Application of Surfactants in Hydraulic Fracturing for Enhanced Oil/Gas Recovery
Received: 10-Dec-2018 / Accepted Date: 08-Jan-2019 / Published Date: 16-Jan-2019 DOI: 10.4172/2472-0518.1000161
Abstract
Surfactants are widely used in the hydraulic fracturing process to enhance oil/gas productivity and reduce energy consumption by controlling for optimal viscosity of fracturing fluids, reducing surface/interfacial tension between the shale formations and the fracturing fluid, assisting fluid recovery after fracturing, altering the wettability of rock and reducing flow friction of fracturing.
The up-to-date progress of the application of surfactants in fracturing fluids for enhancing oil/gas recovery are summarized comprehensively from the aspects of single surfactant system, mixed surfactant system, surfactant combination with nanoparticles and polymers, mainly concentrating on the effect of surface tension reduction, wettability alteration and drag reduction from the point of experimental research and numerical simulation. And the broad application prospects of surfactant compound with nanoparticle and polymer are also outlooked.
Keywords: Surfactant; Oil/gas recovery; Surface tension; Drag reduction; Wettability alteration
Introduction
Since its discovery, oil and gas has remained the world’s major energy source despite the recent contribution from renewable energy [1]. Hydraulic fracturing is normally applied to stimulate low/ultralow permeability reservoirs in order to obtain an economical hydrocarbon production rate [2]. However, 50-95% of the injected fluid is typically reported to remain in the formation due to the capillary effect [3,4], which will hinder hydrocarbon production and cause formation damage [5]. The capillary force, one form of capillarity, could be mainly responsible for the surface tension of fracturing fluid [6]. On the other hand, gas exploited from reservoirs is prone to condense into liquid under pressure conditions below the saturation point [7], then a liquid phase, known as condensate bank is formed and retained by capillary forces in the liquid-wettable rock, reducing the gas permeability and thus the wellbore productivity [8], especially for low-permeability reservoirs and tight reservoirs [9]. Chemical enhanced oil/gas recovery has been proven to be a promising method to recover the trapped gas by reducing the surface tension and altering the wettability of rock surface from liquid to gas by use of specific surfactant [10]. Several surfactant-based fracturing fluid additives have been developed in an attempt to enhance the flow back and oil/gas production [11-13]. Simultaneously, when the concentration of surfactant in the fracturing fluid reach to some extent, worm-like micelle will from, thus reducing the flow resistance of fracturing fluid [14]. In recent years, a viscosity surfactant-based clear fracturing fluid has been applied as the substitute of traditional polymer-based fracturing fluids with characteristics of high flow back rate, high productivity, low friction and low formation damage [15].
Surfactants, one of the most important addictive in fracturing fluid, are used to control for optimal viscosity of fracturing fluids, reduce surface tension between the shale formations and the fracturing fluid, assist fluid recovery after fracturing, alter the wettability of rock and reduce flow friction of fracturing [16]. In some instances, surfactants may also act as biocides or clay stabilizers [17]. The role of surfactants mentioned above are all helpful to enhance oil/gas productivity and reduce energy consumption [18].
The present review about the application of surfactants in fracturing fluid are concentrating on the recent trends and reach progress of compounds of different surfactants [19] and single special surfactants such as polymeric surfactants [20], Gemini sufactants [21] and so on. However, to the best of our knowledge, there is very limited of such a review that comprehensively introduce the various and latest applications of different surfactants compound with different kinds of chemical additives like nanoparticle, polymer to enhance the productivity of oil/gas from low permeability reservoirs, domestically and abroad so far. In the following sections, we will summarize and discuss the up-to-date progress of the application of surfactants in fracturing fluids for enhancing oil/gas production comprehensively, from the point of the interaction of different kinds of surfactants as well as surfactants with nanoparticle and polymer, mainly concentrating on the effect of surface tension reduction, wettability alteration and drag reduction, from the point of experimental research and numerical simulation. The review is expected to be of references for the researchers working in the field.
Single Surfactant System
Traditional surfactant
Experimental study of traditional single surfactant: Typical traditional surfactant such as anionic surfactant sodium dodecyl sulfate (SDS) and cationic surfactant cetyltrimethylammonium bromide (CTAB), cetyltrimethylammonium chloride (CTAC) can reduce surface tension to some extent around critical micelle concentration (CMC). The effects of SDS and CTAB on the interfacial tension (IFT), wettability alteration and spontaneous imbibition of oil and outcrop rock samples obtained from Aghajari reservoir were investigated. The experimental results show the obvious effect of these surfactants at very dilute concentrations [22]. Mirna et al. studied the performance of the cationic surfactant CTAC on the enhancement of the oil recovery factor from heavy oil-impregnated calcite cores. The interaction energies between CTAC and either of some molecules present in oil were theoretically determined within the framework of the Density Functional Theory and experimentally analysed respectively [23].
Mohammad et al. investigate adsorption kinetics and equilibrium of a novel nononic surfactant Glycyrrhiza Glabra in aqueous solutions for EOR and reservoir stimulation purposes [24]. The result shows that the surfactants trend to adsorb onto carbonate, which can be used for enhancing oil recovery from carbonates effectively and economically [25]. Amit et al. synthesize a carboxybetaine based zwitterionic surfactant by quaternization of dodecyldimethylamine with chloroacetate, which is characterized by FTIR, 1HNMR and TGA analysis. The synthesized surfactant is effective in decreasing the IFT between crude oil and water at a low CMC and changing the wettability of oil wet quartz sample to water wet. Sand pack experiments showed additional oil recovery of 27.03% by flooding of surfactant slug at CMC, which evidence the suitability of the synthesized zwitterionic surfactant for application in chemical EOR. Nilanjan et al. synthesize a novel anionic surfactant from coconut oil as an alternative during oil/ gas recovery, which exhibits good salt tolerance levels and long-term thermal stability, as well as lower cost [26].
Numerical simulation of traditional single surfactant: With the development of computer science and technology, people have not satisfied with the physical and chemical properties of substances obtained by experimental methods. Computer simulation studies and experimental studies can support and supplement each other. Experimental studies provide experimental data that is essential for computer simulation, while computer simulations provide data that cannot or difficult to acquire by experiment [27]. Molecular dynamics simulation and molecular fragment dynamics simulation are helpful to understand the self-assembling behaviour and dynamic characteristics of surfactants, which have become the research hotspot of surfactant numerical simulation.
Chanda et al. simulate the surface coverage, surfactant orientation and kinetic details of a single layer of nonionic surfactant C12E2 adsorbed at the air/water interface at CMC and indicates that the surfactant has stronger mobility on the interface plane than in the normal direction of the interface [28]. The hydrophilic head exhibits faster reorientation dynamics due to the shorter hydrophobic tail length and tends to tilt toward the interface plane.
Pang et al. perform molecular dynamics simulations absorption of the surfactants containing the same hydrophobic carbon: anionic surfactant SDS, cationic surfactant dodecyl trimethyl ammonium bromide (DTAB), nonionic surfactant octaethylene glycol lauryl ether (C12E8) and surface activator siloxane modified by oxyethylene and oxypropylene (DSEP) at the air/water interface [29]. The results show that the head groups of SDS and DTAB are entirely hydrated and located in the region of water, not all the oxyethylene groups of C12E8 immerge in the water layer. Due to the large bond angle and long bond length, the Si-O bond can freely rotate and tilt. Therefore, the DESP chain is flexible and spreads easily at the interface, showing a stronger surface activity and increasing the solubility of the remaining surfactant. In the presence of DESP, the SDS monolayer is relatively ordered and compact, and some of the DTAB molecules are in water while the others at the interface are organized, whereas the C12E8 monolayer turns out to be disordered and loose. Protonated oxyethylene group possesses weak positive charge in aqueous solutions.
There is the electrostatic attraction between DESP and SDS, so the electrostatic repulsion between headgroups of SDS can be partially screened. As a result, the monolayers are more closely arranged in the presence of DESP. Meanwhile, there is a weaker electrostatic repulsion between DESP and DTAB. Additionally, DESP, having higher surfaceactivity, is prone to extend at the surface. Consequently, some DTAB molecules dissolve in water, and the others at the surface are well ordered, far apart from each other. DESP mainly depends on Vander waals and hydrophobic interactions to affect the properties of the C12E8 monolayer. Only the oxyethylene groups of C12E8 close to the surface can interact with DESP by hydrogen bond. The simulation results confirm that the aggregation behaviors of hydrocarbon surfactants at the air/water surface are obviously influenced by a siloxane surfactant, which can provide a theoretical fundament to predict the properties of surfactant mixtures.
Truszkowski use molecular fragment dynamics simulation to study the behaviour of non-ionic surfactant polyoxyethylene alkyl ether at the water-air interface [30]. The calculation results show that the longer the carbon chain of the surfactant, the lower the CMC, a longer carbon chain leads to a lower surface tension under the condition of same concentration below CMC. The surface tension tends to fluctuate when the CMC is reached. At the same time, the number of surfactant molecules at the water-air interface per nm² is analysed, the number of surfactant molecules at the interface increases linearly with increasing concentration until the saturated concentration reached. A longer carbon chain leads to a lower concentration at saturation, which is consistent with experimental results [29].
Special surfactants
Traditional surfactants have limited ability to reduce surface tension. Liang et al. investigate the effect of different surfactants on reducing the damage resulting from water block by reducing the interfacial/ surface tension and enhancing permeability [31]. The result show that surfactants providing moderate reductions on interfacial tension has negative impacts on solving the water block, while surfactants providing the ultralow interfacial tension can enhance matrix permeability and hydrocarbon recovery because of the elimination of the matrix-fracture interaction. In order to obtain ultra-low surface tension, special surfactants are developed.
Fluorocarbon surfactant is one of the most important surfactants which has the highest surface activity, good heat-resistant and salt resistance. However, the synthesis is very difficult and the cost is high, so it is commonly used in combination with cheaper hydrocarbon surfactant [32]. Whereas, fluorocarbon surfactant has poor low temperature resistance and weak affinity with most organic solvents, which limits their applications. Therefore, fluorosilicone surfactant has been developed on the basis of fluorocarbon surfactants [33].
Gemini surfactant encompasses a relatively new generation of surfactants that has unlimited scope in terms of flexibility and economic effectiveness [34,35]. The presence of two hydrophilic head groups and two hydrophobic tails confer unique properties that are well-suited to requirements of different industries [36]. Soheila et al. investigate the aggregation behavior, physicochemical properties and morphology of the surfactants of ester-containing cationic Gemini surfactants, dodecyl esterquat and dodecyl betainate Geminis in the absence and presence of NaBr electrolyte [37]. The results show that the position of ester bonds in alkyl tail has a considerable effect on both physicochemical properties and aggregation behavior of the ester-containing Gemini surfactants and the salt addition induced the growth of micelles. Palet et al. investigate the equilibrium and dynamic interfacial properties of Gemini surfactants with different spacer lengths and demonstrate declining trend of the interfacial tension with the increase of spacer lengths [38]. Then the equilibrium adsorption characteristics of Gemini surfactant molecules at the oil-aqueous interface are explained using the Langmuir and Freundlich isotherms, and conclude that Gemini surfactants molecules at low concentration from the bulk to the interface may be described as a diffusion controlled process, but is best decried as a combination of adsorption and diffusion controlled adsorption at higher concentration, till the interface is completely saturated.
Polymeric surfactant represents a very attractive alternative to chemical enhanced oil/gas recovery, because they can provide simultaneously increase in water viscosity and decrease in interfacial tension, both beneficial for the efficiency of the fracturing process [39]. However, the synthesis are often challenging and costly, Patrizao et al. suggest a much more promising way by the introduction of biopolymers and bio-based monomers [40,41].
Mixed Surfactants System
Experimental study of mixture surfactants
Although a single surfactant can reduce the surface tension of the solution, its ability to form micelles is weak, so it is necessary to compound multiple chemical agents to acquire better performance at lower concentration when improving oil/gas recovery. Mixed surfactant systems have gained great interest because of polymorphism of selfassembly structures accessible through simple tuning of composition that have combined properties of the various surfactants in the mixture [42,43].
When ionic surfactant adsorbs on the interface, the strong electrostatic repulsion resulting form the same charged group facilitate the molecular arrangement not tight enough. The non-ionic or zwitter ionic surfactant will be easier to enter the loose ionic surfactant adsorption layer due to the hydrophobic effect and the possible dipoleion interaction. Thus, the electrostatic repulsion will be weakened and the molecules will arrange closely, then the surface activity will be improved [44,45].
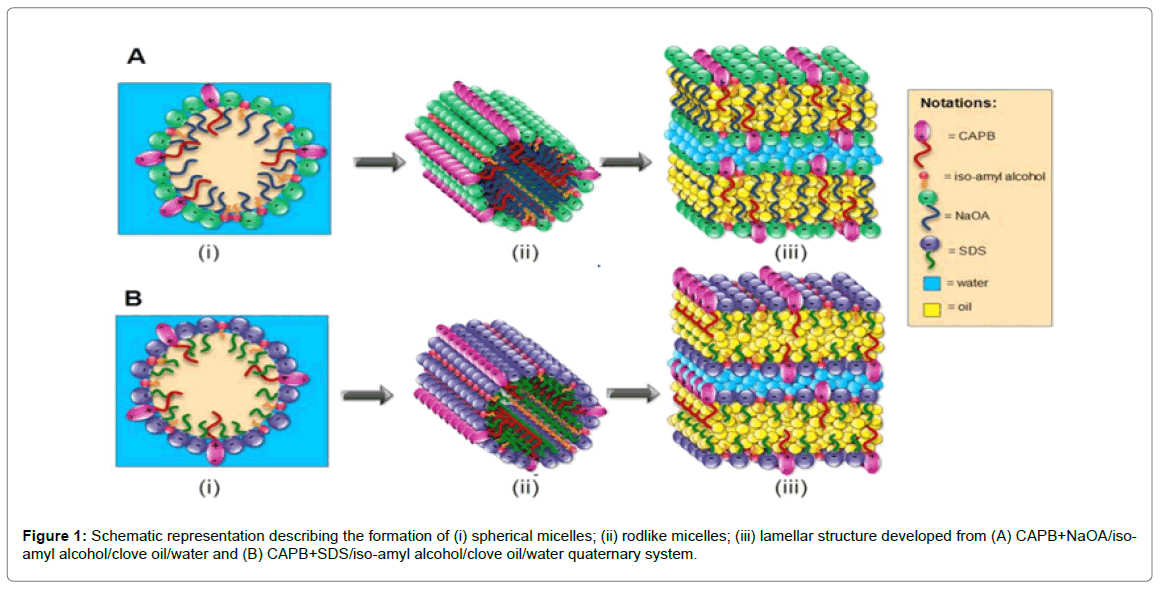
It is reported that zwitterionic-anionic mixed surfactant systems offer synergistic interactions in aqueous medium due to polymorphism of self-assembly structures that have combined properties of surfactants in the mixture. Atrayee et al. study the rheological properties of Viscoelastic Surfactant (VES) based fracturing fluids developed from zwitterionic surfactant Cocamidopropyl betaine(CAPB) mixed with anionic surfactant SDS and sodium oleate (NaOA) respectively [46]. Static and dynamic rheological tests indicate lower CMC and superior viscoelastic properties of the later system (Figure 1).
Numerical simulation of mixture surfactants
Numerical simulation of mixture surfactants can help us to understand interaction mechanism of different surfactant deeply and comprehensively.
AVai et al. study the characteristics of self-assembly spherical micelles formed by onionic surfactants - polyethylene glycol ethers in water and sodium chloride solution, respectively [47]. The results show that the addition of salt has a significant effect on the structure and size of the micelles. In pure water, the hydrophobic hydrocarbon chain radicals of the surfactant accumulate in the centre of the micelle; while in sodium chloride solution, the polar group will gather around the micelle crown. Meanwhile, the rearrangement is accompanied by micelle shrinkage, and the spherical micelle radius will be reduced from 2.0 nm to 1.8 nm.
Wang et aluse molecular dynamics simulation to study the adsorption of cationic surfactant dodecylamine (DDA) and anionic surfactant sodium oleate (NaOL) on the interface [48]. The results show that DDA and NaOL are clearly distributed around the air/water interface, the hydrophobic carbon chain stretches into the air phase, the hydrophilic head group arranges on the air/water interface, and most of the counter ions exist in the interface region near the head group. All heads are hydrated in the aqueous phase, with only a small portion of the carbon chain immersed in the water. Due to the electrostatic interaction between DDA and the NaOL ion-based head group and the hydrophobic interaction between the carbon chains, the mixture display much more compactness and tightness, thus showing stronger surface activity than the pure DDA and NaOL. When the molar ratio of DDA to NaOL is 1:3, will demonstrate a stronger interaction and a lower interfacial tension, which corresponds well with the results obtained from experimental results.
Surfactant Combination with Nanoparticles and Polymers
Chemical flooding has been one of the efficient EOR techniques that use polymer (P) and surfactant-polymer (SP) solutions to improve oil/gas recovery by decreasing interfacial tension/surface tension, increasing capillary number, improving mobility ratio, and increasing macroscopic sweep efficiency [49,50]. Nano fluid are widespread interest in many subsurface engineering applications such as hydrocarbon exploration and production, EOR processes [51-53]. Therefore, Surfactant combination with nanoparticles and polymers is gaining more and more attention.
Surfactant-nanoparticle mixed system
Nanoparticles have a large specific surface area. When added to surfactants solution, nano-emulsions (micro-emulsions) will form and they have better stability due to small-scale effects and Brownian motion. Meanwhile, the nanometer-scale droplets are more likely to enter the tiny voids or cracks of the dense rock, and its large specific surface area can make the nano-droplets fully spread on the rock surface, improve the contact efficiency between the nanoemulsion and the reservoir, and improve the overall application effect of the well fluid [54].
Mohammad et al. compound silica nanoparticles with surfactant surfactant sodium dodecyl benzene sulfonate to prepare NS nanofluids [55]. The interfacial tension between NS nanofluids and crude oil was significantly lower than that of surfactant solution without nanoparticles. It is believed that the electrostatic repulsion between the surfactant and the nanoparticles promotes the diffusion of the surfactant to the interface, while the nanoparticles encapsulated by the surfactant carry the surfactant to the interface due to its Brownian motion, and the surfactant and Surfactant-coated nanoparticles are aligned at the interface to reduce interfacial tension synergistically.
Dong et al. add titanium dioxide nanoparticles directly into water to form nanofluids. As a result, the surface tension of nanofluids decreased firstly and then increased with the increase of titanium dioxide concentration [56]. It is suggested that when the concentration of titanium dioxide nanoparticles is high enough, the attraction between the particles causes capillary forces, which in turn causes the surface tension to rise. The article also explains the reason for the decrease in surface tension from the energy point of view. It also shows that the reduction of surface tension is also a dynamic process because the adsorption of nanoparticles on the surface is a dynamic process.
Jin et al. study the interaction between surfactant and nanoparticles. cationic surfactant CTAB, anionic surfactant SDS, positively charged titanium dioxide nanoparticles and negatively charged silica dioxide are used in the experiment [57]. The results show that when the surfactant and nanoparticle possess same charge, the electrostatic repulsion will drive more surfactants at the interface, and the two will show synergistic effect, then the surface tension is reduced; While when the surfactant and the nanoparticle possess opposite charge, due to the electrostatic attraction, the nanoparticles will adsorb on the surfactant and agglomerate will form, thereby reducing the effective concentration of the surfactant on the interface, and the surface tension will increased instead.
Luo et al. investigate the effect of clean-up addictive compounded by nanoemulsions and surfactants [58]. It is believed that the nanoemulsion has ultra-low surface tension, high solubilisation and small particle size, can effectively enter the pores and fully contact with the solid surface, which can reduce the surface tension and change the surface wetting angle of the solid, then significantly reduce the water absorption of the core, thereby reducing the capillary resistance and effectively improve the fracturing cleanup efficiency.
Studies have shown that due to the unique advantages of nanoparticles, the interaction of surfactants and nanoparticles can change the rheological properties and interfacial properties of the system, reducing the surface tension of the system.
Surfactant-polymer mixed system
When water soluble polymer, surfactant and salt are mixed in aqueous solution, structures known as aggregates may form through polymer-surfactant interaction and can have a drastic effect on the solution’s rheology. The structure of these aggregates described as polymer film will form around surfactant micelles [59].
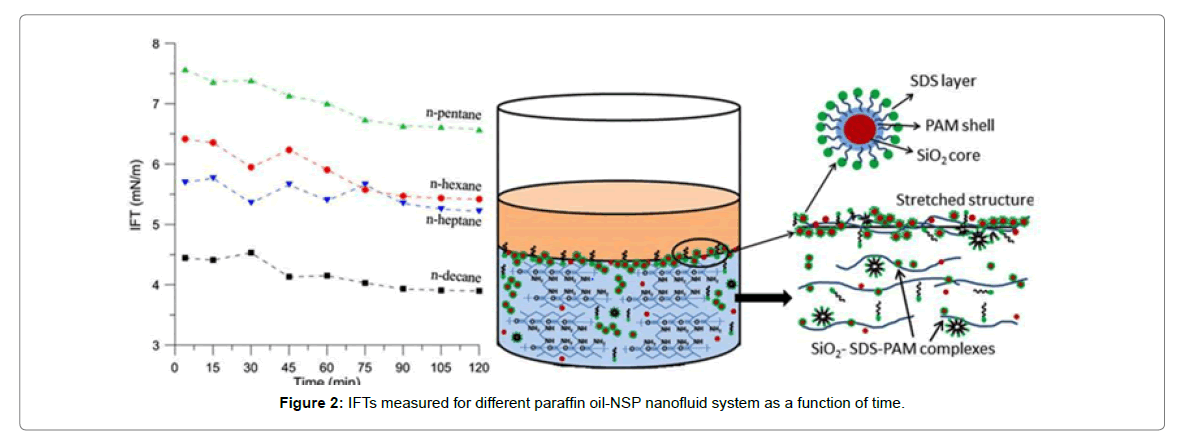
Tushar et al. prepare NP nanofluids by compounding silicon dioxide nanoparticles with polyacrylamide for oilfields, and NSP nanofluids by compounding silicon dioxide nanoparticles, surfactant sodium lauryl sulfate and oilfield polymers polyacrylamide, respectively [60]. The surface tension of nanofluids and the interfacial tension between nanofluids and crude oils are characterized. The results show that the surface tension and oil-water interfacial tension of NP nanofluids and NSP nanofluids are significantly lower than those of traditional oil extraction auxiliaries agent. Furthermore, the surface tension and oilwater interfacial tension of NSP nanofluid are lower than that of NP nanofluid. It is believed that the presence of the surfactant improves the wettability of the nanoparticles, increases the amount of adsorption on the surface, and simultaneously forms the silica nanoparticlespolyacrylamide in the bulk phase due to the Brownian motion of the nanoparticles. The surfactant agglomerates are carried to the surface and arrange directionally on the surface, reducing the surface tension of the system (Figure 2).
Molecular dynamics can be used not only for the simulation of surfactant systems, but also for the study the interaction of surfactantpolymer system to obtain detailed information on the kinetic and structural properties that are not possible in the experiment. Wang et al. use molecular dynamics simulation to study the interaction of cationic surfactant CTAB and the negatively charged polymer sodium polyacrylate (NaPAA) at the air/water interface [61]. The results show that when the surfactant concentration is low, a surfactant-polymer monolayer is formed at the interface, and a multi-molecular layer is formed as the concentration of the surfactant increases. Due to the electrostatic interaction between the negatively charged carboxylate in acrylic acid and the positively charged head group in the surfactant, the oxygen atom of the carboxylate and the hydrogen atom in the water can form a hydrogen bond, which promotes the binding of the surfactant to the polymer chain.
As the concentration of the surfactant increases, due to the hydrophobic interaction between the hydrophobic tails of the surfactant, the layer-layer arrangement is promoted to form a multilayer adsorption structure. Simulation studies have also shown that the polymer-surfactant complex has a dynamic process of ion exchange, consistent with experimental results.
Studies have shown that due to the interaction of surfactants and polymers,the rheological properties and interfacial properties of the system can be improved.
Other Applications of Surfactants in Fracturing Process
Wettability alteration
In gas condensate reservoirs, well productivity reduction resulting from condensate blockage has been one of the most important factors influencing gas and condensate production rates, which is sharper for the low-permeability reservoirs [62]. Many investigators have proposed several methods such as gas recycling, hydraulic fracturing and solvent injection, that is to say using surfactants to mobilize the condensate in the region near wellbore, to restore gas and condensate production rates when condensate blockage occurred [63-65].
In the past few years, many researchers have investigated on surfactants to reduce the surface tension and remove the condensate banking [66,67]. However, these chemicals do not alter the wettability of the rock surfaces from liquid to gas. In recent years, wettability alteration, as a new method, has become more attractive for researchers in industry. Most of the gas condensate reservoirs rocks are naturally liquid-wetting and altering the wettability of the reservoir rock from strongly liquid wetness to preferential gas wetness or intermediatewetting can increase the mobility of condensate and the relative permeability to gas [68]. Then several researchers develop fluorosurfactants and modify the wettability of rocks from liquid to gas [69-71]. A direct relationship with the increase of roughness and heterogeneity of the surface by means of adsorption of the fluorinated surfactant over the rock surface is discussed. They conclude that the surfactant adsorption leads to a reduction of the surface free energy due to the formation of a fluorine atoms network [72,73]. Wang et al. investigate the influence of wettability alteration on the displacement efficiency of gas-condensate by conducting contact angle measurement and gas flood tests [74]. Results show that core wettability can be altered from liquid-wetting to intermediate gas-wetting or preferential gas-wetting by the fluorosurfactant at a very low concentration because the core surface free energy sharply decreased after the treatment of surfactants. And then, oil displacement efficiency, relative permeability, and gas flow in gas–oil systems can be effectively improved by fluorosurfactant treatment due to alteration of wettability. Hassanajili et al. synthesize a fluorinated polymer surfactant to induce gas wetness on reservoir rocks, they used static contact angle (CA) tests on thin sections of rock samples; and water/n-decane-air imbibition tests in core scale to demonstrate the applicability of synthesized chemical. Fahimpour et al. investigate the effect of anionic and nonionic surfactant on the wettability alteration of carbonate respectively, results demonstrate that on positively charged carbonate surfaces, the anionic chemicals are sufficiently effective to repel the liquid phase, whereas the nonionic chemicals showe an excellent stability in brine media [75,76]. Then a new approach of combining anionic and nonionic chemical agents is proposed.
However, Because of the amphiphilic character of surfactant, they tend to form micelle at certain concentration, which could not penetrate all pores, especially in the tight reservoirs [77]. Therefore, it is urgent to develop chemicals with appropriate size which can diffuse into deeper pore throats in low permeability rocks to alter their surfaces from liquid-wet to gas-wet state.
Nanotechnology has recently emerged as an attractive topic of research in the oil and gas industry due to its exceptional characteristics that allow nanoparticles to travel smoothly through porous media without additional risks of pore blockage due to their small size, which can be used to avoid formation damage. At the nano-scale, exceptional properties can be obtained, such as a high surface-area-to-volume ratio and dispensability, in addition to high thermal, chemical stability and dispersibility [78]. Hence, the use of nanofluids based on the interaction of fluorosurfactants and nanoparticles with the appropriate particle size could be a promising alternative for enhancing wettability alteration to gas-wet systems.
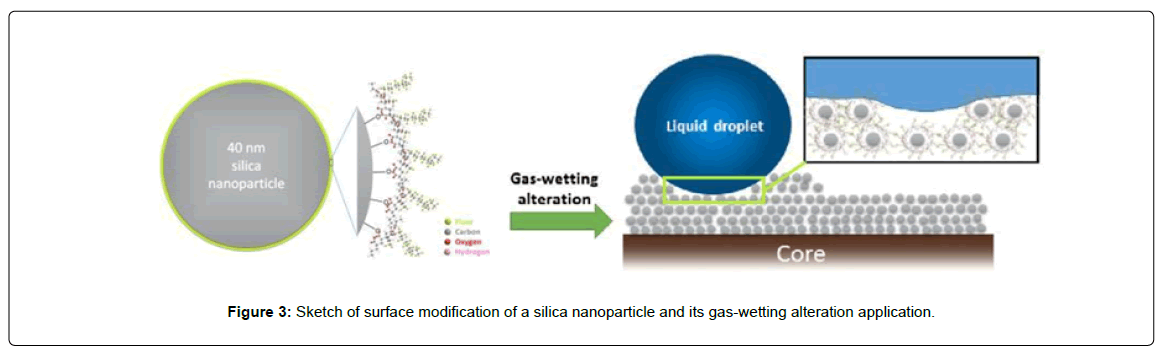
Jin et al. investigate the influence of gas-wetting alterations on cores using silicon dioxide nanoparticles modified with fluorocarbon surfactant [79,80]. The results showed that the wettability of the core could be altered to intermediate gas-wetting or super gas-wetting after nanofluid treatment. They conclude that the dehydration-condensation reactions of silicon dioxide nanoparticles and fluorocarbon surfactant forms a compact layer which can remarkbly alter the wettability from water-wet to gas-wet (Figure 3).
Maribel et al. develop a nanofluid based on the interaction between an anionic surfactant Silnyl®FSJ (SY) and silicon dioxide nanoparticles to alter the reservoir wettability from a liquid-wet state to gaswettability [81]. The results of different concentration of nanoparticles and surfactant are supported by contact angle and imbibition tests on oil-wet and water-wet sandstone samples for the wettability alteration, then the optimum concentration is achieved and performed under tight gas-condensate reservoir temperature and pressure conditions. The result indicate that the synthesized nanofluid can alter the wettability of the system from a strongly liquid-wet to a gas-wet condition, then the formation damage caused by the condensate banking can be reduced,the production of oil and gas can be considerably improved.
At present, there is only few research concerning the wettability alteration resulted from interaction of nanoparticle and surfactant. Nanofluid combining the benefit of nanoparticle and surfactant may be an effective way to change the wettability from water-wet to gas-wet, which can be a research hotspot in the near future.
Drag reduction
In the development of shale gas reservoirs, the addition of surfactants can reduce the viscosity and vortex formation in the shear flow of the wellbore, thus reducing the flow resistance of fracturing fluid [82]. At present, the drag reducer added in the fracturing fluid is mainly linear vegetable gum and poly acrylamide, Whose shear resistance is poor at higher shear rate, and residue content is high, which may causes serious formation damage [83]. Surfactant is a new type of drag reducer different from polymer, the appearance and disappearance of drag reduction is reversible, and shear degradation does not occur, so it has stable drag reduction effect, especially for large-scale and high shear rate fracturing, as well as the recirculation of fracturing fluid [84]. Studies have shown that when the surfactant drag reducing solution reaches the critical micelle concentration (CMC), spherical micelles are first formed in the solution; with the concentration further increased to the transition concentration (CMCII), the spherical micelles in the solution will transform into rod micelles. When the fluid flows, the rod micelles gradually assemble into a shear-inducing structure and a spatial network structure under shear, then the viscosity of the solution increases and becomes a viscoelastic fluid [85]. Yu et al. believe that the addition of surfactants in the turbulent flow has a dual role: on one hand, the introduction of viscoelastic shear stress can increase the friction, on the other hand, the suppression of the turbulent structure perpendicular to the wall resulting reduction of turbulent shear stress can reduce the friction [86]. Since the latter is stronger than the former, the surfactant exhibits drag reducing effect when added.
A single surfactant has a limited temperature range and needs large amount in the drag reduction process, which greatly limits its use for drag reduction. Adding surfactants of different alkyl chains together is an effective method to expand the effective temperature range and reduce the amount of surfactants, because it is more favourable to form a stable linear micelle network structure after mixing [44]. The addition of counter ions and salts to the surfactant solution can promote the formation of rod micelles. Tuan et al. have shown that after adding a certain proportion of salt to the surfactant solution, a stable micelle structure can form and intertwine each other [87]. Subsequently, a network structure appear, which exhibit significant viscoelasticity, known as worm-like micelles. Peng et al. add the counter ion salicylate sodium to dodecyltrimethylammonium (CTAB), which is believed to increase the size of the rod micelles and the space size between them [88]. The electrostatic energy between the micelles is reduced, so that the electrostatic stability of entire rod shape micelles in the aqueous solution is enhanced. On the other hand, another part of the non-rod micelles formed for the reason that the hydrophilic groups can not completely cover the hydrophobic groups can be converted to rod micelles, reducing the CMCII value of the surfactant any further.
Cationic surfactants are widely used for drag reduction for the wide effective temperature range. Cationic surfactants CTAC have excellent light, heat and shear stability, has been one of the currently attractive surfactants. Hadri et al. study the effects of temperature and concentration on the drag reduction performance of low concentration cationic surfactant CTAC and counter ion sodium salicylate (NaSl) during turbulent drag reduction [89]. The results showed that when the CTAC concentration is greater than 50ppm and the average temperature is 20°C, the drag reduction rate reaches the maximum. As the temperature increases, to achieve the same drag reduction effect, the CATC concentration needs to be increased. In addition, the zwitterionic surfactant has the characteristics of low toxicity and high biodegradability, which can be combined with an anionic surfactant to obtain a compound reducer with excellent comprehensive performance, as well as the compound of nonionic surfactant and the cationic surfactant. It is also reported that a kind of a drag reducer composed of twelve alkyl betaine and sodium twelve alkyl sulfate and another kind of drag reducer composed of rapeseed oil oleic acid ethanolamide, lauryl alcohol and sixteen alkyl three methyl salicylic acid ammonium salt both show strong drag reduction performance [90].
When drag reduction occurs for the surfactant solution, the turbulent structure inside the fluid will be inhibited by the induced structure formed by the surfactant, thereby changing the turbulent structure, which provides possible conditions for other drag reduction methods that acquiring drag reduction by influencing turbulent structures, and also provides guidance for combining surfactant drag reduction with other suitable drag reduction methods. Therefore, the study of surfactants combined with other drag reduction methods to realize efficient turbulence drag reduction is also a hot topic in the near future [91].
A nanoparticle-associated surfactant micelle fracturing fluid has been developed recently, which can associate linear micelles to form a three-dimensional network structure. Nanoparticles at very low concentration can greatly improve low shear velocity viscosity, swelling time and elastic storage modulus. It is believed that the surface of the nanoparticle can interact with the end cap of the linear micelle, functioning as a joint bond,then the micelles will be coupled into a micelle network. The nanoparticle can replace the end cap and join the nanoparticle and the worm-like micelle [92].
Maxey et al. study the effect of nanoparticles on improving the rheological properties and fluid loss control properties of surfactant fluids, indicating that surfactant fluids without nanoparticles exhibit significant viscosity, while the surfactant fluids will show significant elasticity as soon as the nanoparticles were added [93]. It is suggested that the addition of nanoparticles increases fluid shear viscosity, improves fluid loss control, and improves shear stability due to the formation of a viscous network structure, exhibiting similar properties to crosslinked polymers. In general, the addition of nonionic surfactants to nanofluids is the best choice because of the negative effects of nanosuspension settling and nanoparticle settling is least.
Matras et al. study the effect of the interaction of nonionic surfactant cocoamidopropyl betaine with high molecular weight polymer polyoxyethylene and anionic polyacrylamide on the drag reduction effect through the parameters like surface tension, viscosity, and electrical conductivity, respectively [94]. The results show that due to the hydrophobic interaction and electrostatic interaction between the high molecular polymer and the surfactant, a polymer film is formed around the surfactant micelle to generate a polymer-surfactant agglomerate, which changes the flow characteristic of the original system. The novel suspension shows higher drag reduction rate, larger range of drag reduction Reynolds number and effective temperature range, stronger shear resistance, and partial recovery after shear failure.
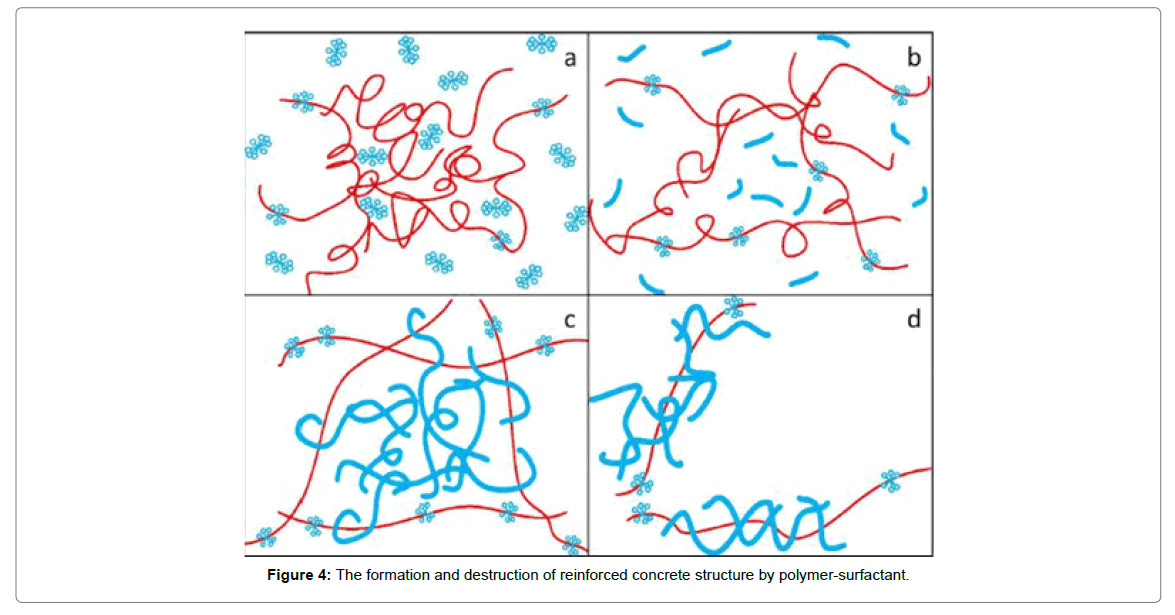
Liu et al. test the mixed aqueous solutions of cationic surfactant CTAB, non-ionic molecular polymer polyacrylamide and NaSal as the counter-ion salt to verify the speculation about their intensification possibilities of drag reduction performance under different conditions [14]. The research show that surfactant molecules form micelles round polymer chains, then a kind of reinforced concrete structures will form(as shown in Figure 4) with the increase of Reynolds number, but exceed which the reinforced concrete structures will be destroyed, the formed reinforced concrete structures were more complex and more effective in restrain vortices, leading to the intensification of drag reduction compared to pure surfactant solutions, the experiment also prove that temperatures were more influential than concentrations.
MalcherT et al. Investigated the drag reduction mechanism and properties of nonionic polyoxyethylene(PEO) and cationic surfactant CTAB agglomerates by computational fluid dynamics Numerical simulation. The results show that the complex structure of CTAB-PEO agglomerates of neutral polymer and surfactant are non-covalently bonded in aqueous solution, with a viscosity higher than that of single surfactant or polymer. In a certain range of Reynolds number, agglomerates will elongate, stretch and transfer internal stress when subjected to shear in the flow, thereby reducing the resistance.
Studies have shown that surfactants can present superior drag reduction effect with nanoparticles and polymers, synergistically. It can retain the advantages of a variety of original single materials, make up for its shortcomings, improve drag reduction efficiency and reduce drag stability, which will be a hot spot in the future research of drag reducers (Figure 4).
Summary and Prospect
The up-to-date progress of the application of surfactants in fracturing fluids for enhancing oil/gas production are summarized comprehensively from the aspects of single surfactant system, mixed surfactant system, surfactant combination with nanoparticles and polymers, mainly concentrating on the effect of surface tension reduction, wettability alteration and drag reduction from the point of experimental research and numerical simulation. Based on the research on the surfactants summarized above, surfactants, as one of the most important clean-up addictive in fracturing, have multiple functions including reducing surface tension, altering wettability, reducing flow friction and so on. However, the effect of single surfactant is poor, which can be improved by the compounding system. Nanoparticles have the advantage of large specific surface area, small particle size, and obvious Brownian motion effect, which can show significant effects when compounded with surfactants. Polymer-surfactant aggregates can have a drastic effect on the solution’s rheology. Therefore, the study on the mechanism and interaction of surfactants, nanoparticles, and polymers in Nano fluids will be the hotspot of research on fracturing fluids in the future.
Hydraulic fracturing consumes a huge amount of water. Faced with the current dual pressure of development cost and environmental protection, it requires 100% reuse of fracturing fluid. The exploitation of “green”cleanup addictive with high flow back rate, excellent resistance, and low damage to formation under high salinity to meet the requirements of on-site fracturing construction will be the focus of future research and will also have broad application prospects.
References
- Kazmerski L (2016) Renewable and Sustainable Energy Reviews. Renew Sust Energ Rev 38: 834-847.
- Zhang GQ, Chen M (2010) Dynamic fracture propagation in hydraulic re-fracturing. J Petrol Sci Eng 70: 266-272.
- Carpenter C (2016) The Role of Induced Unpropped Fractures in Unconventional Oil and Gas Wells. J Petrol Technol 68: 58-65.
- Le DH, Hai NH, Mahadevan J (2012) Gas Recovery From Tight Sands: Impact of Capillarity. Spe J 17: 981-991.
- Longoria RA, Liang T, Huynh UT (2017) Water Blocks in Tight Formations: The Role of Matrix/Fracture Interaction in Hydrocarbon-Permeability Reduction and Its Implications in the Use of Enhanced Oil Recovery Techniques. Spe J 22: 9.
- Sakhaei Z, Mohamadi-Baghmolaei M, Azin R (2017) Study of production enhancement through wettability alteration in a super-giant gas-condensate reservoir. J Mol Liq 233: 64-74.
- Fan L, Harris BW, Jamaluddin AJ (2005) Understanding gas-condensate reservoirs. Oilfield Rev. 10: 16-25.
- Franco CA, Romero RZ, Arango JZ (2012) Inhibited Gas Stimulation To Mitigate Condensate Banking and Maximize Recovery in Cupiagua Field. Spe Prod Oper 28: 154-167.
- Vattuone L, Gambardella P, CemiÄ F (2007) Field Studies of Drilling and Completion Fluids to Minimize Damage and Enhance Gas Production in Unconventional Reservoirs, European Formation Damage Conference. Society of Petroleum Engineers pp: 2490-2502.
- Santanna VC, Curbelo FDS, Castro TN (2009) Microemulsion flooding for enhanced oil recovery. J Petrol Sci Eng 66: 117-120.
- Rui-Dong LI, Wang DM, Zhang ZY (2013) Studies on Surfactant Enhanced Oil Recovery for Low Permeability Oilfield. Oilfield Chem 30: 221-225.
- Liu D, Wang Q, Wei J (218) Experimental study on drag reduction performance of mixed polymer and surfactant solutions. Chem Eng Res Des.
- Baruah A, Pathak AK, Ojha K (2016) Study on rheology and thermal stability of mixed (nonionic–anionic) surfactant based fracturing fluids. Aiche J 62: 2177-2187.
- Guo DH, Cui XD, Yang XP (2017) Advances in surfactant research for oil and gas production. Fine. Specialty Chem.
- Stringfellow WT, Domen JK, Camarillo MK (2014) Physical, chemical, and biological characteristics of compounds used in hydraulic fracturing. J Hazard Marter 275: 37-54.
- Dai C, Wang K, Liu Y (2014) Study on the Reutilization of Clear Fracturing Flowback Fluids in Surfactant Flooding with Additives for Enhanced Oil Recovery (EOR). Plos One 9: e113723.
- Kamal MS, Hussein IA, Sultan AS (2017) Review on Surfactant Flooding: Phase Behavior, Retention, IFT, and Field Applications. Energy Fuel 31: 7701-7720.
- Raffa P, Broekhuis AA, Picchioni F (2016) Polymeric Surfactants for Enhanced Oil Recovery: a Review. J Petrol Sci Eng 145: 723-733.
- Kamal MS (2016) A Review of Gemini Surfactants: Potential Application in Enhanced Oil Recovery. J Surfactants Deterg 19: 223-236.
- Golabi E (2014) The investigation of the Anionic and Cationic Surfactants effects on the enhanced oil recovery in Iran oil reservoir. Int J Chemical Stud 2: 63-71.
- Pons-Jiménez M, Cartas-Rosado R, MartÃnez-Magadán JM (2014) Theoretical and experimental insights on the true impact of C 12 TAC, cationic surfactant in enhanced oil recovery for heavy oil carbonate reservoirs. Colloid Surface A 455: 76-91.
- Ahmadi MA, Zendehboudi S, Shafiei A (2012) Nonionic Surfactant for Enhanced Oil Recovery from Carbonates: Adsorption Kinetics and Equilibrium. Ind Eng Chem Res 51: 98940-9905.
- Kumar A, Mandal A (2017) Synthesis and physiochemical characterization of zwitterionic surfactant for application in enhanced oil recovery. J Mol Liq 243.
- Pal N, Saxena N, Laxmi KVD (2018) Interfacial behaviour, wettability alteration and emulsification characteristics of a novel surfactant: Implications for enhanced oil recovery. Chem Eng Sci 187: 200-212.
- Popova MV, Raev DL (2018) Aggregation Behavior of Monomeric Surfactants and a Gemini Cationic Surfactant by NMR and Computer Simulation Data. Appl Magn Reson 2: 1-12.
- And SB, Chanda J (2003) Monolayer of Monododecyl Diethylene Glycol Surfactants Adsorbed at the Air/Water Interface: A Molecular Dynamics Study. Langmuir 19: 10443-10448.
- Pang J, Wang Y, Xu G (2011) Molecular dynamics simulations of SDS, DTAB, and C12E8 monolayers adsorbed at the air/water surface in the presence of DSEP. J Phys Chem B 115: 2518.
- Truszkowski A, Epple M, Fiethen A (2014) Molecular fragment dynamics study on the water-air interface behavior of non-ionic polyoxyethylene alkyl ether surfactants. J Colloid Interface Sci 6: 140-145.
- Liang T, Achour SH, Longoria RA (2017) Flow physics of how surfactants can reduce water blocking caused by hydraulic fracturing in low permeability reservoirs. J Petrol Sci Eng 157.
- Alâ€Amodi AO, Alâ€Mubaiyedh UA, Sultan AS (2016) Novel fluorinated surfactants for enhanced oil recovery in carbonate reservoirs. Can J Chem Eng 94: 454-460.
- Wang F (2007) Experimental Investigation on Synthesis and Surfactivity of the Silicofluoride Surfactant, Dyestuffs & Coloration.
- Tehranibagha AR, Holmberg K (2010) Cationic Ester-Containing Gemini Surfactants: Physical−Chemical Properties, Langmuir 26: 9276-82.
- Kamboj R, Singh S, Bhadani A (2012) Gemini Imidazolium Surfactants: Synthesis and Their Biophysiochemical Study. Langmuir 28: 11969-78.
- Wang S, Zhao K (2016) Dielectric analysis for the spherical and rodlike micelle aggregates formed from Gemini surfactant: Driving forces of micellization and stability of micelle. Langmuir 32: 7530-7540.
- Javadian S, Aghdastinat H, Tehrani-Bagha A (2013) Self-assembled nano structures of cationic ester-containing gemini surfactants: The surfactant structure and salt effects. J Chem Thermodyn 62: 201-210.
- Nguyen CV, Nguyen TV, Chi MP (2015) Dynamic adsorption of a gemini surfactant at the air/water interface. Colloid Surface A 482: 365-370.
- Raffa P, Wever DA, Picchioni F (2015) Polymeric Surfactants: Synthesis, Properties, and Links to Applications. Chem Rev 115: 8504.
- Zou C, Wu H, Ma L (2010) Preparation and application of a series of novel anionic acrylamide polymers with cyclodextrin sides. J Appl Polym Sci 119: 953-961.
- Raffa P, Broekhuis AA, Picchioni F (2016) Polymeric Surfactants for Enhanced Oil Recovery: a Review. J Petrol Sci Eng 145: 723-733.
- Liu X (2010) Preparation and Laboratory Evaluation of Fracturing Fluid Clean-up Additive GCY-3.Adv Fine Pe.
- Xiaohong C,Yan J, Yang C (2010) Mechanism of the Mixed Surfactant Micelle Formation. J Phys Chem 114: 7808-7816.
- Da SM, Weinzaepfel E, Afifi H (2013) Tuning the viscoelasticity of nonionic wormlike micelles with β-cyclodextrin derivatives: a highly discriminative process. Langmuir 29: 7697-708.
- Lu H, Yuan M, Fang B (2015) Wormlike Micelles in Mixed Amino Acidâ€Based Anionic Surfactant and Zwitterionic Surfactant Systems. J Surfactants Deterg 18: 589-596.
- Baruah A, Shekhawat DS, Pathak AK (2016) Experimental investigation of rheological properties in zwitterionic-anionic mixed-surfactant based fracturing fluids. J Petrol Sci Eng 146: 340-349.
- Vanin AA, Brodskaya EN (2017) Molecular-dynamics simulation of the surface layer of a nonionic micelle. Colloid J 79: 310-316.
- Wang L, Liu R, Hu Y (2016) Adsorption of mixed DDA/NaOL surfactants at the air/water interface by molecular dynamics simulations. Chem Eng Sci 155: 167-174.
- Park S, Lee ES, Wan RWS (2015)Adsorption behaviour of surfactants for chemical flooding in enhanced oil recovery. J Ind Eng Chem 21: 1239-1245.
- Mandal A, Kar S (2016) A thermodynamic assessment of micellization for a mixture of sodium dodecyl benzene sulfonate and Tween 80 surfactants for ultralow interfacial tension. Fluid Phase Equilibria 408: 212-222.
- Jayhooni SMH, Mirvakili A, Rahimpour MR (2012) Nanofluid concept for enhancement of hydrogen utilization and gasoline production in fixed bed reactor Fischer–Tropsch synthesis of GTL (gas to liquid) technology. J Nat Gas Sci Eng 9: 172-183.
- Shamsijazeyi H, Miller CA, Wong MS (2014) Polymerâ€coated nanoparticles for enhanced oil recovery. J Appl Polym Sci 131: 4401-4404.
- Sharma T, Kumar GS, Sangwai JS (2015) Comparative effectiveness of production performance of Pickering emulsion stabilized by nanoparticle–surfactant–polymerover surfactant–polymer (SP) flooding for enhanced oil recoveryfor Brownfield reservoir. J Petrol Sci Eng 129: 221-232.
- Luo M, Sun T, Lü Z (2016) Preparation and performance evaluation of nanoemulsions for water control fracturing in tight gas formations. J China U Petrol.
- Zargartalebi M, Kharrat R, Barati N (2015) Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 143: 21-27.
- And LD, Johnson D (2003) Surface Tension of Charge-Stabilized Colloidal Suspensions at the Water−Air Interface. Langmuir 19: 10205-10209.
- Jin J, Li X, Geng J, Jing D (2018) Insights into the complex interaction between hydrophilic nanoparticles and ionic surfactants at the liquid/air interface. Phys Chem 20: 15223-235.
- Luo M, Liu J, Wen Q, Liu H, Jia Z (2011) Fracturing Clean-up Effectiveness Improved by Environment-Friendly MES Middle Phase Microemulsion. Acta Petrolei Sinica 27: 454-460.
- Kim JT, Kim CA, Zhang K, Jang CH, Choi HJ (2011) Effect of polymer–surfactant interaction on its turbulent drag reduction. Colloid Surface A 391: 125-129.
- Sharma T, Sangwai JS (2017) Silica nanofluids in polyacrylamide with and without surfactant: Viscosity, surface tension, and interfacial tension with liquid paraffin. J Petrol Sci Eng 152: 575-585.
- Wang H, Zhang H, Yuan S, Xu, Zhen X, Liu C (2014) Molecular dynamics study of the structure of an oppositely charged polyelectrolyte and an ionic surfactant at the air/water interface. Colloid Surface A 454: 104-112.
- Asgari A, Dianatirad M, Ranjbaran M, Sadeghi AR, Rahimpour MR (2014) Methanol treatment in gas condensate reservoirs: A modeling and experimental study. Chem Eng Res Des 92: 876-890.
- Jiang J, Younis RM (2016) Compositional modeling of enhanced hydrocarbons recovery for fractured shale gas-condensate reservoirs with the effects of capillary pressure and multicomponent mechanisms. J Nat Gas Sci Eng 34: 1262-1275.
- Hassanzadehkhadar R, Aminshahidy B, Hashemi A, Ghadami N (2013) Application of Gas Injection and Recycling to Enhance Condensate Recovery. Liq Fuel Technol 31: 1057-1065.
- Mahdiyar H, Jamiolahmady M (2014) Optimization of hydraulic fracture geometry in gas condensate reservoirs. Fuel 119: 27-37.
- Mirchi V, Saraji S, Goual L, Piri M (2015) Dynamic interfacial tension and wettability of shale in the presence of surfactants at reservoir conditions. Fuel 148: 127-138.
- Aguirre H, Bonilla R (2004) Chemical Stimulation Experience on a Naturally Fractured Gas Conden sate Reservoir-Field Case[C]Abu Dhabi International Conference and Exhibition. Society of Petr oleum Engineers.
- Sheydaeemehr M, Sedaeesola B, Vatani A (2014) Gas-condensate production improvement using we ttability alteration: a giant gas condensate field case study. J Nat Gas Sci Eng 21: 201-208.
- Chen H, Tang T, Amirfazli A (2012) Fabrication of polymeric surfaces with similar contact angles but dissimilar contact angle hysteresis. Colloid Surface A 408: 17-21.
- NVM, Birjandi FC, Sargolzaei J (2014) Super-non-wettable surfaces: A review. Colloid Surface A 448: 93-106.
- Yin Y, Ning G, Wang C, Rao Q (2014) Alterable Superhydrophobic–Superhydrophilic Wettability of Fabric Substrates Decorated with Ion–TiO2 Coating via Ultraviolet Radiation. Ind Eng Chem Res 53: 14322-14328.
- Cappelletti G, Ardizzone S, Meroni D, Soliveri G, Ceotto M (2013) Wettability of bare and fluorinated silanes: a combined approach based on surface free energy evaluations and dipole moment calculations. J Colloid Interface Sci 389: 284-291.
- Ogihara H, Jing X, Saji T (2015) Controlling surface energy of glass substrates to prepare superhydrophobic and transparent films from silica nanoparticle suspensions. J Colloid Interface Sci 437: 24-27.
- Ma L , Li L, Zhao X, Wang Y (2015) Influence of Wettability Alteration to Preferential Gas-Wetting on Displacement Efficiency at Elevated Temperatures. J Disper Sci Technol 36: 1274-1281.
- Sharifzadeh S, Hassanajili S, Rahimpour MR (2013) Wettability alteration of gas condensate reservoir rocks to gas wetness by sol–gel process using fluoroalkylsilane. J Appl Polym Sci 28: 4077-4085.
- Fahimpour J, Jamiolahmady M (2015) Optimization of fluorinated wettability modifiers for gas/cond ensate carbonate reservoirs. Spe J 20: 729-742.
- Li K, Jing X, He S, Wei B (2016) Static Adsorption and Retention of Viscoelastic Surfactant in Porous Media: EOR Implication. Energy Fuel 30: 9089-9096.
- Franco CA, Zabala R, Cortes FB (2017) Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J Petrol Sci Eng 157: 39-55.
- Jin J, Wang Y, Wang K, Ren J,Bai B et al. (2016) The effect of fluorosurfactant-modified nano-silica on the gas-wetting alteration of sandstone in a CH 4 -liquid-core system. Fuel 178: 163-171.
- Jin J, Wang Y, Nguyen TAH, Nguyen AV, Wei M et al. (2017) The effect of gas-wetting nano-particle on the fluid flowing behavior in porous media. Fuel 196: 431-441.
- Franco-Aguirre M, Zabala R, Lopera SH, Franco CA, Cortés FB (2018) Interaction of anionic surfactant-nanoparticles for gas - Wettability alteration of sandstone in tight gas-condensate reservoirs. J Nat Gas Sci Eng 51: 53-64.
- Wang H, Qiu X, Zhai W (2011) Functioning mechanism of drag reducer used in shale reservoir fracturing. J Xiangtan U 19: 263-270.
- Guo-Yan MA, Shen YD, Kai LI (2016) Performance of Polymer Drag Reducing Agent for Slick-water Fracturing. Fine Chem.
- Wei J, Huang C, Na XU (2016) Research progress concerning turbulent drag reduction of surfactant solution. Chem Ind Eng Prog.
- Lin Z, Zheng Y, Talmon Y, Maxson A, Zakin JL (2016) Comparison of the effects of methyl- and chloro-substituted salicylate counterions on drag reduction and rheological behavior and micellar formation of a cationic surfactant. Rheol Acta 55: 117-123.
- Yu B, Li F, Kawaguchi Y (2004) Numerical and experimental investigation of turbulent characteristics in a drag-reducing flow with surfactant additives. Int J Heat Fluid Fl 25: 961-974.
- Tuan NA, Mizunuma H (2013) High-shear drag reduction of surfactant solutions. J Non-Newton Fluid 198: 71-77.
- Peng CS, Mei LD (2006) Behavior of Drag Reduction for CTAB Surfactant Aqueous Solution. J Pe U 19: 68-71.
- Hadri F, Besq A, Guillou S, Makhloufi R (2011) Temperature and concentration influence on drag reduction of very low concentrated CTAC/NaSal aqueous solution in turbulent pipe flow. J Non-Newton Fluid 166: 326-331.
- Kai DU, Huang FX, Zhuo YI, WenLong Z (2014) Recent advances on friction reducer for slickwater fracturing of shale gas reservoirs. Scientia Sinica Chimica 44: 1696-1704.
- Zhang H, Wang D, Weiguo GU (2009) Progress of surfactant turbulent drag reduction. Chem Ind Eng Prog 28: 1701-1700.
- Nabhani N, Emami M, Moghadam ABT (2011) Application of Nanotechnology and Nanomaterials in Oil and Gas Industry.American Institute of Physics 2011: 128-131.
- Maxey J, Crews J, Huang T (2008) Nanoparticle Associated Surfactant Micellar Fluids.American Institute of Physics 1027: 857-859.
- Matras Z, Malcher T, Gzyl-Malcher B (2008) The influence of polymer surfactant aggregates on drag reduction. Thin Solid Films. 516: 8848-8851.
- Malcher T, Gzylmalcher B (2012) Influence of polymer-surfactant aggregates on fluid flow. Bioelectrochemistry 87: 42-49.
Citation: Chai Y, Li X, Jing D (2019) Application of Surfactants in Hydraulic Fracturing for Enhanced Oil/Gas Recovery. Oil Gas Res 4: 161. DOI: 10.4172/2472-0518.1000161
Copyright: © 2019 Chai Y et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7363
- [From(publication date): 0-2019 - Oct 22, 2025]
- Breakdown by view type
- HTML page views: 6316
- PDF downloads: 1047