Applying Ionizing Radiation to Metal Speciation for the Environmental Assessment of Underground Aquifer Associated with Technogenic Landfill Containing Sludge from A Water Treatment Plant (WTP)
Received: 01-Feb-2018 / Accepted Date: 28-Feb-2018 / Published Date: 02-Mar-2018 DOI: 10.4172/2155-6199.1000434
Abstract
This study was carried out in a technogenic landfill of sludge from the Taiaçupeba Water Treatment Station, Suzano, Brazil. The availability of this residue is a problem, since the presence of heavy metals in the sludge increases the risk of contamination of the physical environment, especially the soils and groundwater. The metal dosage methodologies in the samples took into account the presence of organic matter, and the use of technologies based on Advanced Oxidative Processes (AOPs) were applied to lead to the complete mineralization of metallic chemical elements. The results indicated that the irradiated samples presented higher metals dosages in relation to the non irradiated samples. The application of water analysis techniques through advanced oxidation processes (AOP) such as radiolysis indicated that contents found in environments containing high organic matter concentration, such as the samples collected in the technogenic landfill where the sludge was deposited, presented different values from those analyzed through conventional techniques.
Keywords: Ionizing radiation; Advanced oxidative processes; Metal speciation
Introduction
Assessing samples from aquatic systems requires adopting methodologies able to minimize organic matter interference in analytical results, mainly when it comes to dosing metals whose concentrations are too low to be detected.
Several studies have shown that soils rich in organic matter have superior fixing capacity than poor soils. However, this capacity becomes secondary in comparison to the action of iron, aluminum and manganese oxyhydroxides or even to the action of clayminerals in retaining metals in the soil or in sediments [1-3].
In addition to mineral composition, the occurrence of microbiological contamination and excess of organic matter in groundwater wells, in association with dissolved aluminum (Al3+), form complex organic compounds [4]; pre-preparation methods such as sample acidification only oxidize part of the organic substances found therein.
Complex chemical formations related to nonfully digested organic matter may happen mainly when atomic spectrometry methods such as Atomic Absorption Spectrometry (AAS), Inductively Coupled Plasma Optical Emission Spectrometry (ICPOES), and Inductively Coupled Plasma Mass Spectrometry (ICPMS) are applied to metal quantification [5,6].
The application of metal dosing methodologies to samples derived from aquatic systems should take into consideration the presence of organic matter, since it may compete with the species of interest for the working electrode surface. This fact may reduce measurement sensitivity and/or lead to electrochemical reduction or oxidation and, consequently, increase the residual current to the point of hampering the analysis.
In addition to the instrumental analytical method, procedures adopted as sample pretreatment for metal analysis often comprise dissolution and mineralization, which are susceptible to contamination and/or loss of chemical elements due to volatilization.
The option for the organic matter mineralization method may lead to metal species release into an analytical solution; thus, it is essential checking the total organic carbon (TOC) reduction. The entire organic molecule turns into carbon dioxide (CO2) and water (H2O) during the decomposition of organic compounds, fact that enables total mineralization [7,8].
Classical organic matter destruction methods involve wet mineralization; they consist of heating the sample and adding reagents such as oxidizing acids, mixtures thereof or even hydrogen peroxide to it. However, these methods have the disadvantage of posing substantial risk of contaminating the sample, mainly samples containing metals at trace level, besides requiring high reactant consumption and long mineralization time. Thus, the advanced oxidation mineralization through ionizing radiation is an alternative to the conventional acid mineralization [4,7,9,10].
Advanced Oxidative Processes (AOP) can mineralize organic compounds [9] and have been adopted in the environmental assessment of contaminated areas, mainly in the treatment of wastewater, due to the complex characterization of these wastes.
Technologies based on Advanced Oxidative Processes (AOPs), which promote chemical changes in the substrate, can also lead to the complete mineralization of metallic chemical elements [11,12]. In addition, AOPs may be used to treat contaminants whose concentration is very low (ppb) [13,14], as it happens to some metals.
The interaction between energetically charged particles (electrons, photons, and alpha particles), highenergy photons (gamma rays and xrays) and matter mainly leads to the ionization and excitation of the medium where radiation is absorbed in. Ionized and excited molecules, as well as free electrons, are produced when water molecules are irradiated.
Photochemical reactions generated by radiation are generally radical and based on hydroxyl radical formation (HO•) a highly reactive oxidizing agent capable of breaking covalent bonds and inducing the complete mineralization of organic compounds found in the sample.
In addition, this procedure significantly reduces mineralization time and reagent amounts, fact that minimizes the possibility of contaminating the sample [15,16].
The Production and Disposal of Sludge from Water Treatment Plants (WTP)
Social demands for improvements in quality of life involve issues related to environmental sanitation; thus, the water applied to several human activities must present features able to meet the quality standards and criteria for human consumption, or potability standards. The World Health Organization (WHO) internationally set criteria and parameters for drinking water for human consumption followed by the Brazilian Ministry of Health [17].
The option for water treatment technology should take into consideration the physicochemical features of the raw water to be treated in order to allow removing or reducing certain constituents found in it.
According to NBR 12.216 (ABNT), a Water Treatment Plant (WTP) is the set of units designed to adapt water features to the potability standards set by the Brazilian Ministry of Health. The implementation of water treatment systems depends on environmental licensing, according to Resolution 237 issued by the National Environment Council [18].
The water treatment systems used by most water purification industries in the world, including Brazil, comprise coagulation, flocculation, decantation or sedimentation processes, as well as filtration and disinfection; these systems are generically called conventional water treatment plants [19,20].
The addition of aluminum sulfate or ferrous sulfate leads to coagulation; compounds formed during the coagulation stage present adsorption property. These particles present superficially positive electric charges, whereas the impurities in the water such as suspended matter, colloidal substances, as well as some dissolved salts and bacteria, present negative electric charge. Thus, flakes retain suspended impurities, and it enables decantation in the form of sludge. The produced sludge is a non-Newtonian, gelatinous fluid whose solid fraction consists of aluminum hydroxide or iron hydroxide, inorganic particulate matter, polymers, colloids and several organic compounds; it presents low compressibility, high volume and low solid content.
The production and disposal of sludge derived from WTPs is already taken into consideration in several countries; it has been a worldwide tendency to consider sludge a product, rather than a waste, since it adds value through reuse [21].
Although the sludge generated in WTPs is considered solid waste by the legislation (ABNT, 2014), in many Brazilian regions, this waste is released in natura in areas that were not prepared for such disposal, fact that does not comply with public environmental sanitation policies.
The installation of WTPs should be preceded by a system sizing stage, which may be carried out through tests or at laboratory scale, since raw water composition turbidity, suspended solids, among others changes depending on the season [22].
The seasonal character in the composition of the water to be treated would require a preliminary study about sludge production by the WTP for at least one year, fact that makes this procedure economically unfeasible. Thus, projects end up dimensioning sludge production through empirical formulas, although they do not represent the water resource reality.
The sludge features may be related to environmental aspects (pH, solids, metals, COD, biodegradability, toxicity, pesticides, fertilizers and volatile organic compounds) associated with waste disposals. Also, to geotechnical aspects (particle size and size distribution, plasticity and liquidity limits, specific resistance, heating and cooling responses and sedimentation) related to water removal and to future uses of solids from wastes [23,24].
Study Site
The Taiaçupeba Water Treatment Plant and Dam (Taiaçupeba WTP), which is managed by São Paulo State Basic Sanitation Company (SABESP Cia. de Saneamento Básico do Estado de São Paulo), is located in Suzano County, Metropolitan Region of São Paulo (RMSP Região Metropolitana de São Paulo), Brazil. The dam composes the Alto Tietê Producer System (SPAT Sistema Produtor Alto Tietê), which is the third largest Integrated Water Production System whose adductors supply the RMSP.
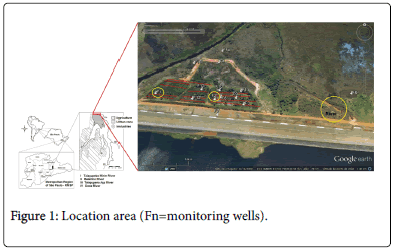
The SPAT is formed by Tietê River headwaters, which are regularized by Ponta Nova (Tietê and Claro Rivers), Paraitinga (Paraitinga River), Biritiba (Biritiba River), Jundiaí (Jundiaí, Rio Grande and Doce Rivers) and Taiaçupeba Dams (Taiaçupeba Mirim, Balainho and TaiaçupebaAçu Rivers) (Figure 1).
The sludge generated at Taiaçupeba WTP from 1992 to 2004 was discarded in the reservoir located downstream of the WTP catchment. At the request of São Paulo State Basic Sanitation Company (CETESB Companhia de Saneamento Básico do Estado de São Paulo), the sludge once discarded in the lake was partially removed from February 2005 on. Part of it was taken to be treated along with the sludge generated in the desiccators in the sludge densification and disposal system (ADSL), at the ratio of 60 tons of WTP sludge to 90 tons of sludge from the lake. The material was sent to the drying area, in covered furrows and, after drying, it was stored in landfill cells as SABESP's environmental liability.
A larger volume was collected and accumulated in dikes located in the area next to the dam spillway, near the massif without following environmental norms and formed a technogenic landfill. Part of the sludge remains in the lake enclosed in cofferdams (Figure 2).
The scenario described above, which comprises the succession of events from the sludge generation to its deposition inside the lake wherein it was studied and characterized, evidenced the presence of metals above the levels set by Brazilian environmental standards [25].
The partial sludge removal from the lake and its disposal in an area adjacent to the dam did not comply with the environmental norms for the installation of technogenic landfills.
Materials and Methods
Monitoring wells were set in the area where the technogenic landfill was installed in order to collect samples from the deposited material column, as well as to collect underground water. The setting of the wells was preceded by the application of geophysical methods. Those methodologies were used to estimate the groundwater depth level, the thickness of the sedimentary package (sludge+technogenic landfill covering soil), as well as to map and monitor organic and inorganic compounds such as contaminant plumes, slurry, among others, in the geological environment [26].
The geophysical methods adopted in the current study were the electrical resistivity method, which used the Vertical Electrical Sounding (VES) technique, and the electromagnetic method, which used the EM31 technique. The points for the drilling, and subsequent groundwater sampling, were located according to a geophysical survey (resistivity and electromagnetic map), which indicated regions presenting anomalies.
Physicochemical variables such as water sample pH, Eh, dissolved oxygen, electrical conductivity; salinity and temperature were set in the field by using a portable multiparameter probe, which was previously calibrated with the respective solutions.
Water samples were collected for anions and cations analysis; unfiltered and no acidified water samples subjected to anions analysis (fluoride, chloride, nitrate, bromide, nitrite, sulfate and dihydrogen phosphate), whereas samples not subjected to acid treatment underwent cations analysis (aluminum, barium, strontium, lead, cadmium, zinc, copper, nickel, chromium, manganese, iron, lithium, sodium, potassium, magnesium and calcium).
The inductively coupled plasma optical emission spectrometry (ICPOES) was adopted as metal dosing method, according to the Brazilian standards ABNT NBR ISO/IEC 17025, ISO 14001 and OHSAS 18001 standards.
Water samples were selected from two monitoring wells (F1 and F2) and subjected to radiation method; whereas a surface water sample called “River” water collected at the dam spillway was used for comparative purposes.
The samples were irradiated at the chemistry laboratory of the Radiation Technology Center (CRT Centro de Tecnologia das Radiações) belonging to the Nuclear and Energy Research Institute of the National Nuclear Energy Commission (IPEN Instituto de Pesquisas Energéticas e Nucleares / CNEN Comissão Nacional de Energia Nuclear). The CRT has all safety requirements for analytical procedures.
Sample preparation consisted in transferring them to 100 ml glass vials, which were symmetrically arranged to the radiation source in order to receive the radiation dose as evenly as possible, since samples in the gammacell 20 device are surrounded by 60 Co radioactive bars.
Radiation was carried out at room temperature (25°C) based on the Gamma cell source (60 Co), at maximum dose rate 1.5 kGy/h, and at single absorption dose 20 kGy. Samples were chemically analyzed through ICPOES after radiation in order to determine aluminum, zinc, manganese, iron and lead levels.
Results and Discussion
Samples were irradiated in order to "disrupt" the organic matter and release fixed metals into the water. Subsequently, they were analyzed through UVV is in order to check the effective elimination of organic compounds. Samples were analyzed through ICPOES (metal determination in duplicate); one sample was not irradiated and the other one was subjected to radiation.
In the Table 1 shows the anion composition and physic-chemical parameters of the contamination in the wastewater in this study.
| Samples | F- | Cl- | NO2- | NO3- | Br- | HPO4-2 | SO4-2 |
|---|---|---|---|---|---|---|---|
| F1 | 0,14 | 28,53 | 0,005 | 0,75 | 2,46 | 0,14 | 1,19 |
| F2 | 0,05 | 58,32 | 0,04 | 0,25 | 3,83 | 0,05 | 10,53 |
| River | 0,05 | 2,83 | 0,013 | 0,3 | 0,17 | 1,54 | 30,34 |
| LoQ | 0,01 | 0,01 | 0,005 | 0,011 | 0,001 | 0,01 | 0,02 |
| Ref. 1 | ND | ND | ND | 10 mg/L-N | ND | ND | ND |
| Ref. 2 | 1,4 mg/L | 250 mg/L | 1 mg/L-N | 10 mg/L-N | ND | ND | 250 mg/L |
| Samples | CE (μЅ/cm) | pH | T (°C) | STD (ppm) | Salinidade (‰) | Eh (mV) | |
| F1 | 372,5 | 5,96 | 21,51 | 186 | 0,18 | 8,9 | |
| F2 | 638 | 5,89 | 24,88 | 319 | 0,31 | -18,9 | |
| River | 89 | 4,96 | 21,78 | 44 | 0,04 | -11 | |
| Ref. 3 | 6 a 9,5 | ||||||
Table 1: Anions concentration in water samples (mg/L) and physical chemical parameters. Ref. 3=CETESB/2014, São Paulo State; Portaria
2.914/11, Brazil. Non-detected (ND); limit of quantification (LoQ); Ref.1=reference values established by CETESB/14, São Paulo State, or
CONAMA 396/08, Brazil; Ref. 2=CONAMA 430/11.
The Table 2 shows the results of the ICPOES technique applied to irradiate (20 kGy) and no irradiated samples (0 kGy) for three toxic metals according the reference values established by Brazilian environment agencies: Al, Pb, Mn and Zn.
| Sample | Al dissolved | Pb dissolved | Zn dissolved | Mn dissolved | Fe dissolved | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 kGy | 20 kGy | 0 kGy | 20 kGy | 0 kGy | 20 kGy | 0 kGy | 20 kGy | 0 kGy | 20 kGy | |
| F1 | ND | 0,2 ± 0,03 | ND | <LoQ | 0,04 | 0,555 ± 0,077 | 0,9 | 0,999 ± 0,15 | 45,20 | 50,070 ± 7,57 |
| F2 | ND | 0,31 ± 0,045 | ND | <LoQ | 0,04 | 0,372 ± 0,052 | 1,85 | (2,595 ± 0,38)*5 | 64,61 | 71,240 ± 10,77 |
| River | ND | 0,28 ± 0,041 | ND | <LoQ | 0,10 | 0,185 ± 0,026 | 0,05 | 0,052 ± 0,0076 | 0,42 | 0,427 ± 0,065 |
| LoQ | 0,05 | 0,005 | 0,005 | 0,005 | 0,05 | |||||
| Ref. | 0,1 mg/L | 0,01 mg/L | 1,8 mg/L | 0,1 mg/L | 0,3 mg/L | |||||
Table 2: Anti-nutritional factors of germinated finger millet.
The analysis of the nonirradiated (F1 and F2) samples showed undetected Al and Pb; Zn=0.04 mg/L in both samples; Mn=0.9 mg/L and 1.85 mg/L; as well as Fe=45.2 mg/L and 64.61 mg/L, respectively.
The same samples presented Al=0.2 mg/L and 0.31 mg/L; Pb within the quantification limit in both samples; Zn=0.55 mg/L and 0.37 mg/L; Mn=0.99 mg/L and 12.97 mg/L; as well as Fe=50.07 mg/L and 71.24 mg/L, respectively, after irradiation.
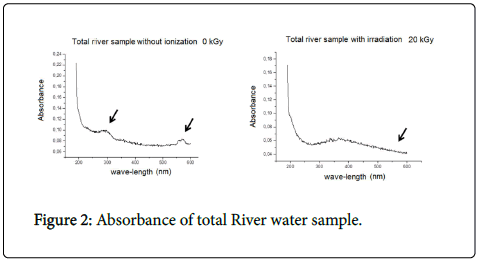
The UVV is technique was applied to investigate absorption in the samplings. The comparison between the total nonirradiated sample and the total sample irradiated with 20 kGy showed small absorbance decrease in the irradiated sample, fact that evidenced organic matter release in the herein analyzed matrix; such release was indicated by sample color change from brown to colorless (Figure 2).
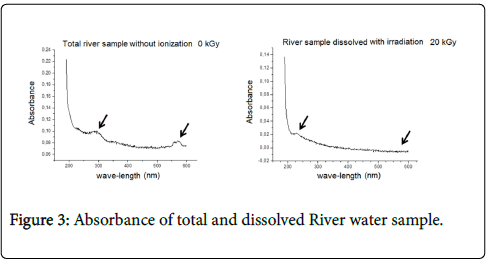
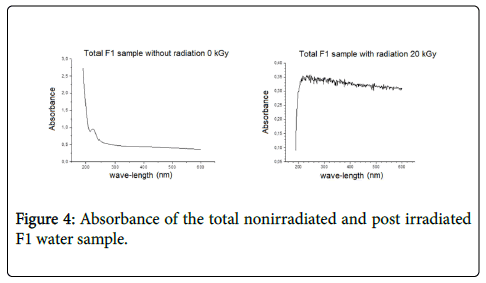
Accordingly, the absorbance in the total nonirradiated water sample and in the nonirradiated dissolved sample was compared. Results showed lower organic matter in the filtered sample, i.e., the dissolved sample presented lower absorbance than the nonirradiated one (Figure 3). The absorbance in the total irradiated F1 sample and in the irradiated filtered F1 sample was also compared both at the dose 20 kGy. The dissolved sample presented low absorbance (Figure 4), which enabled making adjustments to apply the methodology for sample dilution.
From the classical filtration process, metal ions are obtained in the samples. However, when an energy charge emitted through the radiations is applied, the concentration of these same metals is increased due to the breaking of the complex organic matter ions present. Therefore, the option of organic matter mineralization method allowed the release of the metal species to the analytical solution, because in the decomposition of the organic compounds allows full mineralization.
Conclusions
The traditional process comprising nitric acid addition to acidify, digest and store samples in order to subsequently apply analytical techniques able to quantitatively analyze these samples does not assure results without organic interferers. It is known that analytical systems, such as ICPOES, reach elevated temperatures, as well as that, despite this condition, the complex organic matter is not fully mineralized.
However, whenever an energy charge emitted through radiation is applied, the concentration of these very it metals increases due to the disruption of complex organic matter ions found in the solution.
The radiolytic degradation applied to water samples from polluted environments is a recent technique used to treat natural and polluted waters, as well as effluents from distinct sources.
The application of water analysis techniques through advanced oxidation processes (AOP) such as radiolysis indicated that metal contents found in environments containing high organic matter concentration, such as the samples collected in the dam spillway (river) and in the wells implanted in the technogenic landfill where the sludge was deposited in, presented different values from those analyzed through conventional techniques.
In fact, the analyzed metals increased in the samples after the organic matter was “disrupted” through radiation; the post radiation values of some metals such as aluminum and manganese classified the samples as above the environmental parameters set by the Brazilian legislation. However, the analyses were not able to find these metals before irradiation.
The application of radiolysis to characterize the presence of metals in water samples presenting organic matter is a procedure that may help conducting environmental assessments in degraded areas.
Acknowledgments
The São Paulo Research Foundation, FAPESP–Process 2013/01507-1. Dra. Aurea Beatriz Cerqueira Geraldo-Energetic and Nuclear Research Institute - IPEN/CNEN-SP.
References
- Kalbasi MG, Racz J, Loewen Rudgers LA (1978) Mechanism of Zn adsortion by iron and aluminum oxides. Soil Science 125: 146-150.
- Chlopecka A, Bacon JR, Wilson MJ, Kay J (1996) Forms of cadimium, lead, and zinc in contaminated soils from Southwest Poland. Journal of Environmental Quality, Madison, 25: 69-79.
- Imbernon RAL (2000) Geochemical behavior of Heavy Metals in the Soil System of the Taiaçupeba dam Environmental Control. FEHIDRO Technical Report.
- Bazante-Yamaguishi R, Moura E, Manzoli JE, Geraldo AB (2014) Radiation-grafted, chemically modified membranes part I–Synthesis of a selective aluminum material. Radiation Physics and Chemistry 94: 133-136.
- Knapp G, Maichin B, Fecher P, Hasse S, Schramel P (1998) Iodine determination in biological materials options for sample preparation and final determination. Fresenius J Anal Chem 362: 508-513.
- Machat J, Otruba V, Kanicky V (2002) Spectral and nonspectral interferences in the determination of selenium by inductively coupled plasma atomic emission spectrometry. Journal of Analytical Atomic Spectrometry 17: 1096-1102.
- Duarte CL, Mori MN, Baumgartner JB (2004) Treatment of Cutting Oil by the Advanced Oxidation Process by Ionizing Radiation. Brazilian Congress of Science and Technology in Waste and Sustainable Development, Costão do Santinho, Florianópolis, Santa Catarina, ICTR, p: 9.
- Carvalho AMX, Vale HMM, Ferreira EM, Cordeiro AFP, Barros NF, et al. (2008) Microbial activity of soil and litter in areas populated with Pinus elliottii and Terminalia ivorensis. Soil Science Journal 32: 2709-2716.
- Borrely SI, Sampa MHO, Pedroso CB, Oikawa H, Silveira CG, et al. (2000) Radiation processing of wastewater evaluated by toxicity assays. Radiation Physics and Chemistry 57: 507-511.
- Duarte CL, Sampa MHO, Rela PR, Oikawa H, Silveira CG, et al. (2002) Advanced Oxidation Process by Electron Beam Irradiation Induced Decomposition of Pollutants in Industrial Effluents. Radiat Phys Chem 63: 647-651.
- Sarria V, Parra S, Adler N, Péringer P, Benitez N, et al. (2002) Recent developments in the coupling of photoassisted and aerobic biological processes for the treatment of bio recalcitrant compounds. Catalysis Today 76: 301-315.
- Tabrizi GB, Mehrvar M (2004) Integration of advanced oxidation technologies and biological processes: recent developments, trends, and advances. Journal of Environmental Science and Health, Part A 39: 3029-3081.
- Domènech X, Jardim WF, Liftter MI (2001) dvanced oxidation processes for the elimination of contaminants. In: Elimination of Contaminants by Heterogeneous Photocatalysis, Graph, La Plata, Argentina.
- Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Advanced in Environmental Research 8: 553-597.
- Moraes MCF (2004) Study of the effects of ionizing radiation on the acute toxicity of anionic surfactant effluents. Dissertation (MSc in Applications in Nuclear Techniques), Institute of Energy and Nuclear Research, p: 106.
- Meeroff DE, Waite TD, Kazumi J, Kurucz CN (2004) Radiation Assisted Process Enhancement in Wastewater Treatment. Journal of Environmental Engineering 130: 155-166.
- Libânio M (2010) Fundamentals of water quality and treatment. Campinas, Brazil, p: 949.
- Cordeiro JS (2001) Processing of sludge from Water Treatment Plants (ETAs). Sanitation Solid Waste: Processing, Recycling and final disposal 2: 121-142.
- Derisio JC (2012) Introduction to environmental pollution control. Office of texts, São Paulo, Brazil, p: 234.
- APHA, AWWA, WEF (1999) Standard methods for the examination of water and wastewater. 20th edn, Washington, USA.
- Katayama VT (2012) Quantification of the production of sludge from full-cycle water treatment plants: a critical analysis. Dissertation (Master in Engineering)-Polytechnic School of the University of São Paulo, São Paulo, Brazil, p: 144.
- Di Bernardo L, Dantas AB, Voltan PEN (2012) Methods and Techniques of Treatment and Disposal of Waste Generated in Water Treatment Plants.
- Di Bernardo L, Dantas A, Di B (2005) Methods and Techniques of Water Treatment 2nd edn. São Carlos: RiMa 2: 792.
- Franco DR, Berquó TS, Imbernon RAL, Partiti CSM, Enzweiler J (2007) Environmental monitoring of magnetic iron phases of urban water reservoir lake sediments (Taiaçupeba Lake, metropolitan region of São Paulo, Brazil) by using Mossbauer spectroscopy. Environ Geol 52: 831-842.
- Moreira CA, Braga ACDO (2009) Application of geophysical methods in the monitoring of contaminated area under natural attenuation. Sanitary and Environmental Engineering 14: 257-264.
Citation: Imbernon RAL, Yamaguishi BR and Muchimbane ABDA (2018) Applying Ionizing Radiation to Metal Speciation for the Environmental Assessment of Underground Aquifer Associated with Technogenic Landfill Containing Sludge from A Water Treatment Plant (WTP). J Bioremediat Biodegrad 9: 434. DOI: 10.4172/2155-6199.1000434
Copyright: © 2018 Imbernon RAL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3727
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2910
- PDF downloads: 817