Case Report Open Access
Application of X-ray Fluorescence Spectrometers for Biopharmaceutical Forensics
Jessica P Mondia1*, Matthew Marrichi2, Amr S Ali1, Austen Ng3 and Maureen Lanan4
1Deparment of Cell Culture Development, Biogen, Cambridge, MA 02142, USA
2Department of Manufacturing Sciences, Biogen, Cambridge, MA 02142, USA
3Department of Protein Biochemistry, Biogen, Cambridge, MA 02142, USA
4Department of Analytical and Cell Culture Development (formerly), 31 Jefferson St, Newton, MA 02458, USA
- *Corresponding Author:
- Jessica P Mondia
Deparment of Cell Culture Development, Biogen
250 Binney Street, Cambridge
MA 02142, USA
Tel: 617-679-2961
E-mail: jessica.mondia@biogen.com
Received date: September 02, 2015; Accepted date: September 22, 2015; Published date: September 29, 2015
Citation: Mondia JP, Marrichi M, Ali AS, Austen Ng, Lanan M (2015) Application of X-ray Fluorescence Spectrometers for Biopharmaceutical Forensics. J Anal Bioanal Tech 6:280. doi:10.4172/2155-9872.1000280
Copyright: © 2015 Mondia JP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In biopharmaceutical drug development and production, a portable energy dispersive x-ray fluorescence (EDXRF) spectrometer can be used for identification of foreign particles, investigation of discolored solutions, and improvement of process understanding. The spectrometer is a safe, small, robust, rapid, easy-to-use, multielemental diagnostic tool. Examples using the EDXRF for detecting metal composition in particulates, precipitants, liquids, and resins-with rapid resolution of investigations-are described.
Keywords
EDXRF; Biopharmaceutical; Forensics; Manufacturing; Investigations; Discoloration; Particulates
Introduction
Portable energy-dispersive x-ray fluorescence (EDXRF) spectrometers are safe, inexpensive, versatile, easy-to-use, rapid instruments for multi-elemental analysis. They are employed in fields such as geoarcheology (to date artifacts) [1,2], environmental applications (e.g., measuring heavy metal content in soils) [3,4], and the food industry (to determine toxic inorganics in FDA regulated products) [5,6]. Their use in the (bio-) pharmaceutical industry is relatively recent. In one study, portable EDXRF spectrometers were used to determine toxic metals (Cr, As, Pb and Hg) in pharmaceutical tablets [7]. In another study, an EDXRD spectrometer was used to measure metal variability in nutrient powders used in cell culture, to improve bio-therapeutic product consistency [8]. However, the potential of EDXRF as a forensic tool has yet to be fully explored.
Biopharmaceutical forensics refers to the application of science to help resolve issues in the development and manufacturing of biotherapeutic drugs. These investigations may encompass a wide variety of goals, such as enhancing process understanding during drug development, resolving process deviations during manufacturing, or ensuring suitability of raw materials for drug production. Issues that arise during an investigation may be associated with costly delays in the development of a process and/or the release of a drug batch. Thus, there is a desire for rapid and information-rich diagnostic tools to understand the root cause and the risks of the problem.
Common forensic tools employed by the industry for organic identification include Raman spectroscopy, nuclear magnetic resonance (NMR), and liquid chromatography [9]. But in a number of cases, such as discolored substances, metal composition is often more useful for elucidating the root cause. In biopharmaceuticals, metals are typically detected by inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma atomic emission spectroscopy (ICP-AES) or atomic absorption spectroscopy (AAS); in all cases, sample preparation or sample digestion is required. However, sample preparation is not always feasible, as in the case of plastic or metal particulates and chromatography resins. Here we show how EDXRF can be used as a metal analysis tool to generate fast, meaningful results to help resolve or guide biopharmaceutical forensic investigations for many types of materials.
X-ray fluorescence is the emission of secondary radiation from a material after being excited by x-rays at a higher energy. Because each element has a unique orbital structure, the energy of the emitted radiation identifies the element, while the fluorescence intensity correlates to its abundance. Like ICP-MS, EDXRF is a multi-elemental analysis tool, which is able to measure elements ranging from Na- U. Neither ICP-MS nor EDXRF can distinguish between oxidation states of metals. The strength of ICP-MS is to quantitatively measure concentrations as low as parts per billion (ppb) levels, but its utility is limited to samples that can be solubilized or digested. In contrast, EDXRF is able to measure all matrices including powders, liquids, viscous solutions, resins, particulates and solids in minutes, with minimal to no sample preparation. Moreover, the spectrometer’s small footprint makes it ideal to transport through the manufacturing plant, an impractical task with analysis tools such as ICP-MS. The limitations of EDXRF are that it requires external reference standards of a similar composition for calibration, and that it has higher limits of detection (LOD). For elements of interest in the biopharmaceutical industry, the LODs are approximately 100 ppm for elements ranging from sulfur to calcium (S-Ca), 10 ppm from titanium to iron (Ti-Fe), and 1 ppm from cobalt to selenium (Co-Zr) [8]. These limitations however, have not hindered the swift resolution of many problems typically encountered in the biopharmaceutical industry. To that end, this paper explores three types of investigations: (i) foreign particle identification, (ii) discolored solution composition assessment, and (iii) process development support.
Materials and Methods
The Tracer III-SD energy dispersive handheld XRF spectrometer (Bruker AXS Handheld, Inc., Kennewick, WA) outfitted with a rhodium (Rh) x-ray tube and a 10 mm2 XFlash silicon drift detector was used for all EDXRF analysis. The multichannel analyzer resolution is approximately 20 eV per channel and the instrument resolution is <155 eV (Mn Kα, 1500 cps). The spectrometer operates at a voltage of 40 kV and a current of 30 μA for integration times ranging from 60-300 sec. A filter composed of 0.001” Ti and 0.012” Al is used to optimize the excitation conditions for elements ranging from titanium to silver (Ti-Ag); a second filter composed of Ti is used to optimize excitation from magnesium to iron (Mg-Fe). A collimated elliptical x-ray beam, ~10 mm × 7 mm, is emitted at 53° onto the samples. XRF sample cups (SC-4231: Lab Premier Supply, Port St. Lucie, FL) sealed with ultralene film (SPEX Certiprep, Metuchen, NJ) are used to store liquids, powders and particulates for metal analysis.
All liquid samples were calibrated using standards composed of ultrapure water mixed with varying concentrations of 1000 ppm singleelement solutions purchased from SpexCertiPrep. Note that since resins are stored in solution to maintain their functionality and their interactions with x-rays are similar to liquids, the liquid calibration standards can be employed.
Results and Discussion
Foreign particle identification
Small particulates can enter the manufacturing process through various means, such as improper dissolution of raw materials, raw material milling impurities, chips from storage containers or from worn down manufacturing components. Particulates come in many different shapes and typically range from 0.5 to 10 mm. Traditionally, particles that dissolve can be analyzed by laborious techniques such as ICP-MS or NMR to determine composition and potential for process impact. If the particles cannot be dissolved, they would typically be captured by filters and pose little to no process risk, but their origin would be unknown. Using the EDXRF, an initial (60 sec) assessment with minimal sample prep, (i.e., particle is placed in the center of an XRF sample cup) provides the metal content and guides the next steps to determining root cause.
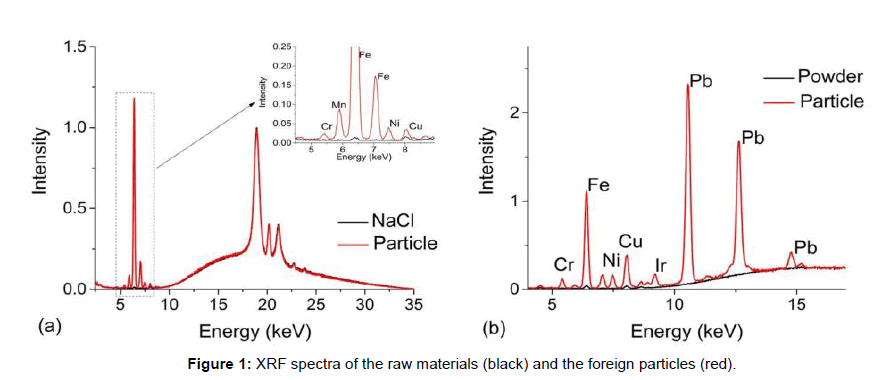
Figure 1 show two case studies of foreign particles found in raw materials used for cell culture. The first set of spectra, Figure 1a, are from sodium chloride crystals (black line) used as a common component in cell culture media overlaid with a foreign metallic looking particle (red line). The entire spectral range can be used to screen metals from Na to U, but for biopharmaceutical investigations, the typical range of interest is from 2.309 keV to 14.165 keV, i.e., the first order transition lines for S to Sr [10]. The broad background between 10-35 keV and the peaks located at 18.9 keV, 20.7 keV and 21.1 keV are associated with instrument-generated x-ray lines that are backscattered from the sample. These features are present in all EDXRF spectra. The sharp peak at 6.4 keV is associated with Fe in the particle and because of its abundance, multiple transition lines are observed such as that at 7.06 keV (Multiple transitions occur from different orbitals, their effiecies being related to the excitation energy and analyte concentration [10]). In this case, the secondary transition could potentially mask a Co transition line and due to the abundance of Fe, there may also be some absorption/reemission interactions between nearby elements. Nonetheless, low levels of Cr, Mn, Ni and Cu were also observed in the foreign particle (Figure 1a). Such metallic particulates are commonly found in salt and fortunately, do not pose a risk because they do not dissolve in standard manufacturing solutions and are easily captured by filters prior to the process stream.
Figure 1b, compares a brownish/black flake (red line) to the nutrient powder (black line) in which it was found. Several metals were detected but the presence of Pb was particularly of note and then led to a discussion with the vendor and a subsequent investigation into the raw materials manufacturing process. Through this communication, it was determined that the particulate was a small flake from a rotary blade used to mill the powder. Again, the particles were easily removed from the solution via filtration. Because both the origin and composition of the particle were known, an assessment of biotherapeutic manufacturing process impact was readily made.
The absence of strong metal signatures can also be useful to direct an investigation. For example, white flakes floating in solution could result from storage containers or deteriorated rubber/Teflon/silicone components used in manufacturing. From previous experience, if the foreign particle results from a worn component used in manufacturing, it will contain large levels of Ti whereas chips from plastic containers used to store raw materials do not have any metal signatures. Raman spectroscopy, which provides a unique fingerprint for each type of plastic, can then be employed to determine the exact origin of the foreign particle by comparing it to a potential source.
Discolored solutions
In manufacturing processes, a discolored solution is often related to corrosion or leeching from process components, leakage from instruments such as pH meters, or grease. Two case studies will be considered here, involving: (1) blue solutions found in Ar gas lines and (2) a discolored chromatography resin.
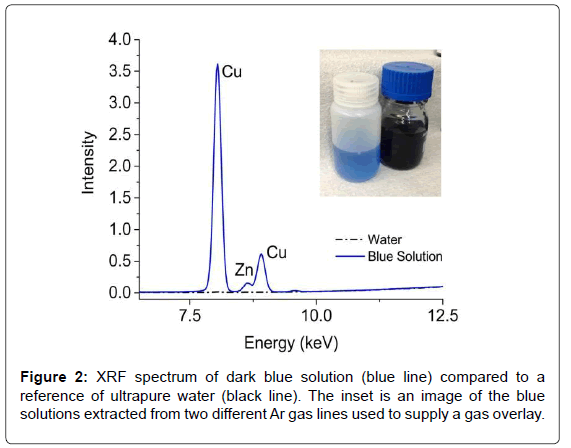
Blue solutions (Figure 2) were observed during routine cleaning of two different Ar gas supply lines. Although the solution did not enter the process, it warranted further investigation. Initial speculations suggested the presence of cibacron blue dye, which is often used with proteins and enzymes. A quick (~1 min) EDXRF measurement of the dark blue solution revealed large concentrations of Cu (~4300 ppm) and Zn (~30 ppm) while the lighter blue solution contained half as much Cu and Zn; neither component is natively found in the dye. Further analysis of the discolored solutions via Raman and NMR also showed no evidence of cibacron blue. However, the NMR analysis revealed the presence of acetic acid, which is used in the washing of purification columns. Since many of the overlay lines use copper piping, the acetic acid likely formed copper(II) acetate (Cu2(CH3CO2-)4(H2O)2), which has an intense blue color [11]. With a straightforward and rapid EDXRF measurement that required little sample preparation, the metal content of the solution was obtained and helped lead to a quick resolution of the investigation.
In a different investigation, the discoloration of resins used in chromatography columns for protein purification was studied. Chromatography resins are typically made of small polymer beads or silica gel. They are often expensive and can take a considerable amount of time and effort to pack into glass or stainless steel columns for use in manufacturing. Hence, resins are cycled (i.e., re-used) for processing multiple batches of drug product and are constantly monitored for process suitability [12]. Understanding the cause of the discoloration and devising prevention methods may reduce leaching of contaminates and prolong the life of the packed chromatography resin.
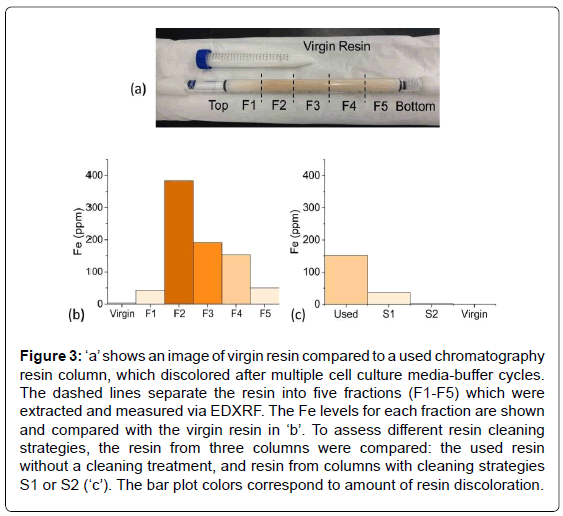
Figure 3a shows a test tube containing virgin resin, which is white in color. Underneath the test tube in this image is a chromatography resin column after multiple cell culture media-buffer cycles, whose discoloration increased with each cycle. Based on the color, the resin material was removed from the column in five fractions and placed into EDXRF sample cups. The resin near the very top of the column appeared slightly discolored; the next fraction had the deepest orange/ yellow discoloration and each subsequent fraction became progressively less discolored. EDXRF of the fractions revealed that Fe correlated with the intensity of the discoloration (Figure 3b).
Figure 3: ‘a’ shows an image of virgin resin compared to a used chromatography resin column, which discolored after multiple cell culture media-buffer cycles. The dashed lines separate the resin into five fractions (F1-F5) which were extracted and measured via EDXRF. The Fe levels for each fraction are shown and compared with the virgin resin in ‘b’. To assess different resin cleaning strategies, the resin from three columns were compared: the used resin without a cleaning treatment, and resin from columns with cleaning strategies S1 or S2 (‘c’). The bar plot colors correspond to amount of resin discoloration.
Once the cause of the discoloration was determined, several cleaning strategies using various chelating agents and wash cycles were assessed. Again, the resin was removed from the columns and measured via EDXRF. Although both cleaning strategies S1 and S2 removed much of the Fe, S2 was significantly better, i.e., near the limit of detection (Figure 3c). Again the EDXRF was very useful toward a rapid resolution of the issue, especially compared to traditional metal analysis techniques which would have required digestion or liquid extraction of the resin beads.
Process development
An alternative use of the EDXRF spectrometer has been to assist with process development. Two very different examples will be given here: one assists in the development of new cell culture media and another looks at cleaning strategies related to pipe rouging.
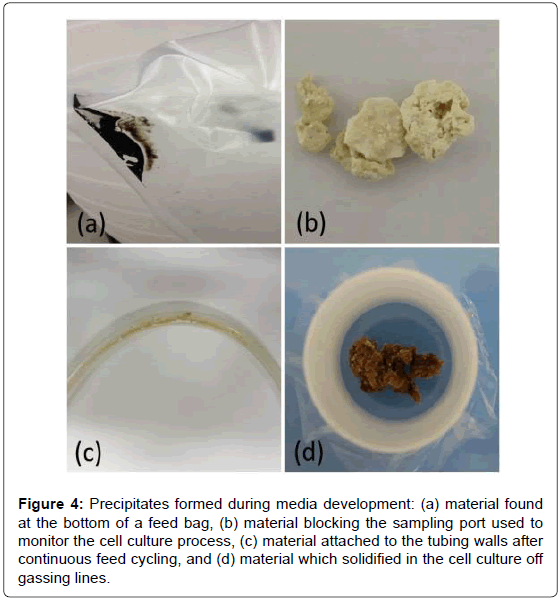
Optimization of cell culture media is crucial to increasing productivity, not only for the cells but also with regards to feeding strategies, whereby more concentrated media translates to fewer feedings [13]. Figure 4 shows images of precipitates encountered during media formulation development. In each case, the EDXRF was used to determine the metal content of the precipitates. The sludge-like material at the bottom of the feed bag in Figure 4a was found to contain large levels of sulfur likely associated with one of the sulfur additives. The rock-like precipitate in Figure 4b, which clogged a sampling port used to monitor the cell culture process, contained large levels of Cl likely associated with some form of salt. EDXRF coupled with NMR measurements of the precipitate formed by cycling feed through the tubing, shown in Figure 4c, contained low levels of metals but a large concentration of one of the amino acids. In contrast, the precipitate shown in Figure 4d was a concentrated form of all the metals contained in the cell culture media. It was found in one of the lines used for off-gassing during the cell culture process. This sample most likely formed due to aerosols entering the off-gas lines, condensing and then solidifying into a pasty material. By assessing the major inorganic and organic components of the precipitates, the cause for nucleation and thus precipitation can often be deduced and corrected in the media formulation, thus improving process understanding.
Figure 4: Precipitates formed during media development: (a) material found at the bottom of a feed bag, (b) material blocking the sampling port used to monitor the cell culture process, (c) material attached to the tubing walls after continuous feed cycling, and (d) material which solidified in the cell culture off gassing lines.
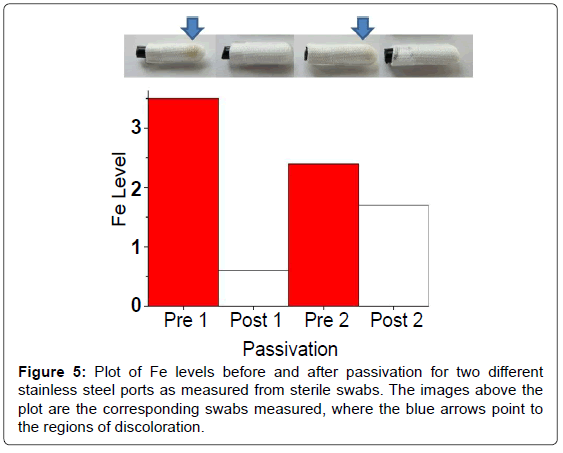
The use of the EDXRF has also assisted with detecting rouging and characterizing pipe passivation procedures. Rouging in biopharmaceutical applications is the formation of reddish-brown residue in stainless steel pipes or tanks, caused by corrosion of the metal surface due to exposure to liquids [14]. The residue is typically high in iron content due to the composition of the stainless steel. Passivation is a technique which coats the stainless steel surface to mitigate corrosion. To help quantify rouging, the EDXRF was used to measure metal levels found on sterile swabs taken from the ports of the pipes before and after passivation. The photographs in Figure 5 are of four swabs collected pre- and post-passivation at two different locations. The impact of passivation on rouging is clearly evident by the color saturation on the swabs sampled before and after passivation. Site 1 is more intense than Site 2, but the impact is apparent at both locations. EDXRF spectra of the swabs were then used to provide a more quantitative analysis of metal content on the swabs. Since iron was the most abundant element, it was selected as the target element to control. The results for pre- and post-passivation of Fe content are shown in Figure 5. Iron content on swabs was reduced upon passivation at both sites although the decrease at Site 2 was less dramatic. This example illustrates how the EDXRF can be used to rapidly detect Fe levels from swabs and hence assist visual inspection when monitoring rouging and ensuring proper passivation.
EDXRF is an effective qualitative tool to quickly assess metal composition, but unlike ICP-MS, which requires internal standards, EDXRF requires external calibration standards to quantify concentrations. Two complications arise for accurate quantitation. First, the samples should fill the entire 10 mm × 7 mm excitation window. Second, the calibration standards should have a similar composition to the samples being measured. That is, materials with different densities, compositions, and structures will influence the absorption, emission and reflection properties of the x-rays. Hence, an accurate concentration is difficult to achieve for small foreign particulates with odd shapes, but relative comparison between swabs tips, for example, is attainable. In short, the EDXRF is well suited as a first-pass diagnostic tool to help focus investigations.
Conclusion
The portable EDXRF spectrometer has proven to be a very powerful diagnostic tool for a variety of different biopharmaceutical forensic applications. For the majority of the case studies presented here, involving particulates, discolored solutions and resins, a 1-5 minute scan was sufficient to determine the composition of metals ranging from sulfur to strontium. Not only are the EDXRF spectra informative, but the spectrometer is also easy to use and transport. Since the EDXRF spectrometer can measure any solid or liquid material, the metal content of small particles, resins, solutions, or even odd shapes such as swabs are easily acquired by placing the sample as-is into an XRF sample cup. The main limitations of the EDXRF compared to other metal analysis methods such as ICP-MS are higher limits of detection (ppm) and the need for reference standards of the same matrix for quantitative concentrations. However, given that a number of issues relate to samples that cannot be digested and contain high metal concentrations, a tool such as EDXRF is ideal. EDXRF allows for rapid, qualitative metal analysis that is highly valuable in resolving biopharmaceutical forensic investigations.
Acknowledgements
Authors would like to acknowledge support from CCD, MS and PBC groups at Biogen in particular Jason Dickens, Brandon Berry, Kyle McElearney, Wenqi Xie, Richard Gilmore III, Orlando Jaquez, and Rob Gronke.
References
- Liritzis I, Zacharias N (2011) X-Ray Fluorescence Spectrometry (XRF) in Geoarchaeology. Springer, New York. pp: 109-142.
- Beckhoff B, Kanngiesser B, Langhoff N, Wedell R, Wolff H (2006) Handbook of Practical X-Ray Fluorescence Analysis. Springer-Verlag, Berlin, Germany.
- Carr R, Zhang C, Moles N, Harder M (2008) Identification and mapping of heavy metal pollution in soils of a sports ground in Galway City, Ireland, using a portable XRF analyser and GIS. Environ Geochem Health 30: 45-52.
- Qiao M, Cai C, Huang Y, Liu Y, Lin A, et al. (2011) Characterization of soil heavy metal contamination and potential health risk in metropolitan region of northern China. Environ Monit Assess 172: 353-365.
- Palmer PT, Jacobs R, Baker PE, Ferguson K, Webber S (2009) Use of field-portable XRF analyzers for rapid screening of toxic elements in FDA-regulated products. J Agric Food Chem 57: 2605-2613.
- Perring L, Andrey D, Basic-Dvorzak M, Blanc J (2005) Rapid multimineral determination in infant cereal matrices using wavelength dispersive X-ray fluorescence. J Agric Food Chem 53: 4696-4700.
- Arzhantsev S, Li X, Kauffman JF (2011) Rapid limit tests for metal impurities in pharmaceutical materials by X-ray fluorescence spectroscopy using wavelet transform filtering. Anal Chem 83: 1061-1068.
- Mondia JP, Goh F, Bryngelson PA, MacPhee JM, Ali AS, et al. (2015) Using X-ray fluorescence to measure inorganics in biopharmaceutical raw materials. Anal Methods 7: 3545-3550.
- Skoog DA, Holler FJ, Crouch SR (2007) Principles of Instrumental Analysis. 6th edn. Thomson Brooks/Cole, Belmont, USA.
- Deslattes R, Kessler Jr. EG, Indelicato P, de Biliy L, Lindroth E, et al. (2005) X-ray Transition Energies. Natl Inst Stand Technol, Gaithersburg.
- Richardson HW (2000) Copper Compounds. In: Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim, Wiley-VCH Verlag GmbH & Co. KGaA, Germany.
- Sofer G, Yourkin J (2007) Cleaning and Cleaning Validation in Process Chromatography. Bioprocess Int.
- McCoy RE, Costa NA, Morris AE (2015) Factors that determine stability of highly concentrated chemically defined production media. Biotechnol Prog 31: 493-502.
- Fleming JR, Kemkes D, DeVoe DW, Crenshaw L, Imbalzano JF (2001) Material of Construction for Pharmaceutical and Biotechnology Processing: Moving into the 21st Century. Pharm Eng 21: 1.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20589
- [From(publication date):
December-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 15868
- PDF downloads : 4721