (2013) Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev 22: 553-560.
- Tay SK, Ho TH, Lim-TanSK (1990) is genital human papillomavirus infection always sexually transmitted. Aust NZJ Obstet Gynaecol 30: 240-242.
- Mammas IN, Dalianis T, Doukas SG, Zaravinis A, Achtsidis V, et al. (2019) Paediatric virology and human papillomaviruses: An update. Exp Ther Med 17: 4337-4343.
- Hong Y, Li SQ, Hu YL, Wang ZQ (2013) Survey of human papillomavirus types and their vertical transmission in pregnant women. BMC Infect Dis 13.
- Iaconelli M, Petricca S, Libera SD, Di Bonito P, La Rosa G (2015) First detection of human papillomaviruses and human polyomaviruses in river waters in Italy. Food Environ Virol 7: 309-315.
- Della LS, Petricca S, Iaconelli M, Sanguinetti M, Graffeo R, et al. (2015) A large spectrum of alpha and beta papillomaviruses is detected in human stool samples. J Gen Virol 96: 607-613.
- Dunyo P, Effah K, Udofia EA (2018) Factors associated with late presentation of cervical cancer cases at a district hospital: a retrospective study. BMC Public Health 18: 1156.
- Mlange R, Matovelo D, Ramba P, Kidenya B (2016) Patient and disease characteristics associated with late tumour stage at presentation of cervical cancer in northwestern Tanzania. BMC Womens Health 16.
- Wistuba II, Thomas B, Behrens C, Onuki N, Lindberg G (1999) Molecular abnormalities associated with endocrine tumors of the uterine cervix. Gynecol Oncol 72: 3-9.
- Ries LAG, Young JL, Keel GE (2007) SEER survival monograph: cancer survival among adults: US SEER program, 1988-2001, patient and tumor characteristics. NIH Pub 111-22.
- Frisch M, Biggar RJ, Goedert JJ (2000) Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 92: 1500-1510.
- Parham G (2010) cervical cancer prevention in HIV-infected women in resource-limited settings. HIV Therapy 4: 625-628.
- Arbyn M, Weiderpass E, Bruni L, De Sanjose S, Saraiya M, et al. (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. THE LANCET-Global Health 8: 191-203.
- Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, et al. (2023) Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet 2023; 11: 197-206.
- Zhao J, Xu L, Sun J, Song M, Wang L, et al. (2023) Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncology 2: 49.
- Fernandez E (2020) Climate Change Will Give Rise to More Cancers. UCSF Research Journal.
- Kingsland J (2020) how might climate change affects the spread of viruses? MedicalNewsToday.
- Smith JS, Green J, Berrington de Gonzalez A, Appleby P, Peto J, Plummer M, et al (2003) Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet 361: 1159-67.
- Rabkin CS, Biggar RJ, Baptiste MS, Abe T, Kohler BA, et al. (1993) Cancer incidence trends in women at high risk of human immunodeficiency virus (HIV) infection. Int J Cancer 55:208-12.
- Mapanga W, Brown GB, Singh E (2019) Knowledge, attitudes and practices of young people in Zimbabwe on cervical cancer and HPV, current screening methods and vaccination. BMC cancer 19: 843.
- Coutinho RA (2000) highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 92: 1823.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ajwang A, Ogutu G, Rogo K, Otoi S, Beltman J, et al. (2023) Are Non-Sexual and Vertical HPV Transmission Increasing the Incidence of Early OnsetCancers in HIV Negative Teenagers in Western Kenya? – A Case Study ofJaramogi Oginga Odinga Teaching and Referral Hospital. Cervical Cancer, 8: 185.
Copyright: © 2023 Ajwang A, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2090
- [From(publication date): 0-2023 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1857
- PDF downloads: 233