Research Article Open Access
Application of the Formula for Rational Antimicrobial Therapy (FRAT) to Community-Acquired Pneumonia
JM Blondeau1,2* and N Theriault31Department of Clinical Microbiology, Royal University Hospital and the Saskatoon Health Region, Saskatoon, SK, Canada
2Departments of Microbiology and Immunology, Pathology and Ophthalmology, University of Saskatchewan, Saskatoon, SK, Canada
3Collegeville, Pennsylvania, USA
- *Corresponding Author:
- JM Blondeau
Department of Microbiology, Royal University Hospital
103 Hospital Drive, Saskatoon, Saskatchewan, Canada
Tel: 306-655-6943
Fax: 306-655-6947
E-mail: joseph.blondeau@saskatoonhealthregion.ca
Received date: January 17, 2017; Accepted date: January 30, 2017; Published date: February 01, 2017
Citation: Blondeau JM, Theriault N (2017) Application of the Formula for Rational Antimicrobial Therapy (FRAT) to Community-Acquired Pneumonia. J Infect Dis Ther 5:313. doi:10.4172/2332-0877.1000313
Copyright: © 2017 Blondeau JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Community-acquired pneumonia (CAP) remains a common condition for which patients seek medical advice in the outpatient setting and where antimicrobial agents are prescribed empirically–often based on therapeutic guideline recommendations. Antimicrobial resistance impacts on therapeutic choices as resistance is associated with clinical failure which in turn may impact morbidity and mortality. The Formal for Rational Antimicrobial Therapy (FRAT) considers etiology and antimicrobial susceptibility to generate a factor to predict the likely activity of an antimicrobial agent in CAP or any other infection for which etiology and susceptibility data can be considered. In considering the FRAT formula in CAP, amoxicillin and macrolides offer a predictability of 52.6-78.3% whereas for trimethoprim/sulfamethoxazole the predictability was 45.2% as compared to 90.1% for tetracyclines/doxycycline and 98.2% for levofloxacin (and moxifloxacin) . The FRAT formula clearly differentiates antimicrobial agents based on spectrum of activity and impact of antimicrobial resistance and provides yet another factor for consideration in the selection of an antimicrobial agent for treatment of CAP.
Keywords
Pneumonia; Antimicrobial Resistance; Antibiotics; Empiric; Comorbidity
Introduction
Community acquired pneumonia (CAP) is one of the most common acute infections in North America, responsible for an estimated 5.2 million adult cases in 2014 [1]. Pneumonia and influenza is the eighth leading cause of death in the United States, seventh in Canada [2], accounting for 50,622 deaths in the USA in 2014 [3]. The age-adjusted death rate for pneumonia and influenza increased significantly between 2012-2013 by 10.4% to 15.8 per 100,000 people [4], however this rate has decreased an average of 3.8 percent per year since 1999 [5]. Pneumonia commonly affects the young (<5 years old) and the elderly (65+ years old) , this older population 65+ years has the highest mortality rate from pneumonia (and influenza) ; 85% of pneumonia deaths are in this demographic.
Pneumonia is a huge burden on healthcare systems, 4.2 million outpatient visits were reported in 2006 [6], with about 25% of patients requiring hospitalization [7]. In 2010, more than 1 million hospitalizations were due to pneumonia and in 2014 the hospitalization rate for pneumonia was 36.6 per 10,000; slightly higher in women and highest in those 65 years or older. Hospitalization for pneumonia is associated with increased complications, costs and negative health outcomes; in 2011 alone pneumonia had an aggregate cost of nearly $10.6 billion for 1.1 million hospital stays [8]. A recent literature review by File and Marrie indicated that of hospitalized pneumonia patients 10-20% are admitted to an intensive care unit [9,10], the mean length of hospital (non-ICU?) stay was ≥ 5 days, and the 30 days re-hospitalization rate as high as 20% [6]. In 2013, an estimated $19.9 billion was spent on pneumonia and influenza health care; $16.2 billion on pneumonia and $3.7 billion on influenza. Emergency room visits and medication costs were higher for influenza cases, however, outpatient/office visit expenditures, home health care costs and hospitalization costs were >11 times higher for pneumonia cases [5]. While CAP is most frequent among the very old and the very young, recent data has shown that it is also a costly infection among working-age adults (age 18-64 years) , the mean annual healthcare costs for patients with CAP were $20,961 compared to $3,783 for those without [11]. These costs increased with an individual’s age and higher risk factors. Comorbidities are a critical factor in CAP treatment and health outcomes-diabetes, chronic heart disease, and smoking are frequently occurring comorbidities in CAP patients [12] and among patients hospitalized for CAP, comorbidities are the cause of more than half of readmission cases to hospital [13].
CAP is caused by both typical and atypical pathogens; Streptococcus pneumoniae is the predominant causative bacterial pathogen for CAP and this pathogen has increasing drug resistance to a broad range of antibiotic therapies. Haemophilus influenzae and Moraxella catarrhalis are also common bacterial causative pathogens with the most common atypical pathogens including (#1) Mycoplasma pneumoniae, Legionella spp. and Chlamydophila pneumoniae [14].
Almost all antibiotic treatment for CAP occurs initially and empirically in the primary care setting, this is due to the difficulty in obtaining appropriate specimens to identify pathogens, particularly atypical pathogens that cannot be cultured with regular sputum or blood, and serological tests are infrequently performed. In larger centers and reference laboratories molecular based assays such as polymerase chain reaction (PCR) and other costly laboratory testing is available [6] but results are rarely able to direct initial therapy due to timing. Thus the current IDSA/ATS guidelines are evidenced based recommendations for selecting antibiotics empirically for a variety of patients with pneumonia [15]. The most commonly prescribed antibiotics in the treatment of CAP are macrolides (43.6% of prescriptions) , the fluoroquinolone levofloxacin (36.9% of prescriptions) , beta-lactam compounds (6.5% of prescriptions) and tetracyclines (5.5% of prescriptions) [16]. This reflects the IDSA/ATS Guideline recommendations of initial macrolide therapy, unless a patient has recent antibiotic treatment or comorbidities and then a fluoroquinolone or beta-lactam plus macrolide therapy is the initial treatment recommendation [15]. However, these antibiotics are not equally effective against combating all CAP pathogens and increasing pneumococcal resistance has been found in global and national North American surveillance studies [17,18]. The CDC reports that pneumococcal bacteria are resistant to one or more antibiotics in 3 out of every 10 cases of pneumonia [19]. Moreover the tolerability profiles of each class and drug needs to be factored into any empirical decision-making.
Antibiotic resistance is a growing burden on society, with significant clinical and economic implications. The CDC reported that drug resistant Streptococcus pneumoniae alone is responsible for almost 1,200,000 illnesses, approximately 7,000 deaths, and 19,000 additional hospitalizations each year, resulting in excess costs of $96 million [20]. Penicillin resistant S. pneumoniae and coincidental co or cross resistance to other drug and drug classes remains a concern [21-23]. In a recent study by Classi, et al. [24], the macrolide monotherapy failure rate was more than 1 in 5 adult patients (22.9%) and in elderly patients (≥ 65 years of age) and patients with multiple comorbidities this rate increased to almost 1 in 3. Treatment failure is associated with higher case fatality, longer hospital stays and higher total hospital charges [25]. Zhanel et al. reported from phase 3 clinical trial data that significantly more patients infected with azithromycin susceptible S. pneumoniae and treated with azithromycin had clinical cure (89.4%) compared to those infected with azithromycin resistant S. pneumoniae (68.6%) (p=0.003) [26]. They predicted that an additional 3.1 clinical failures/100 subjects treated with azithromycin would occur due to azithromycin resistance. Most recently, Mandel suggested the increasing pneumococcal resistance to macrolides may diminish the use of these drugs as monotherapy for CAP [27]. IDSA guidelines for empiric treatment of pneumonia suggest selecting a different antibiotic if resistance is >25% [15].
Measuring Resistance
The standard in vitro measurement of antimicrobial susceptibility or resistance is the minimum inhibitory concentration (MIC) . In this measurement, an inoculum of 105 (100,000 organisms) colony forming units per milliliter (CFU/ml) is exposed to varying drug concentrations (usually doubling dilutions, i.e. 0.25, 0.5, 1, 2, 4 μg/ml, etc.) and following incubation under ideal conditions (media, temperature, atmosphere, time, drug potency) , the lowest drug concentration blocking visible growth is recorded as the MIC. The measurement of MIC is, indeed, challenging as it utilizes an inoculum of bacteria that may not represent organism density at the site of infection during acute or chronic infections. If an MIC is determined on an organism density less than the density present during acute infection, then it may under represent that amount of drug necessary to inhibit the growth of all organisms present in the high-density bacterial population. Such high-density bacterial populations have been shown to exist in pneumonia [28] and other respiratory tract infections [29], meningitis [30,31], urinary tract infections [32] and most likely other infections as well.
Concerns over resistance selection from high-density bacterial populations was shown in a landmark study by Dong et al. [33]. In this report, increasing bacterial populations were exposed to increasing drug concentrations of fluoroquinolone antibacterials in vitro. The majority of cells within the population were inhibited by the MIC drug concentration but a subpopulation of cells not inhibited by the MIC drug concentration required a higher drug concentration for inhibition of growth. These subpopulations were found to contain mutations in the gene (s) for fluoroquinolone antimicrobials (de novo resistance) and the drug concentration inhibiting their growth became known as the mutant prevention concentration (MPC) . It is important to point out that this designation is not the mutation prevention concentration as antimicrobials do not induce nor prevent mutations from occurring but rather these drugs may block mutant cells from growing. The MPC measurement is fundamentally the same as an MIC measurement except that instead of a 105 CFU/ml inoculum, a higher density of organisms are tested–on the order or 109-1010 bacterial cells (1 billion-10 billion bacterial cells) . The rational for this density of bacteria relates to the mutation frequency of various Bug-Drug combinations. For many bacteria, spontaneous mutations arise over a frequency of 1 × 10-7 to 1 × 10-9, meaning that for every 107 to 109 bacterial cells in a population, spontaneously occurring mutants may be present even in populations not exposed to antibacterial drugs [34]. As such, an MIC measurement utilizing a bacterial density <107 CFUs will not detect less susceptible or resistant subpopulations that are only detected when high densities of bacterial cells are tested. MPC measurements allow for the determination of drug concentrations necessary to block growth of mutant subpopulations and with the exception of oxazolidinolones, MPC values are invariably higher than MIC values [35].
It needs to be noted that MPC measurements are currently more technically demanding than MIC measurements and such technical issues are an impediment for such testing to be routinely offered in clinical microbiology laboratories [35,36]. A microtiter plate, liquid based assay has and is being investigated by one of the authors (JMB) .
In our opinion, MPC appeared to have answered a number of questions. In a study published by Blondeau et al. where more than 100 clinical strains of Streptococcus pneumoniae were tested by MIC and MPC to various fluoroquinolones, clear differences were seen between the various agents tested (gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin and trovafloxacin) and the drug concentrations necessary to block mutant cell population growth by MPC testing [37]. Levofloxacin was the least active agent (higher MPC values) by these measurements and it was predicted that levofloxacin was more likely to select for resistant subpopulations than the other agents; of the agents tested, only levofloxacin and moxifloxacin remain in clinical practice. In 2002, Davidson et al. [38], reported on the selection of levofloxacin resistant S. pneumoniae in patients infected with this organism and treated with levofloxacin and numerous subsequent reports have identified the same pattern of resistance selection during antimicrobial therapy with macrolides [39-41].
A more recent report has focused on macrolide antibacterial agents and selection of resistance. Data in the literature appeared to suggest that the use of a once daily macrolide agent was associated with resistance selection more than with other macrolide agents. Indeed, Davidson et al. investigated macrolide resistance in Canadian provinces. In their study, they compared 2 time points and investigated azithromycin use versus clarithromycin and erythromycin use. Interestingly, they reported that provinces that had higher azithromycin use, had a higher increase in macrolide resistance over the two time points but the same was not true for provinces that used more clarithromycin/erythromycin. To this point we focused on macrolide resistance using the MPC measurement. In that study, 191 clinical isolates of S. pneumoniae were tested by MPC to azithromycin, clarithromycin and erythromycin. All isolates were susceptible to the macrolide agents based on current CLSI breakpoints at the time of the study. In the study, MPC values were compared to current susceptibility breakpoints and clear differences were seen between the 3 drugs tested and the likelihood for the selection of subpopulations requiring higher drug concentrations for inhibition of growth than the susceptibility breakpoint. Azithromycin was statistically more likely to select for non-susceptible populations than was clarithromycin (p<0.0001) or erythromycin (p<0.0001) and clarithromycin was less likely to select for resistant subpopulations than was erythromycin (p<0.0001) . These studies suggested that azithromycin was the driver of macrolide resistance with S. pneumoniae [42].
Interestingly, Ovetchkine et al. [43] suggested azithromycin should not be used to treat acute pharyngitis, acute otitis media or community-acquired pneumonia in otherwise healthy children except in cases of atypical bacterial causes or in cases of life threatening beta-lactam allergy and acute pharyngitis by macrolide susceptible strains of S. pyogenes. Concerns over breakthrough pneumococcal bacteria in patients treated with azithromycin, occurrence of intravascular pneumococcal infection despite treatment and increasing resistance rates were reasons given to avoid azithromycin use.
Susceptibility programs have monitored changes in susceptibility and resistance data for the past 25 years. In Canada, national surveillance for susceptibility of respiratory pathogens showed that for isolates collected between 2007-2011 [44], 100% of 1881 strains of S. pneumoniae were susceptible to linezolid and vancomycin, 93.7%-99.3% were susceptible to extended spectrum beta-lactam agents (2nd, 3rd generations, cephalosporins and carbapenems) , 99.9% susceptible telithromycin and 99.1% susceptible to fluoroquinolones (moxifloxacin, levofloxacin) . Susceptibility to clarithromycin, penicillin and TMP/SMX were 80.3%, 81.7% and 84.8% respectively.
More recent data on S. pneumoniae (n=2502) isolates collected between 2007-2015 showed that 82.4%, 78.6% and 84.8% of strains were susceptible to penicillin, clarithromycin and TMP/SMX respectively. Susceptibility to 2nd, 3rd generation cephalosporins, carbapenems and fluoroquinolones ranged from 93.5% to 99.8% and were 100% susceptible to linezolid and vancomycin.
For 1038 H. influenzae isolates collected between 2002-2011, susceptibility to ampicillin was 81.2%, 86.8% to clarithromycin and 83.3% to TMP/SMX; susceptibility to amoxicillin/clavulanic acid, extended spectrum cephalosporins and carbapenems, fluoroquinolones and telithromycin ranged from 96.5%-100%.
In the USA, susceptibility data on S. pneumoniae (n=4567) for clinical isolates collected between 2008-2014 [45] showed 57.2% of isolates (oral or meningeal breakpoints) were susceptible to penicillin but this value increased to 92.7% using parenteral non-meningeal breakpoints. Only 51.3% of strains were susceptible to azithromycin, 88.4% to amoxicillin/clavulanic acid, 81-93% to ceftriaxone (meningitis versus non-meningeal breakpoints) , 98% to moxifloxacin, 75% to tetracycline, 69.1% to TMP/SMX and 100% to linezolid and vancomycin. In this same report, the MIC90 value for solithromycin (an investigational compound) was 0.25 μg/ml and no organism had an MIC >1 μg/ml to this compound.
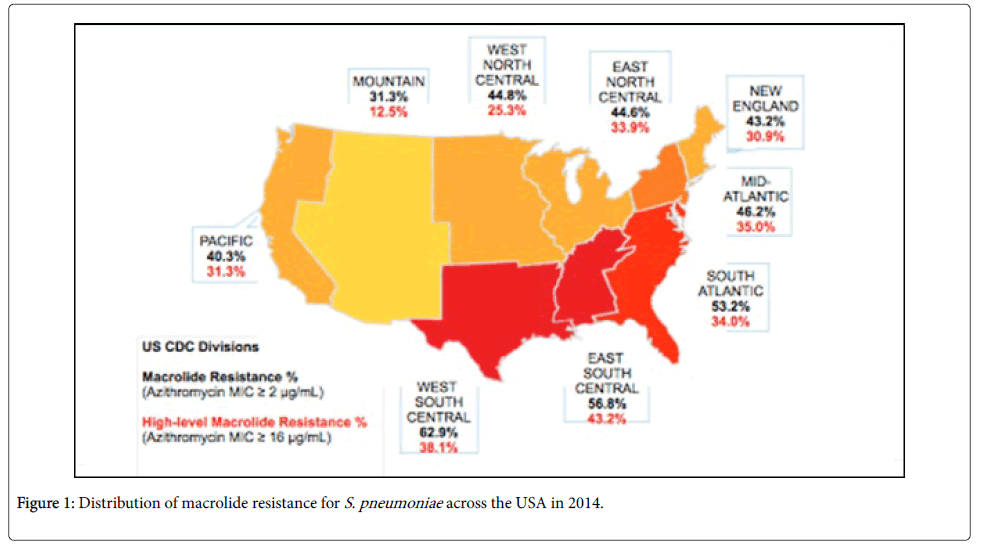
Using the Center for Disease Control (CDC) regional divisions, macrolide resistance was further explored (Figure 1).
Macrolide resistance was defined as an azithromycin MIC of >2 μg/ml and high level resistance was defined based on an azithromycin MIC >16 μg/ml. Macrolide resistance was highest in the West South Central region at 69.9% followed by East South Central (56.8%) and South Atlantic (53.2%) and the lowest rate of resistance was in the mountain region at 31.3%. The region with the highest percentage of strains (25%) that were both macrolide and penicillin resistant was East South Central with the remaining regions having penicillin/ macrolide resistance rates between 6-15.4%. High level macrolide resistance ranged from 12.5% to 43.2% over all the regions and was highest in East South Central (43.2%) , West South Central (38.1%) and Mid Atlantic (35%) . Finally, nationally, macrolide resistant S. pneumoniae has increased from 39.7% in 2008 to 48.4% in 2014.
Primary care practitioners are often unaware of national and local antibiotic resistance patterns and the common causative organisms, which can result in inappropriate and ineffective prescriptions [46]. Therefore, prescribing an initial antibiotic which is active against pneumococcal organisms and other pathogens and considers local and regional resistance would be preferable. A tool which highlights the etiology of pneumonia and the relevant susceptibly rates would enable more targeted empiric prescribing within the community setting. We describe the application and potential benefits of such a tool to CAP.
Methods
Susceptibility rates for the relevant pathogens for the USA and Canada were taken from various data sources [44,45,47-53] and data from two current global CAP studies [54,55] were integrated to create aggregated etiology patterns. The relevant pathogens included S. pneumoniae, H. influenzae, M. pneumoniae, C. pneumoniae (previous Chlamydia pneumoniae) Legionella pneumoniae, Klebsiella pneumoniae, and methicillin-susceptible Staphylococcus aureus. As pointed out earlier certain co-morbidities have been shown to adversely impact outcomes in CAP and include diabetes, smoking and heart disease. The CDC reported regional prevalence rates of these comorbidities were overlaid with USA regional pneumonia pathogen susceptibility patterns. Additionally, the rate of antibiotic prescriptions and macrolide prescriptions per 1,000 people, by state/CDC census regions, were also cross-referenced with the susceptibility patterns [56].
The formula to help select rational antimicrobial therapy (FRAT) was published by Blondeau and Tillotson in 1999 [57]. In fact, the original title intended for this formula was Formula for Empiric Antimicrobial Therapy (FEAT) but that title was rejected by the reviewers because of the use of the word “empiric”. In practice, most antimicrobials are prescribed empirically, often based on evidencebased guidelines and without culture and susceptibility data on potential infecting organisms.
The FRAT (or FEAT) formula uses two data items to develop a factor (F) which depends on the incidence (I) of specific bacterial pathogens and the percent susceptible (S) of those organisms to the antimicrobial agent or agents being considered. As such, the formula FS=%I × %S/100+%I × %S/100+%I × %S/100+ etc. where I is the % incidence of the pathogens in a given infection, S is the % susceptible to the antimicrobial agent and F is the sum of F derived for each bacterial species [57]. Table 1 is a summary of the FRAT (FEAT) formula applied to community-acquired pneumonia and select antimicrobials used for treatment.
| Organism | % Incidencea | Amoxicillin | Ceftriaxone | Macrolide | Levofloxacin | Tetracycline | TMP/SMX* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % S | F | % S | F | % S | F | % S | F | % S | F | % | F | ||
| S. pneumoniae | 20.5 | 88.4 | 18 | 80.8 | 17 | 50 | 10 | 99 | 20 | 74 | 15.1 | 69.1 | 14.2 |
| H. influenzae | 10 | 74.5 | 7.5 | 99 | 9.9 | 94 | 9 | 100 | 10 | 99 | 9.9 | 70.7 | 7.1 |
| M. catarrhalis | 3.4 | 100 | 3.4 | 99 | 3.3 | 99 | 3 | 100 | 3.4 | 100 | 3.4 | 99 | 3.2 |
| M. pneumoniae | 8.6 | 0 | 0 | 0 | 0 | 99 | 9 | 99 | 8.5 | 99 | 8.5 | 0 | 0 |
| C. pneumoniae | 6.1 | 0 | 0 | 0 | 0 | 99 | 6 | 99 | 6 | 99 | 6 | 0 | 0 |
| L. pneumophila | 9.3 | 0 | 0 | 0 | 0 | 99 | 9 | 99 | 9.2 | 99 | 9.2 | 0 | 0 |
| K. pneumoniae | 1.4 | 98.4 | 1.4 | 99 | 1.3 | 99 | 0 | 99 | 1.3 | 83 | 1.16 | 95 | 1.3 |
| S. aureus** | 2 | 98 | 2 | 99 | 1.9 | 94 | 2 | 94 | 1.8 | 98 | 1.96 | 93 | 1.86 |
| TOTAL | 61.3 | 32.3 | 33.4 | 48 | 60.2 | 55.2 | 27.7 | ||||||
| PREDICTABILITY | 52.6 | 54.5 | 78.3 | 98.2 | 90.1 | 45.2 | |||||||
| *trimethoprim/sulfamethoxazole **methicillin-susceptible aDoes not add to 100% as respiratory viruses not considered and for some patients, a pathogen is not identified. |
|||||||||||||
Table 1: Example of FRAT (FEAT) applied to community-acquired pneumonia [54,55,57-63].
Results
Regional resistance rates in the USA
Overall, regional differences were seen in levels of antibiotic resistance throughout the USA and Canada, however, for the most common causative pathogen, S. pneumoniae resistance to macrolides ranged from 31.3% to 62.9%. Penicillin and macrolide resistance ranged from 6-25%.
FRAT application
For the FRAT Table example summarized in Table 1, a comparison is shown for 6 antimicrobial agents representing five different drug classes. The total pathogen incident added to 61.3% and will not add to 100% as viruses are not considered in the calculation nor were organisms whose occurrence is infrequent. Clearly, such bacteria at lower incidences could be added and included in the calculation if desired. In considering amoxicillin, resistance with S. pneumoniae was approximately 12% and resistance to influenzae was approximately 25% and this combined with a lack of activity against atypical pathogens yields an overall factor of 32.2%; when considered with the incidence of the pathogens summarized gives an overall predictability factor of 52.6%. Similarly for ceftriaxone, susceptibility of S. pneumoniae was approximately 81% and lack of activity against atypical pathogens provided a factor of 33 and the predictability was 54.5%. Despite a high incidence of macrolide resistance at 50% for S. pneumoniae, the overall impact factor was 38.1 with a predictability of 78.3% and this in part is attributed to the activity against atypical pathogens summarized in the Table. A low predictability for trimethoprim/sulfamethoxazole was also seen at 45.2% and again was accounted for by resistance amongst Streptococcus pneumoniae and Haemophilus influenzae strains and lack of activity against atypical pathogens. For the fluoroquinolone levofloxacin, a factor of 60.5 was seen giving a predictability of 98.2% and again owing for the lack of resistance amongst S. pneumoniae and H. influenzae strains and activity against atypical pathogens. A similar factor could be seen for the fluoroquinolone moxifloxacin.
The varying macrolide resistance rates seen throughout the various CDC geographical divisions clearly demonstrate how the predictability factor could change. For West South Central with macrolide resistance at 62.9%, the predictability would be 74.2% whereas for Mountains (31.3% of macrolide resistance) the predictability would be 84.7%. Interestingly, these differences on predictability are based on varying susceptibility of S. pneumoniae; clearly varying susceptibility to other pathogens would also influence the predictability of the antimicrobial agent.
It should be clear that if different incidence data was used such that the percentages of the various pathogens summarized was higher or lower then this along with varying susceptibility or resistance would clearly change the calculation of the factor which when divided by the percent incidence would change the overall predictability for any or all of the antimicrobial agents. In fact, the formula is best used when applied to the most recent and local susceptibility or resistance and etiology data that may be available for analysis.
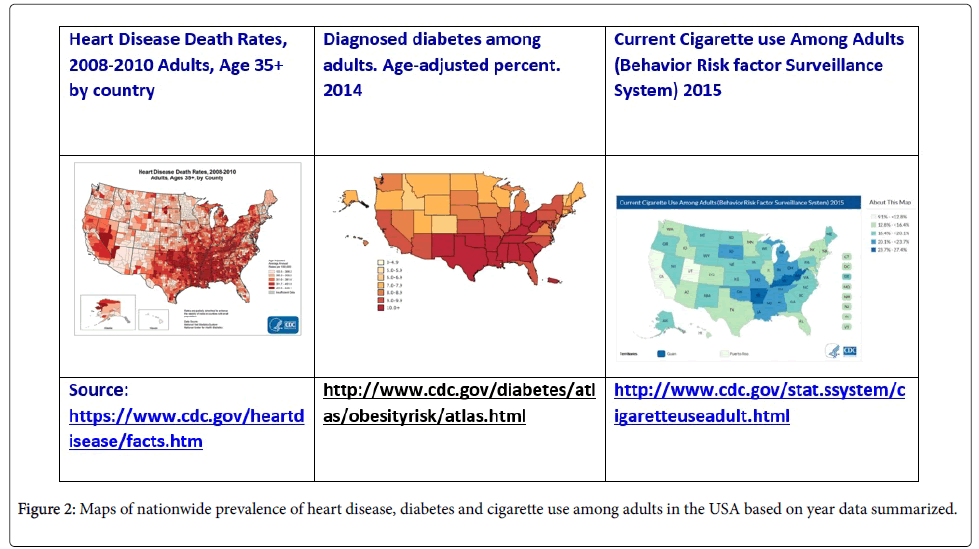
In addition to different susceptibility rates identified in the 9 CDC Census regions, demonstrated differences are also seen in prevalence of chronic heart disease, diabetes and smoking. Comparing all data from these regions identifies a trifecta; high prevalence of comorbidities, high rates of pneumococcal resistance and macrolide failure, and high prescription rates-particularly of macrolides, suggesting that there are areas where more effective prescribing is needed. This trifecta was demonstrated with the highest percentages in the East South Central region; diagnosed diabetes age-adjusted percent ranged from 9->10%, prevalence of heart disease among adults ranged from 6.4%-8.2%, of adults 20.1-27.4% smoke cigarettes and S. pneumoniae macrolide resistance was 56.8% [45,58].
Regional comorbidities
Interestingly, West Virginia state has higher prevalence rates of smoking and heart disease than the majority of its surrounding states. Regions with the highest community antimicrobial prescribing rates are also the same regions with the highest rates of antibiotic resistance.
Figure 2 shows the prevalence of heart disease, diabetes and cigarette use among adults in the USA in 2014. The South Central region was identified as the region with the highest antibiotic prescribing rate across all age groups [59], and relevant to our comparisons, counties with a high proportion of obese persons were associated with increased prescribing rates. Overall, Southern regions had the highest prevalence of heart disease and diabetes, however the East South Central region had the highest prevalence of all three comorbidities-heart disease, diabetes, smoking, as well as the highest macrolide resistance rate and macrolide prescription rate. The Mountain region had antibiotic prescription rates below the national average (502-778 per 1,000 population) , lowest macrolide resistance rate (31.3%) and the lowest prevalence of diabetes except for the two most southern states in this region Arizona and New Mexico.
Discussion
Concern over antimicrobial resistance and therapeutic failure have been well documented and debated in the peer reviewed literature. Antimicrobial resistance may be associated with failure to eradicate the bacterial pathogens, additional days of antimicrobial therapy, change in antimicrobial therapy, clinical deterioration and death. Davidson et al. reported on the emergence of levofloxacin resistant S. pneumoniae in patients with pneumonia and treated with levofloxacin [38]. Lonks et al. reported breakthrough bacteremia during macrolide (erythromycin) or azalide (azithromycin) therapy is more likely to occur among patients infected with erythromycin-resistant pneumococcus and macrolide resistance was clinically relevant [39].
Coenen and Goossens [60] commented on antibiotic treatment failure in primary care based on a study by Currie and colleagues [61] whereby 11 million antibiotic monotherapies for upper and lower respiratory tract infections, skin and soft tissue infections and acute otitis media over the years of 1991-2012. The data was extracted from the United Kingdom primary care data base. The results suggest that >1/10 monotherapies were associated with failure and that failure rates had increased (particularly in lower respiratory tract infections) with most of the increases in failure occurring in more recent years. Regarding increased mortality and antimicrobial resistance, Cosgrove summarized U.S. data showing an increase in mortality associated with the emergence of resistance during hospitalization and treatment [62]. de Kraker et al. reported on increased fatal outcomes more frequently with MRSA blood stream infections versus MSSA blood stream infections [63]. As such the clinical relevance of drug resistant bacteria and treatment with an antimicrobial to which the infecting pathogen is resistant to (or resistance develops during therapy) has been clearly established.
The continuing trend of increasing antimicrobial resistance among CAP pathogens, particularly S. pneumoniae to commonly used macrolides and β-lactam agents if of great concern [64]. Findings from recent surveillance studies [54,55,65] support the observations from PROTEKT and SENTRY surveillance studies; S. pneumoniae and Mycoplasma resistance to antimicrobial agents overall is changing and differs by region. Macrolide resistance rates are higher in the Southeast, North Central and South Central Regions, and a retrospective cohort study identified residing in the southern United States as a risk factor for macrolide-nonsusceptible pneumococci [66]. The identification of a positive relationship between macrolide-resistant S. pneumoniae and macrolide treatment failure in patients with CABP [24] demonstrates the current impact of resistance and continued prescribing of ineffective treatments. Variables not considered include quality of life indicators and lost productivity due to absenteeism from work. Such variables have been discussed elsewhere [67-69].
Cillonez et al. showed that mortality increased with age in patients with community-acquired pneumonia [12]. Torres et al. commented on higher mortality rates in elderly patients with CAP and also stated that lifestyle factors are associated with an increased risk of CAP including smoking, alcohol abuse and other conditions such as diabetes mellitus, liver and renal disease, cardiovascular and cerebrovascular disease and other conditions including immunocompromised patients [70].
The existing challenge of appropriate and effective antibiotic treatment for CAP patients, in light of regional differences in causative pathogens and resistance rates, is further compounded by the presence of comorbidities in patients. Initial empiric management of CAP in regions where comorbidity prevalence is highest is critically important as these patients are already at higher risk for CAP [71], as well as the severity and outcome of the episode [65]. Research has shown that patients with chronic disease comorbidities, such as diabetes or who smoke, are more likely to receive inappropriate therapy, have longer hospital stays, are more likely to be re-admitted in 30 days [25], and are at increased risk of death due to CAP over both short (30 days) and long term (1 year) time frames [72]. Recommendations suggest such patients should be given initial therapy of fluoroquinolones or a combination of beta-lactam+macrolide agents [5]. However, if one adds the impact of antimicrobial resistance to this complex calculation it is likely that initial empiric prescribing can be a challenge.
The East South Central region of the USA has the highest prevalence of S. pneumoniae antimicrobial resistance, as well as heart disease, diabetes and smoking. Interestingly, this region also has the highest rates of antibiotic prescribing in the country [59].
It has been suggested that US physicians consistently underestimate the local and national levels of resistance; macrolide resistance was estimated at 21% rather than the accurate 31-64% [54,55], and perhaps not surprisingly, the states with the highest macrolide resistance also dispensed the most macrolide antibiotic prescriptions in 2014 (240 per year vs the national 154) [73].
As the aging population of the USA increases, and incidence of chronic diseases increases, a larger at risk population for CAP emerges [6] which could contribute to creating the perfect storm of antibiotic resistance in pneumonia and the potential for inappropriate initial empiric therapy.
The percentage of the USA population with diabetes varies by state, however, in ages 65-74 years (higher CAP risk group) South Central, South East and East Central regions had the highest prevalence ranging from 23.5%-37.1% in 2014 (CDC USA Diabetes Surveillance System, 2016) . Diabetes is considered a predisposing condition to CAP and up to 25% of CAP patients have diabetes [74,75]. Overall, diabetes is associated with increased risk of hospitalization, requires longer hospital stays, higher mortality among hospitalized patients [76] and re-hospitalization within 30 days of hospital discharge [65]. Diabetes was significantly associated with development of pleural effusion and mortality in a study by Falguera et al. [77], and was also among 12 comorbidities associated with increased risk for hospitalization within 28 days after an outpatient CAP episode [78]. There is a high burden of diabetes patients hospitalized for CAP. Studies have found prevalence of pre-existing diabetes ranging from 16.2% to 25.9% and the burden of diabetes in episodes of CAP is increasing [76].
Diabetes contributes more than just adverse health outcomes. Patients with diabetes have been found to have different clinical features of CAP compared to other patients [79], are at risk for CAP not just during winter months, but throughout the year [65], and patients with both diabetes and CAP also have a higher frequency of other conditions such as COPD [76], adding complexity to diagnosis and effective antimicrobial therapy.
Interestingly, Martins et al. [76] found that diabetes had a more adverse impact upon younger people (under 20-39 years) and in women with CAP, this impact on younger adults was also found in a study by Kornum et al. [80]. This data highlights the potential impact of increasing diabetes prevalence and S. pneumoniae resistance to antimicrobials in not only older patients, but also in younger people as well suggesting physicians should be more cognizant of the association of diabetes and CAP across varying age groups.
Heart disease is more common in middle age and elderly individuals and is the leading cause of death in the USA, responsible for approximately 610,000 deaths annually [81]. Pneumonia can trigger new cardiac events and contributes to acute exacerbations of pre-existing heart conditions [82,83]. It is estimated that half of the elderly hospitalized CAP patients have concurrent heart disease [84], this dual condition significantly increases mortality due to pneumonia [83]. However, there is a tendency among physicians to seek a unifying diagnosis [83], but CAP and cardiac diseases are mutually aggravating conditions [85]. Smoking and diabetes are risk factors for heart disease [86].
Smoking is associated with negative outcomes in CAP patients, as well as increasing a patient’s risk of developing additional complicating comorbidities. The risk for pneumococcal pneumonia is significantly higher among adults who smoke compared to non-smokers or those not currently smoking [87]. Smoking has been shown to increase the risk of heart disease as well as increase the likelihood to develop type 2 diabetes by 30%-40% and also reduces the ability to control diabetes and insulin dosing [88].
In individuals 65 years or older, Jackson et al. [89] calculated the proportion of cases to particular risk factors, reporting 2.4% of CAP cases are due to current smoking, but this increases to 5.5% of cases in patients with no cardiopulmonary disease. In a study by Millet et al. investigating factors associated with hospitalization within 28 days of CAP diagnosis (a‘post-CAP’ hospitalization) , smokers had nearly three times the odds of hospitalization than non-smoker, and after adjustments for comorbidities, 96% higher odds of hospitalization than non-smokers [78]. Smoking is an independent risk factor for mortality associated with CAP; in a study of patients hospitalized with CAP, current smokers had a fivefold increased risk of 30-day mortality from pneumococcal CAP compared to non-smokers and ex-smokers [72].
Use of a tool such as FRAT/FEAT would be not only beneficial for guiding empiric prescribing for general patients, but in regions where both resistance and comorbidities are high, it could be particularly important and potentially increase the probability that an antibacterial agent active against the infecting pathogens could be empirically chosen.
Continued monitoring and new surveillance efforts are integral to collective accurate local and regional resistance and susceptibility trends to aid clinicians in selecting the best drug treatment for empiric therapy, as well as assist in judicious antibiotic use [90].
Empirical prescribing by definition has to be based on broader information and then patient specific data. General practitioners (GP) need to know local resistance and susceptibility trends. Physicians appear to be aware of antimicrobial resistance and with its clinical importance but may underappreciate its prevalence nationally or locally [91]. If for example, knowledge on macrolide resistance locally is underappreciated the potential consequences of choosing a drug where resistance is high and susceptibility low may lead to more failures. The FRAT tool can help prescribers have a better understanding of the disease and which drugs are most likely to be effective, enabling practical application of regional data to assist in a greater understanding of local resistance and susceptibility trends and better predictability with empiric prescribing.
However, antibiotics carry a cost in terms of adverse events, these vary by class and by patient type, which includes age, and comorbidities. In order to account for these critical underlying factors, the busy practitioner could benefit from methods which make the prescribing process less complex. Regarding the distribution of the various factors in the USA, Figures 1 and 2 illustrate the general incidence of resistance, diabetes, smoking and heart disease.
While antimicrobial resistance is global in distribution and nationwide, the Southern region of the USA is of most concern as resistance of pathogens and susceptibility to antimicrobials is layered on the high prevalence of comorbidities. Using Louisiana as an example; 1117 prescriptions dispensed vs the national 835 rate, 240 per 1000 population macrolide prescriptions were dispensed-for comparison Oregon’s rate was 90 per 1000 population [73] but S. pneumoniae macrolide resistance is 56.8% [45]. Considering comorbidities, the heart disease death rate and diabetes prevalence rate are highest ranging from 452.0-846.1 per 100,000 adults and 23.5%-37.1% respectively, and the smoking rate among adults is 20.1- <23.7%. Our thinking is that the FRAT formula calculation along with therapeutic guideline recommendations used together come closer to ensuring adequate empiric antimicrobial prescribing.
Conclusion
The FRAT tool has been developed to support empiric antibiotic prescribing in the Primary Care setting to integrate regional and local susceptibility data against the key bacterial pathogens. Its simplicity allows for easy calculation once incident data and susceptibility data is available. Indeed, the FRAT formula can be readily applied to susceptibility data generated in hospital or community laboratories, state or provincial laboratories or from regional and national surveillance studies. In fact, the only limitation to use of this formula is access to recent and meaningful susceptibility data for the target pathogens of interest. The FRAT formula, as a tool, is one more approach along with therapeutic guidelines, to help select appropriate antimicrobial therapy for infected patients.
Acknowledgement
We thank Glenn Tillotson for ongoing discussions regarding the FRAT formula and for sections of this manuscript. We also thank Deb Hills for excellent clerical assistance. This manuscript was supported in part by an unrestricted educational grant from Cempra Pharmaceuticals.
References
- Rozenbaum MH, Mangen MJ, Huijts SM, van der Werf TS, Postma MJ (2015) Incidence, direct costs and duration of hospitalization of patients hospitalized with community acquired pneumonia: A nationwide retrospective claims database analysis. Vaccine 33:3193-3199.
- http://www.statcan.gc.ca/daily-quotidien/081204/dq081204c-eng.htm
- Kochanek KD, Murphy SL, Xu J,Tejada-Vera B (2016) Deaths: Final Data for 2014. Natl Vital Stat Rep 65:1-122.
- Xu J, Murphy SL, Kochanek KD, Bastian BA (2016) Deaths: Final Data for 2013. Natl Vital Stat Rep 64:1-119.
- American Lung Association (2015) Trends in pneumonia and influenza morbidity and mortality.
- File TMJr, Marrie TJ (2010) Burden of community-acquired pneumonia in North American adults. Postgrad Med 122:130-141.
- Brar NK, Niederman MS (2011) Management of community-acquired pneumonia: a review and update. TherAdvRespir Dis 5:61-78.
- http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/infectious-disease/community-acquired-pneumonia/
- Marrie TJ, Shariatzadeh MR (2007) Community-acquired pneumonia requiring admission to an intensive care unit: a descriptive study. Medicine (Baltimore) 86:103-111.
- Restrepo MI, Mortensen EM, Velez JA, Frei C, Anzueto A (2008) A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest 133:610-617.
- Broulette J, Yu H, Pyenson B, Iwasaki K, Sato R (2013) The incidence rate and economic burden of community-acquired pneumonia in a working-age population. Am Health Drug Benefits 6:494-503.
- Cilloniz C, Polverino E, Ewig S, Aliberti S, Gabarrus A, et al. (2013) Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 144:999-1007.
- Prina E, Ranzani OT, Torres A (2015) Community-acquired pneumonia. Lancet 386:1097-1108.
- Ramirez JA, Anzueto AR (2011) Changing needs of community-acquired pneumonia. J AntimicrobChemother 66 Suppl 3:iii3-iii9.
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, et al. (2007) Infectious Disease Society of America/American Thoracic Society Consensus Guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44:S27-S72.
- Landsman-Blumberg P, Carroll C, Murty S, Slaff S, Classi P, et al. (2016) Meeting Abstracts - Academy of Managed Care Pharmacy Nexus 2016. J Managed Care and Specialty Pharmacy 22:S63.
- Hoban D, Baquero F, Reed V,Felmingham D (2005) Demographic analysis of antimicrobial resistance among Streptococcus pneumoniae: worldwide results from PROTEKT 1999-2000. Int J Infect Dis 9:262-273.
- Jenkins SG, Brown SD, Farrell DJ (2008) Trends in antimicrobial resistance among Streptococcus pneumoniae isolated in the USA: update from PROTEKT US years 1-4. Ann ClinMicrobiolAntimicrob 7:1-11.
- https://www.cdc.gov/pneumococcal/about/diagnosis-treatment.html
- http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf#page=79
- Blondeau JM (1999) A review of the comparative in vitro activity of 12 antimicrobial agents with a focus on 4 new "respiratory quinolones". J AntimicrobChemother 43:1-11.
- Reynolds CA, Finkelstein JA, Ray GT, Moore MR, Huang SS (2014) Attributable healthcare utilization and cost of pneumonia due to drug-resistant streptococcus pneumonia: a cost analysis. Antimicrob Resist Infect Control 3:16.
- Pfaller MA, Farrell DJ, Sader HS, Jones RN (2012) AWARE Ceftaroline Surveillance Program (2008–2010) : Trends in Resistance Patterns Among Streptococcus pneumoniae, Haemophilusinfluenzae, and Moraxella catarrhalis in the United States. Clin Infect Dis 55:S187-S193.
- Classi P, Landsman-Blumberg P, Carroll C, Murty S, Slaff S, et al. (2016) The relationship between macrolide-resistant Streptococcus pnuemoniae and treatment failure in adults with community-acquired bacterial pneumonia by CDC Region in the USA. In: Academy of Managed Care Pharmacy (AMCP) Nexus Meeting. National Harbor, Maryland 2016.
- Micek ST, Lang A, Fuller BM, Hampton NB, Kollef MH (2014) Clinical implications for patients treated inappropriately for community-acquired pneumonia in the emergency department. BMC Infect Dis 14:61.
- Zhanel GG, Wolter KD, Calciu C, Hogan P, Low DE, et al. (2014) Clinical cure rates in subjects treated with azithromycin for community-acquired respiratory tract infections caused by azithromycin-susceptible or azithromycin-resistant Streptococcus pneumoniae: analysis of Phase 3 clinical trial data. J AntimicrobChemother 69:2835-2840.
- Mandell LA (2016) Something New for Community-Acquired Pneumonia? Clin Infect Dis 63:1681-1682.
- Frisch AW, Tripp JT, Barrett Jr CD, Pidgeon BE (1942) The specific polysaccharide content of pneumonic lungs. J Exp Med 76:505-510.
- Fagon J, Chastre J, Trouillet JL, Domart Y, Dombret MC, et al. (1990) Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Use of the protected specimen brush technique in 54 mechanically ventilated patients. Am Rev Respir Dis 142:1004-1008.
- Bingen E, Lambert-Zechovsky N, Mariani-Kurkdjian P, Doit C, Aujard Y, et al. (1990) Bacterial counts in cerebrospinal fluid of children with meningitis. Eur J ClinMicrobiol Infect Dis 9:278-281.
- Feldman W (1976) Concentrations of bacteria in cerebrospinal fluid of patients with bacterial meningitis. J Pediatr 88:549-552.
- Coulthard MG, Kalra M, Lambert HJ, Nelson A, Smith T, et al. (2010) Redefining urinary tract infections by bacterial colony counts. Pediatrics 125:335-341.
- Dong Y, Zhao X, Domagala J,Drlica K (1999) Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob Agents Chemother 43:1756-1758.
- Blondeau JM, Hansen GT, Metzler KL, Hedlin P (2004) The role of PK/PD parameters to avoid selection and increase of resistance: mutant prevention concentration. J Chemother 16:1-19.
- Hesje C, Tillotson GS and Blondeau JM (2007) MICs, MPCs and PK/PDs: A match (sometimes) made in hosts. Exp Rev Resp Med 1:7-16.
- Blondeau JM (2009) New concepts in antimicrobial susceptibility testing: the mutant prevention concentration and mutant selection window approach. Vet Dermatol 20:383-396.
- Blondeau JM, Zhao X, Hansen GT, Drlica K (2001) Mutant prevention concentrations (MPC) of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 45:433-438.
- Davidson RJ, Cavalcanti R, Brunton JL, Bast DJ, De Azavedo J, et al. (2002) Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med 346:747-750.
- Lonks JR, Garau J, Gomez L, Xercavins M, Ochoa de Echaguen A, et al. (2002) Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin Infect Dis 35:556-564.
- Kelley MA, Weber DJ, Gilligan P, Cohen MS (2000) Breakthrough pneumococcal bacteremia in patients being treated with azithromycin and clarithromycin. Clin Infect Dis 31:1008-1011.
- Musher DM, Dowell ME, Shortridge VD, Jorgensen JH,Magueres PL, et al. (2002) Emergence of macrolide resistance during treatment of pneumococcal pneumonia. N Engl J Med 346:630-631.
- Metzler KL, Drlica K,Blondeau JM (2013) Minimal inhibitory and mutant prevention concentrations for azithromycin, clarithromycin and erythromycin with clinical isolates of Streptococcus pneumoniae. J AntimicrobChemother 68:631-635.
- Ovetchkine P, Rieder MJ (2013) Azithromycin use in paediatrics: A practical overview. Peadiatr Child Health 18:311-316.
- Zhanel GG, Adam HJ, Baxter MR, Fuller J, Nichol KA, et al. (2013) Antimicrobial susceptibility of 22746 pathogens from Canadian hospitals: results of the CANWARD 2007-11 study. J AntimicrobChemother 68 Suppl 1:i7-22.
- Keedy K, Li J, Nenninger A, Sheets A, Fernandes P, et al. (2016) Antibiotic susceptibility of Streptococcus pneumoniae in the US in 2014. In: ID Week. New Orleans 2016.
- Sanchez GV, Roberts RM, Albert AP, Johnson DD, Hicks LA (2014) Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerg Infect Dis 20:2041-2047.
- Bandet T, Whitehead S, Blondel-Hill E, Wagner K, Cheeptham N (2014) Susceptibility of clinical Moraxella catarrhalis isolates in British Columbia to six empirically prescribed antibiotic agents. Can J Infect Dis Med Microbiol 25:155-158.
- Tomic V, Dowzicky MJ (2014) Regional and global antimicrobial susceptibility among isolates of Streptococcus pneumoniae and Haemophilusinfluenzae collected as part of the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.) from 2009 to 2012 and comparison with previous years of T.E.S.T. (2004-2008) . Ann ClinMicrobiolAntimicrob 13:52.
- Paukner S, Sader HS, Ivezic-Schoenfeld Z, Jones RN (2013) Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother 57:4489-4495.
- Taylor-Robinson D, Bebear C (1997) Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J AntimicrobChemother 40:622-630.
- Jacobs MR, Bajaksouzian S, Windau A, Good CE, Lin G, et al. (2004) Susceptibility of Streptococcus pneumoniae, Haemophilusinfluenzae, and Moraxella catarrhalis to 17 oral antimicrobial agents based on pharmacodynamic parameters: 1998-2001 U S Surveillance Study. Clin Lab Med 24:503-530.
- Sanchez GV, Master RN, Clark RB, Fyyaz M, Duvvuri P, et al. (2013) Klebsiellapneumoniae antimicrobial drug resistance, United States, 1998-2010. Emerg Infect Dis 19:133-136.
- Critchley IA, Jones ME, Heinze PD, Hubbard D, Engler HD, et al. (2002) In vitro activity of levofloxacin against contemporary clinical isolates of Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumoniae from North America and Europe. ClinMicrobiol Infect 8:214-221.
- File TM Jr, Rewerska B, Vucinic-Mihailovic V, Gonong JR, Das AF, et al. (2016) SOLITAIRE-IV: A Randomized, Double-Blind, Multicenter Study Comparing the Efficacy and Safety of Intravenous-to-Oral Solithromycin to Intravenous-to-Oral Moxifloxacin for Treatment of Community-Acquired Bacterial Pneumonia. Clin Infect Dis 63:1007-1016.
- Barrera CM, Mykietiuk A, Metev H, Nitu MF, Karimjee N, et al. (2016) Efficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: a global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial (SOLITAIRE-ORAL) . Lancet Infect Dis 16:421-430.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, et al. (2016) Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA 315:1864-1873.
- Blondeau JM, Tillotson GS (1999) Formula to help select rational antimicrobial therapy (FRAT) : its application to community and hospital acquired urinary tract infections. Int J Antimicrob Agents 12:145-150.
- https://gis.cdc.gov/grasp/PSA/AUMapView.html
- Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, et al. (2015) US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 60:1308-1316.
- Coenen S, Goossens H (2014) Antibiotic treatment failure in primary care. BMJ 349:g5970.
- Currie CJ, Berni E, Jenkins-Jones S, Poole CD, Ouwens M, et al. (2014) Antibiotic treatment failure in four common infections in UK primary care 1991-2012: longitudinal analysis. BMJ 349:g5493.
- Cosgrove SE (2006) The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 42 Suppl 2:S82-S89.
- de Kraker ME, Wolkewitz M, Davey PG, Koller W, Berger J, et al. (2011) Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 55:1598-1605.
- Jones RN, Farrell DJ, Mendes RE, Sader HS (2011) Comparative ceftaroline activity tested against pathogens associated with community-acquired pneumonia: results from an international surveillance study. J AntimicrobChemother 66:iii69-iii80.
- Torres A, Blasi F, Dartois N, Akova M (2015) Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 70:984-989.
- Neuman MI, Kelley M, Harper MB, File TM Jr, Camargo CA Jr(2007) Factors associated with antimicrobial resistance and mortality in pneumococcal bacteremia. J Emerg Med 32:349-357.
- Linder JA, Singer DE (2003) Health-related quality of life of adults with upper respiratory tract infections. J Gen Intern Med 18:802-807.
- Dalager-Pedersen M, Koch K, Thomsen RW, Schonheyder HC, Nielsen H (2014) The effect of community-acquired bacteraemia on return to workforce, risk of sick leave, permanent disability pension and death: a Danish population-based cohort study. BMJ Open 4:e004208.
- Mangen MJ, Bonten MJ, de Wit GA (2013) Rationale and design of the costs, health status and outcomes in community-acquired pneumonia (CHO-CAP) study in elderly persons hospitalized with CAP. BMC Infect Dis 13:597.
- Torres A, Peetermans WE, Viegi G, Blasi F (2013) Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 68:1057-1065.
- Shea KM, Edelsberg J, Weycker D, Farkouh RA, Strutton DR, et al. (2014) Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis 1:ofu024.
- Naucler P, Darenberg J, Morfeldt E, Ortqvist A and HenriquesNormark B (2013) Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax 68:571-579.
- http://www.cdc.gov/getsmart/community/pdfs/annual-reportsummary_2014.pdf
- Lepper PM, Ott S, Nuesch E, von Eynatten M, Schumann C, et al. (2012) Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 344:e3397.
- Falcone M, Tiseo G, Russo A, Giordo L, Manzini E, et al. (2016) Hospitalization for Pneumonia is Associated With Decreased 1-Year Survival in Patients With Type 2 Diabetes: Results From a Prospective Cohort Study. Medicine (Baltimore) 95:e2531.
- Martins M, Boavida JM, Raposo JF, Froes F, Nunes B, et al. (2016) Diabetes hinders community-acquired pneumonia outcomes in hospitalized patients. BMJ Open Diabetes Res Care 4:e000181.
- Falguera M, Pifarre R, Martin A, Sheikh A, Moreno A (2005) Etiology and outcome of community-acquired pneumonia in patients with diabetes mellitus. Chest 128:3233-3239.
- Millett ER, De Stavola BL, Quint JK, Smeeth L, Thomas SL (2015) Risk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: a cohort study. BMJ Open 5:e008737.
- Di Yacovo S, Garcia-Vidal C, Viasus D, Adamuz J, Oriol I, et al. (2013) Clinical features, etiology, and outcomes of community-acquired pneumonia in patients with diabetes mellitus. Medicine (Baltimore) 92:42-50.
- Kornum JB, Thomsen RW, Riis A, Lervang HH, Schonheyder HC, et al. (2008) Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 31:1541-1545.
- http://rockitfuelradio.com/wp-content/uploads/2015/05/Blacks-Underlying-Cause-of-Death-1999-2013-Results-Form.pdf
- Corrales-Medina VF, Madjid M, Musher DM (2010) Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 10:83-92.
- Musher DM, Rueda AM, Kaka AS, Mapara SM (2007) The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis 45:158-165.
- Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ (2005) Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA 294:2712-2719.
- Dutt TS, Tousheed SZ, Mohan BV (2014) Community acquired pneumonia and cardiac diseases: a fatal association. Indian J Chest Dis Allied Sci 56:153-156.
- Fryar CD, Chen TC, Li X (2012) Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010. NCHS Data Brief 103: 1-8.
- Flory JH, Joffe M, Fishman NO, Edelstein PH, Metlay JP (2009) Socioeconomic risk factors for bacteraemic pneumococcal pneumonia in adults. Epidemiol Infect 137:717-726.
- https://www.ncbi.nlm.nih.gov/books/NBK179276/
- Jackson ML, Nelson JC, Jackson LA (2009) Risk factors for community-acquired pneumonia in immunocompetent seniors. J Am GeriatrSoc 57:882-888.
- Stein CR, Weber DJ,Kelley M (2003) Using hospital antibiogram data to assess regional pneumococcal resistance to antibiotics. Emerg Infect Dis 9:211-216.
- Tennant I, Nicholson A, Gordon-Strachan GM, Thoms C, Chin V, et al. (2010) A survey of physicians' knowledge and attitudes regarding antimicrobial resistance and antibiotic prescribing practices at the University Hospital of the West Indies. West Indian Med J 59:165-170.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 7204
- [From(publication date):
February-2017 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 6252
- PDF downloads : 952