Research Article Open Access
Application of Microbial Culture and Rhamnolipid for Improving the Sedimentation of Oil Sand Tailings
Soroor Javan Roshtkhari and Catherine N Mulligan*Civil and Environmental Engineering, Concordia University, Montreal, Québec, Canada
- *Corresponding Author:
- Catherine N Mulligan
Civil and Environmental Engineering
Concordia University, Montreal, Québec, Canada
Tel: 5148482424 extn. 7925
E-mail: mulligan@civil.concordia.ca
Received date: April 13, 2016; Accepted date: June 30, 2016; Published date: July 01, 2016
Citation: Roshtkhari SJ, Mulligan CN(2016) Application of Microbial Culture and Rhamnolipid for Improving the Sedimentation of Oil Sand Tailings. J Bioremed Biodeg 7:358. doi:10.4172/2155-6199.1000358
Copyright: © 2016 Roshtkhari SJ, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Densification of oil sand tailings deposited in the tailing ponds and recovering water from them are two major challenges in the oil sands surface mining industry. A small increase in the tailings settlement rate (which normally is very slow) can improve the densification of tailings and significantly reduce water consumption and the volume of the tailing ponds. In this work, the objective was to evaluate the role of a mixed culture of two microbial strains isolated from weathered oil and rhamnolipid (JBR 425) together with these strains in the sedimentation of fine tailing particles. It has been found that a mixed culture of two microbial strains isolated from weathered oil increased the sedimentation. Rhamnolipid (0.5%) together with these two microbial strains at 15°C ± 2°C showed significant increases in sedimentation (by a factor of 5.1), the concentration of larger particles (by a factor of 2.63), the particle mean diameter (by a factor of 2.70) and flocculation in the tailings samples compared to the control while the zeta potential is still negative. This means that the mechanism of flocculation is probably due to increasing the hydrophobicity of the particles, interaction of biosurfactant and high molecular weight microbial organic compounds through a bridging mechanism with clay particles. This work shows the potential of using rhamnolipid and microbial culture in order to increase the oil sand sedimentation through flocculation and microbial activity in a more environmentally friendly densification process.
Keywords
Biosurfactant; Rhamnolipid; Sedimentation; Oil sands tailings; Microbial culture
Introduction
"Tails" or "tailings" are the by-products from the extraction of bitumen from the sand by surface mining method which are pumped into tailings ponds for storage. This tailing suspension is a mixture of process affected water, sand, clays, salts, metals, residual bitumen and hydrocarbon diluents [1]. In the pond the toxic water forms the top layer which can be recycled into the extraction process [2,3]. Coarse sand grains (larger than 44 microns [4,5]) settle out quickly. TFT will settle and within two or three years a layer of mature fine tailings (MFT) develops which is a mixture of fine clay particles (under 44 microns in size) and water, with approximately 30%-60% solids and has a consistency similar to yogurt. Complete settling of MFT is very slow [2,3,6] (almost a century). Now more than 170 km2 of tailings ponds exist in Alberta. Toxic impacts of tailing ponds can affect ecosystem and human health [1]. An increase in tailings settlement rate and remediation of tailing ponds can increase the efficiency of water recycling and reduce the volume of tailings ponds and their environmental impacts. There are natural, physical or chemical/biological methods in order to treat tailings ponds and increase sedimentation. In chemical/ biological methods densification can be achieved by addition of agents such as calcium sulfate (gypsum), and some microbial activity such as methanogenesis [2,7]. Currently most of the industrial methods for oil sand tailings densification are based on clay particle flocculation by addition of polymeric flocculants [5,8,9]. Biological methane formation (methanogenesis) by anaerobic microbes as a low cost technology can improve tailings densification [10-13]. The rising of gas bubbles in the tailings can produce channels which make it easy for water to drain due to excess pore pressures within the tailings [14]. It has been reported that microbial cells and exopolysaccharides or extracellular polymeric substances (EPS) can improve the clay aggregation and flocculation [15]. EPS are compounds secreted by microorganisms into their environment. These compounds are important in biofilm formation and cell attachment to surfaces. There are also interactions between soil bacteria and clay particles which can form clay aggregates with the appearance of 'hutches' housing the bacteria [16]. There are different classes of microorganisms growing in the tailings ponds that contribute to increased tailings aggregation and sedimentation. In addition to methanogen bacteria, sulfate-reducing bacteria (SRB) and nitrate-reducing bacteria (NRB) have also been found in tailing ponds environment [17,18]. The tailings pond microbial communities are dynamic and change rapidly when the input of fresh tailings and/ or gypsum is stopped [19]. Bordenave et al. observed that microbial cells can absorb the clay particles on their surfaces and within the EPS, causing the aggregation of fine particles [7]. In this way the tailings sedimentation under gravity will increase [7]. According to their report, the active cultures of M. barkeri (methanogen) or Thauera sp. strain N2 (NRB) show strong bonds to clay particles and an increase in sedimentation. Nitrate addition also can reduce methane production by methanogenic bacteria. These observations show the potential for increasing tailing sedimentation by using microbial biomass to aggregate and reduce methane production by in situ addition of nitrate [7]. Addition of calcium ions in the form of Ca(NO3)2 and lactate to tailing pond samples can increase the densification rate by 15% (v/v). Lactate significantly boosted microbial activity with increased methanogenesis, sulfate reduction or nitrate reduction [20]. The sulfide emissions by SRB activity in the gypsum treated pond are also limited as they are highly soluble and will oxidize in surface waters which suggests that the production of hydrogen sulfide might be a self-limiting process, which will begin to decrease after a period of time [13,21,22]. The performance of the flocculants is an important issue in flocculation based methods. However the recycle water quality, start up and operational costs, experienced operators and careful operational control, in some cases should be considered [5]. In the microbial activity and methanogenesis method, CH4 and CO2 (greenhouse gases) emissions (up to 104 m3 day– 1 for methane) from tailings ponds should be controlled [12,13]. It has been reported that synthetic surface active agents (surfactants) showed the ability for dewatering of slurries when they are combined with polymers as flocculants. They can change surface wetting characteristics of particles and lead to an increase in flocculation and dewatering [23-26]. Surfactants can reduce surface and interface tension by forming molecular film at the interface of air and water or two liquid phases (i.e., oil/water). There are some surfactants which are produced by living natural sources and known as biosurfactants [27,28]. Biosurfactants (such as rhamnolipids (RLs) which is the most intensively studied biosurfactants) have more advantages over synthetic surfactants such as low or non-toxicity and biodegradability. They are also more economic than the other surface active agents in some cases due to efficiency and have potential to decrease the environmental impacts of oil sands [28-34]. In this work the main objective is to evaluate the use of microorganisms (by inoculation or naturally present) together with rhamnolipid biosurfactant to enhance the sedimentation in tailing ponds, and understanding the mechanism of sedimentation within this approach. In this way sedimentation will increase through flocculation and microbial activity without producing large amounts of CH4 and having the limitations of other methods while taking advantage of the biosurfactants for remaining water and sediment bioremediation.
Materials and Methods
Origin of the oil sand tailings
The tailings pond sample was provided by Maria Demeter (Lab Manager/Environmental Engineering Technologist), Civil & Environmental Engineering Department, University of Alberta. It is a by product of the extraction of bitumen from oil sand which was prepared by Industrial Hygiene. Tailings samples were provided in 20 L plastic pail and stored at room temperature. It is comprised of bitumen (1-2 wt%), naphtha (<0.1 wt%), clay (30-60 wt%), and water with the pH in the range of 7.3-7.8. The clay content of 30-60 wt% shows that the samples are taken from mature fine tailing layer from the depth below 10 m of the tailing pond [35].
Rhamnolipid
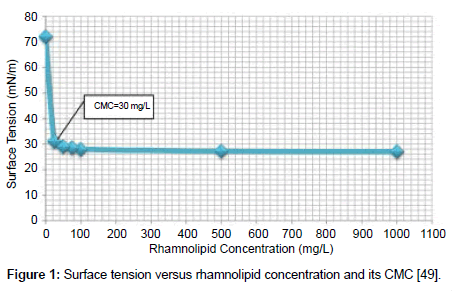
Rhamnolipid biosurfactant (JBR 425 from Jeneil Biosurfactant Co., USA) was used to investigate its effect on oil sands tailings. It is a mixture of two forms of rhamnolipid, at 25 wt% in water, with the CMC value of 30 mg/l at the lowest surface tension of 28 mN/m [36-38].
Microbial cultures
A Bacillus subtilis strain and cultures of two microbial strains isolated (by growing on R2A nutrient agar medium (Sigma-Aldrich, for microbiology) and Bushnell Hass medium by one of my colleagues in the lab for her own research) from weathered oil (including light crude oil, diesel and biodiesel/B 100) were used for this study [39]. Characterization of the natural microbial communities was conducted by pyrosequencing of 16S rRNA. Characterization of bacteria isolated from the BD, D and L oil by 16S rRNA pyrosequencing showed that the Firmicutes was the dominant phylum in biodiesel (100%) and diesel (53%). The Actinobacteria was dominant in the diesel (47%) and the Proteobacteria (97%) and Actinobacteria (3%) were the two dominant phyla in the light crude oil [39]. The strains used in this study belong to Firmicutes and Proteobacteriaphyla and were identified as orders of Bacillales and Sphingomonadales [39]. The microbial cultures were grown aerobically in 25 ml of medium containing mineral salts of nitrogen (sodium nitrate) and phosphorus (monobasic and dibasic potassium phosphates) at C:N:P ratio of 100:10:1 [40], at 37°C for 24 hours without shaking [41].
Experimental approach
The effect of rhamnolipids and microbial cultures on sedimentation was evaluated through sedimentation experiments and the feasibility of biosurfactant production was evaluated through batch experiments.
Sedimentation experiments: The tailings densification process includes consolidation and sedimentation processes near the bottom and top of a tailings column, respectively [42]. The sedimentation process can be easily monitored as the downward movement of the boundary between clear liquid and suspended tailings. Its rate of movement, the ‘‘hindered settling velocity” [42], is orders of magnitude smaller than the Stokes’ single particle settling velocity (35 cm/day for a 2 micron diameter particle with a density of 2.65 g/cm3 [7]; tailings sedimentation rates in the experiments (about 0.1 cm/day) were much smaller than this). In this way, the sedimentation (S) was determined according to the following equation [7]: where h is the position of the boundary and H is the total height of the liquid column [7]. Each test was repeated three times (in triplicate) and the average data are reported.
S(%)=1-h/H
Sedimentation experiments by microbial cultures at 23°C ± 2°C: These experiments were performed with 13-15 g of tailings diluted in 5 mL of deionized water in 20 mL glass tubes (15 cm) closed with a compressed layer of paper towel in order to prevent liquid evaporation. However there are still small amounts of liquid lost due to evaporation but it ressembles the aerobic conditions at the surface of actual tailing ponds. Sedimentation experiments were performed at room temperature (23°C ± 2°C). Sedimentation experiments were also performed with diluted tailing pond samples inoculated with cultures of the biosurfactant producer Bacillus subtilis strain, and a mixed culture of two microbial strains isolated from weathered oil. Five mL of microbial culture were added to the diluted tailing pond samples. Sedimentation tubes with diluted tailings samples and 5 mL of deionized water served as the control. pH was adjusted to 8 by adding 0.1N NaOH. Six homogenized sedimentation tubes were incubated at room temperature (23°C ± 2°C) and the measurement of the sedimentation in unshaken tubes was performed every 10 days for a period of 50 days.
Sedimentation experiments by rhamnolipid and microbial cultures at 15°C ± 2°C: These experiments were performed in four 500 mL glass columns. 100 ml rhamnolipid at 0.5% concentrations, 100 ml of deionized water and 10 ml of mixed culture of two microbial strains isolated from weathered oil (grown previously aerobically in 25 ml of NB medium) were added to 200 g of oil sand tailing pond samples (three columns). A sedimentation column with 200 g of tailings samples and 210 mL of deionized water served as the control. The columns were covered with a paper towel in order to prevent liquid evaporation. The pH of the samples were measured at the starting time. Homogenized sedimentation columns were incubated at 15°C ± 2°C and measurement of the sedimentation in unshaken columns was performed every 10 days over a period of 50 days.
Feasibility of in situ biosurfactant production: The feasibility of biosurfactant production by the indigenous microorganisms of oil sand tailings pond, the biosurfactant producer Bacillus subtilis strain, and two microbial strains isolated from weathered oil was evaluated through batch experiments.
Indigenous microorganisms of oil sand tailings pond: In this set of experiments there are two batches: control batches and nutrient amended batches. The control batches are tailings and deionized water only, and were performed to evaluate the natural ability of tailings to produce biosurfactants. The nutrient amended batches were performed to improve the natural biosurfactant production in tailings. In this approach mineral salts of nitrogen (sodium nitrate) and phosphorus (monobasic and dibasic potassium phosphates) were added to the tailings to attain a C:N:P ratio of 100:10:1 [40,43]. A buffer solution was added to maintain a constant pH in accordance with that of the control batches. The concentration of added phosphorus was doubled to account for its precipitation [43,44]. For each batch, approximately 20 g of tailings and 100 mL of deionized water were placed in a 250 mL Erlenmeyer flask. Foam stoppers were used to fit the flasks top in order to prevent the entry of microorganisms and dust into the samples while allowing aeration. Flasks and media were sterilized by autoclaving at 121°C for 20 min before each experiment run. Aeration was achieved by rotating the flasks at 200 rpm on an orbital shaker (Thermolyne AROS 160) for 50 days. Samples covering days 0, 5, 15, 30, 45 and 50 were taken to measure the surface tension. All tests were performed in triplicate.
Bacillus subtilis strain and two microbial strains isolated from weathered oil: In this set of experiments, there were two batches: tailings and deionized water batches inoculated with 5 mL of microbial culture of biosurfactant producer Bacillus subtilis strain and tailings and deionized water batches inoculated with 5 mL of two microbial strains isolated from weathered oil. For each batch, approximately 20 g of tailings and 100 mL of deionized water were placed in a 250 mL Erlenmeyer flask. Foam stoppers were used to fit the flasks top in order to prevent the entry of microorganisms and dust into the samples while allowing aeration. Flasks and media were sterilized by autoclaving at 121°C for 20 min before each experiment run. Aeration was achieved by rotating the flasks at 200 rpm on an orbital shaker (Thermolyne AROS 160) for 50 days. Samples on days 0, 5, 15, 30, 45 and 50 were taken to measure the surface tension. All tests were performed in triplicate.
Analytical methods
Settled tailings and tailings process water from each set of experiments were separated using a pipette in order to perform analysis on them.
Settled tailings
Particle size distribution: Fines are defined as mineral particles smaller than 44 μm. The fines to solids ratio was the weight percent of fines in the whole solid mass. The size distribution of tailings was measured using a Horiba model LA-950V2 laser scattering particle size analyzer which uses Mie Scattering (laser diffraction) to measure particle size in the span of 0.01-3000 μm [45]. This equipment has a standard centrifugal pump to suspend and circulate the particles in the cell. Both the circulation and the agitation speeds were adjusted to 5. The in-built 130 watt ultrasonic probe delivers highest dispersing capability during the measurements. Both the ultrasonic power and the ultrasonic times were adjusted to 5. Dried tailings samples from all parts of the sediment column (Rock crystal with refractive index (R): 1.540) were dispersed in deionized water (water with refractive iIndex (R): 1.333) before introducing them into the particle size analyzer.
Zeta potential measurement: The zeta potential (or electrophoresis mobility) of the oil sands tailings particles in a diluted suspension (after using rhamnolipid and/or microbial cultures, and before it) was measured by Zeta-Meter System 3.0+ (USA). It works based on energizing the electrodes and watching and tracking one of the colloid particles (which are placed in a viewing chamber called an electrophoresis cell) as it moves across a grid in the microscope. The cell was filled with the sample (about 20 ml). Electrodes were inserted and were connected to the Zeta-Meter 3.0+ unit. The specific conductance of the sample was determined and according to it the appropriate voltage would be selected. The electrodes were energized and moving colloids across a grid were watched in the microscope. One moving colloid was tracked by simply pressing a button and holding it down while the colloid moves across the grid. When the button was released, the colloid’s zeta potential (or electrophoretic mobility) was instantly displayed [46]. Fifteen measurements were done for each sample and the average value is presented.
Tailings process water
Surface tension measurement: The surface tension of the filtered supernatant was measured using a Fisher Scientific ensiometer. This device works based on the ASTM method D1331-89 [47] which employs the du Noüy ring method for direct results with no calculations [48]. The accuracy of this method is ± 0.25 mN/m. The device was calibrated as instructed by the manufacturer and its accuracy checked by measuring the surface tension of deionized water at room temperature and comparing it to the 72 mN/m which is reported in the literature [28]. The reduction in surface tension is related to the biosurfactant concentration below the defined critical micelle concentration (CMC). The CMC can be used as an indicator for biosurfactant production levels. Above the CMC the surface tension does not change with rhamnolipid concentration. The concentration in this range was determined by serial dilution which brings the concentration below the CMC. Figure 1 shows the surface tension versus rhamnolipid concentration and its CMC [49]. Below the CMC the concentration was determined according to Figure 1 which was obtained by Ref. [38].
Results and Discussion
Results of sedimentation experiments
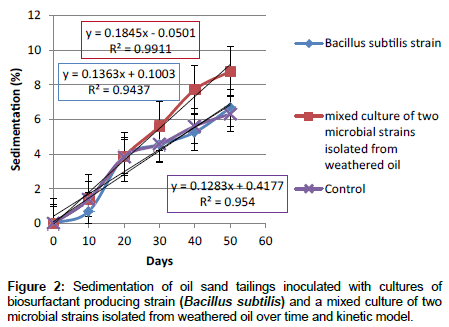
Role of microbial cultures in tailings sedimentation (at 23°C ± 2°C): The role of microbial cultures to increase sedimentation was analyzed by comparing sedimentation of diluted tailing pond samples inoculated with cultures of the biosurfactant producer Bacillus subtilis strain, and a mixed culture of two microbial strains isolated from weathered oil (Figure 2). The presence of a mixed culture of two microbial strains isolated from weathered oil increased the sedimentation compared to the control. However, the sedimentation tubes inoculated with the Bacillus subtilis strain gave almost the same sedimentation amount as the control. Results for kinetics of sedimentation suggest a linear kinetics for sedimentation of oil sand tailings inoculated with cultures of the biosurfactant producing Bacillus subtilis strain, and a mixed culture of two microbial strains isolated from weathered oil (Figure 2).
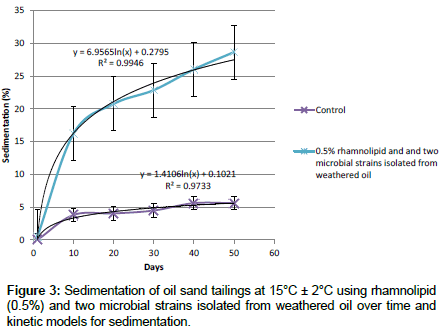
Role of mixture of rhamnolipid and microbial cultures in tailings sedimentation at (15°C ± 2°C): Sedimentation of tailings amended with the rhamnolipid (0.5%) and two microbial strains isolated from weathered oil were compared to the control at 15°C ± 2°C in order to evaluate the role of lower temperature in sedimentation of tailing pond samples (Figure 3). All of these show an increase in sedimentation compared to the control. It means that microbial cultures together with rhamnolipid can significantly increase the sedimentation of tailings compared to the amount of sedimentation of tailings amended only with rhamnolipid even at lower temperature. Results for kinetics of sedimentation suggest a logarithmic kinetics for sedimentation (Figure 3). Table 1 summarizes the results of kinetic rates for sedimentation for different samples. The predicted results are very close to the experimental data.
| Samples | Kinetic rate (sedimentation%/day) | Final sedimentation (%) | ||
|---|---|---|---|---|
| Experimental | Predicted | |||
| 23 ºC±2ºC | Bacillus subtilisstrain | 0.1363 | 6.67 | 6.92 |
| mixed culture of two microbial strains isolated from weathered oil | 0.1845 | 8.77 | 9.17 | |
| 15ºC±2ºC | 0.5% rhamnolipid and two strains isolated from weathered oil | 6.9565 | 28.67 | 26.94 |
Table 1: Results of kinetic model for sedimentation for different samples over a 50 day period.
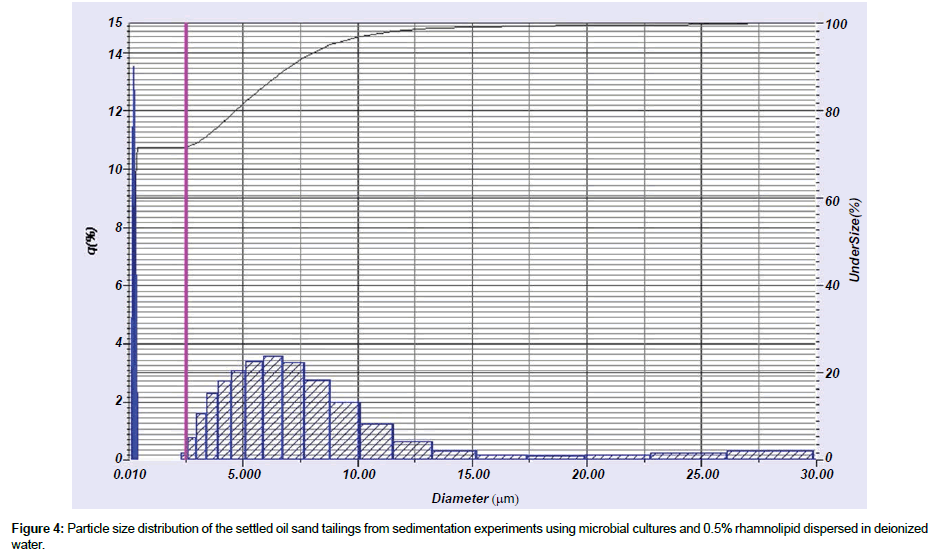
Particle size distribution: Using the particle size analyzer, the particle aggregation and flocculation were evaluated. Dried settling tailings samples from sedimentation experiments using microbial cultures and 0.5% rhamnolipid were dispersed in deionized water. Figure 4 shows the particle size distribution using 0.5% rhamnolipid and microbial cultures as the sedimentation agents at 15°C ± 2°C. The measured particle diameter based on cumulative% (90%) and the mean diameters are respectively 7.03 μm and 2.11 μm. These values are actually the diameters of the flocculation of tailings particles. Compared to the measured particle diameter based on cumulative% (90%) (2.67 μm) and particle mean diameter (0.78 μm) of control experiments which were simply composed of deionized water and tailings without any sedimentation agents, one can conclude that rhamnolipid and microbial cultures mixed with oil sand tailings can improve effectively the aggregation and flocculation of tailings particles in this case. It seems that lowering the temperature did not result in a significant change in measured particle diameter based on cumulative% (90%) and particle mean diameter and flocculation compared to the result of particle size distribution at room temperature. The results at lower temperature showed the strong potential of using rhamnolipid and microbial culture according to the temperature at the site.
Zeta potential measurement: The results of zeta potential measurement of dried settling tailings samples at 15°C ± 2°C (gained from sedimentation experiments using microbial cultures and 5% rhamnolipid) resuspended in deionized water showed the amount of -52.2 mV for microbial cultured amended samples and -42.2 mV for control.
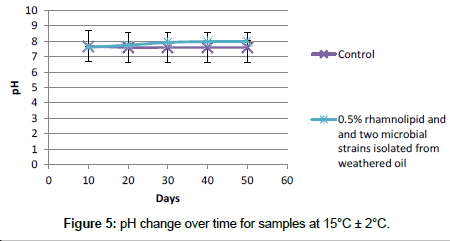
pH measurement: In this work in order to understand better the role of the microbial culture, the pH of samples was measured. Figure 5 shows the pH change during the time for samples at 15°C ± 2°C. The pH slightly increased with time. According to the pH measurements (during the 50 days) increase in the ionic strength (I) of the pore water and reduction in the thickness of the DDL of clay particles is not responsible for increasing the sedimentation as dissolution of MFT carbonate minerals and releasing divalent cations could not occur at pH higher than 7.5. Methanogenesis in MFT might be responsible for the observed pH increase as CO2 was consumed [50]. Gas production resulted in ebullition of bubbles dominated by CH4 (due to the poor solubility of CH4 in water), creating transient channels for escape of pressurized pore water, particularly in the MFT near the mud line [50].
Results of in situ biosurfactant production
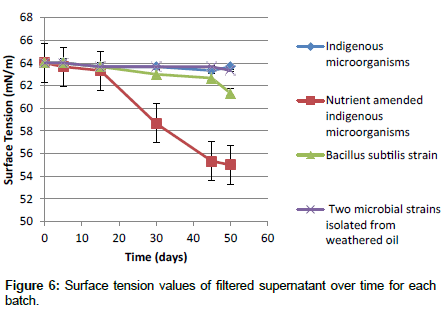
Surface tension measurement: Surface tension measurement can be applied to determine the biosurfactant concentration indirectly as there is a relation between biosurfactant concentration below the CMC and the surface tension of sample. In order to demonstrate biosurfactant production, the surface tension of filtered supernatant from each set of batch experiments was measured. Figure 6 shows the results for surface tension for each batch over time. The initial surface tension for all samples was about 64 mN/m. This value was reduced to about 63 mN/m for indigenous microorganisms of oil sand tailings pond in the control experiment and was lowered to 55 mN/m after 50 days for indigenous microorganisms of oil sand tailings pond in the presence of nutrients which means that the amount of produced biosurfactant by indigenous microorganisms is very low. Bacillus subtilis strain and two microbial strains isolated from weathered oil reduced the surface tension of supernatant to the values of 61 mN/m and 63 mN/m respectively after 50 days which means a very low amount of biosurfactant could be produced by them. However with a longer time it may be possible to achieve more biosurfactant production.
Discussion
The results of the cell surface hydrophobicity (CSH) in the control showed that the isolated bacteria recovered from the biodiesel had hydrophilic properties (negative CSH, tendency to interact with the hydrophilic compounds), while the isolated bacteria recovered from the diesel and light crude oils had hydrophobic properties (positive CSH means a tendency to interact with the hydrophobic compounds). For example, the hydrophobicity values of -50%, 16% and 2% were obtained following 1 h of incubation of bacterial cells on the biodiesel, diesel and light crude oil, respectively [39]. This result and other studies on the effect of hydrocarbons on the bacterial cell surface properties bacteria show that they are capable of modifying their cell surface structures based on the availability and the compositions of hydrocarbons [51-54]. The cell surface hydrophobicity is modified in order to help the microorganisms avoid contact with toxic compounds [51-55] or to uptake food (e.g., hydrocarbons). For example, some bacteria release vesicles (which have an intercellular structure and an outer membrane of a lipid bilayer) from the outer membrane [51-54,56], some release lipopolysaccharide (LPS) to change the cell surface hydrophobicity [57], and some form an exopolysaccharide (EPS) matrix to create a stable environment and optimal conditions for growth (exopolymer microdomains as a structural agent for heterogeneity within microbial biofilms) [58]. In one study, the adhesion to a sandy soil and a clay loam soil of a series of Lactobacillus strains with various cell surface characteristics were investigated [59]. Bacterial cell surface hydrophobicity, as determined by the bacterial adherence to octane and polystyrene, was the major parameter influencing the adhesion to the sandy soil. The cell surface charge of the bacteria was of minor importance in the adhesion to the sandy soil [59]. It has been reported that the attachment to the benthos is facilitated by the common action of both coflocculation and hydrophobic interactions. EPS also can help the bacteria to adhere to the surface and can serve as flocculants to bind small clay particles [58]. The results of zeta potential and particle size distribution supported the idea that rhamnolipid has the potential to be used as a flocculating agent for oil sand tailings sedimentation. It is well known that the particle hydrophobicity has a significant effect on flocculation [24,60-62]. Increased surface hydrophobicity, which is dependent on increasing the concentration of surfactant could increase flocculation of clay particles [24]. The rhamnolipid anions adsorb on the oil sand tailings surfaces, rendering the surfaces hydrophobic and resulting in the flocculation of oil sand clay particles due to the hydrocarbon chain association [24] when the rhamnolipid adsorption layers on particles contact each other. The increase in negative zeta potential should give rise to the increase of the energy barrier, preventing the particle aggregation. However, the increase in the surface charge by the rhamnolipid adsorption on the particle surfaces did not lead to a decrease in the flocculation of tailing particles and even led to improving their flocculation which means that the rhamnolipid adsorption onto the tailing particle surfaces improved the hydrophobic interaction between the particles much more strongly than the electrical double layer repulsion. Rhamnolipid (which is a biosurfactant produced by Pseudomonas aeruginosa) mixed with microbial cultures showed strong flocculating activity, while zeta potential still remained negative. It means that the mechanism of flocculation is not charge neutralization. Microbial activity can increase MFT by microbial cells and/or EPS secreted by microbial cells [7] and/ or biogenic gas production [12,63]. Macromolecules (such as EPS) could be viewed as naturally produced flocculants [64]. Addition of macromolecules to stabilize inorganic dispersions (kaolinite, silica, or alumina) could increase flocculation [65]. Yu et al. showed that EPS causes aggregation of particles through a bridging mechanism which can be viewed as the result of the interaction of naturally produced, high molecular weight, and long chain organics with kaolin clay particles in the way that the macromolecules bridge the individual clay particles into an aggregate. It is possible that the strong flocculating activity of rhamnolipid mixed with the microbial cultures was probably due to the biosurfactant and high molecular weight microbial organics. Bioflocculants with high molecular weights involved more adsorption sites, stronger bridging, and higher flocculating activity [66]. Another possible reason for the strong flocculating activity of rhamnolipid mixed with microbial culture could be due to the change in chemistry of pore water as a result of microbial metabolism. Siddique et al. showed that microbial metabolism could alter the chemistry of pore water that in turn influences the consolidation of clay particle suspensions [50]. They showed that dissolution of MFT carbonate minerals (presumably calcite/dolomite) increased Ca2+ and Mg2+ concentrations in pore water. They also observed a relatively higher concentration of HCO3- in the pore water of amended MFT, presumably due to dissolution of biogenic CO2 in pore water and/or dissolution of carbonate minerals [67]. The dissolution of entrapped CO2 reduced pore water pH, thereby dissolving carbonate minerals and releasing divalent cations [50]. Results for pH measurement support that during this experiment's time (50 days) at 15°C ± 2°C, there could not be any significant change in ionic strength (I) of the pore water and the thickness of diffuse double layer (DDL) of clay particles due to dissolving carbonate minerals and releasing divalent cations. So flocculation is not a result of double layer compression or cation (such as (Ca2+)) bridging between particles. However there is the possibility of an increase in the consolidation due to small amounts of CH4 production as in thicker layer of mud. However in this case rhamnolipid mixed with the microbial culture could improve the hydrophobic interactions and increase the flocculation.
Mechanism of sedimentation using rhamnolipid and microbial culture
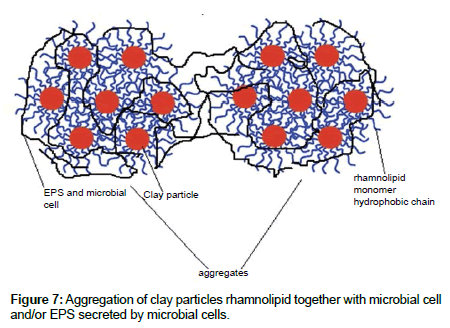
Microbial activity can increase sedimentation by microbial cells and/ or EPS secreted by microbial cells. Exopolysaccharides or extracellular polymeric substances (EPS) are compounds secreted by microorganisms into their environment [16]. Formation of exopolysaccharide (EPS) matrix help them to create a stable environment and optimal conditions for growth (exopolymer microdomains as a structural agent for heterogeneity within microbial biofilms) [58] as they can modify their cell surface hydrophobicity according to the availability and the composition of hydrocarbons. In this way their attachment to the clay particle is facilitated by the common action of both coflocculation and hydrophobic interactions. EPS can help the bacteria to adhere to the surface and causes aggregation of particles through a bridging mechanism in the way that the macromolecules bridge the individual clay particles into an aggregate. Using rhamnolipid together with microbial culture had a stronger activity than rhamnolipid by itself. This was mainly due to the improvement of hydrophobic interactions by microbial culture and by rhamnolipid adsorption on the clay particle and high molecular weight microbial organics interaction with clay particles as bioflocculants with high molecular weights involved more adsorption sites, stronger bridging, and higher flocculating activity (Figure 7). Besides these, there is also the possibility of a small increase in consolidation due to small amounts of CH4 production.
Conclusions
The results obtained from sedimentation tests showed that a mixed culture of two microbial strains isolated from weathered oil increased the sedimentation while the Bacillus subtilis strain at 23°C ± 2°C gave almost the same sedimentation amount as the control. The results obtained from sedimentation tests and particle size distribution analysis indicate that the presence of rhamnolipid (0.5%) together with these two microbial strains could lead to significant increases in sedimentation at 15°C ± 2°C (by a factor of 5.1) the concentration of larger particles (by a factor of 2.63) particle mean diameter (by a factor of 2.70) and flocculation in the tailings samples amended with them compared to the control. The results of zeta potential and particle size distribution at 15°C ± 2°C supported the idea that rhamnolipid adsorption on the particle surfaces increase the negative surface charge while it improved the hydrophobic interaction between the particles much more strongly than the electrical double layer repulsion. It means that the mechanism of flocculation is not charge neutralization and probably it is due to the interaction of the biosurfactant and high molecular weight microbial organics through a bridging mechanism with clay particles in the way that the macromolecules bridge the individual clay particles into an aggregate. According to the pH measurements strong flocculating activity of rhamnolipid mixed with microbial culture could not be as a result of double layer compression or by cation (such as Ca2+) bridging but there might be small amount of CH4 production at deeper layer of mud which could create transient channels for escape of pressurized pore water and increase the consolidation of tailings. However rhamnolipid mixed with microbial cultures could improve the hydrophobic interactions and increase the flocculation in this way. In situ biosurfactant production was investigated and surface tension measurements shows that indigenous microorganisms (even in the presence of nutrients), Bacillus subtilis strain and two microbial strains isolated from weathered oil could produce a very low amount of biosurfactant.
Acknowledgements
The authors would like to acknowledge the funding from a NSERC Discovery Grant and Concordia University.
References
- Timoney KP, Lee P (2009) Does the Alberta Tar Sands Industry Pollute? The Scientific Evidence. Open Conservation Biology Journal 3: 65-81.
- Masliyah JMG, Murray Gray (2007) Extracting and Upgrading of Oilsands Bitumen. Course Pack, In: University of Alberta.
- MamerM(2010) Oil Sands Tailings Technology: Understanding the Impact to Reclamation. In.Suncor Energy Inc.
- Energy Resources Conservation Board (2009) Directive 074: Tailings performance criteria and requirements for oil sands mining schemes. ERCB, Calgary, Alberta, Canada.
- BGC Engineering Inc (2010) Oil Sands Tailings Technology Review. Edmonton, Alberta, Canada: University of Alberta 136.
- World Wildlife Fund's final report(2010) Tailings, a lasting oil sands legacy.
- Bordenave, Kostenko V, Dutkoski M, Grigoryan A, Martinuzzi RJ, et al. (2010) Relation between the activity of anaerobic microbial populations in oil sands tailings ponds and the sedimentation of tailings.Chemosphere 81: 663-668.
- Hunter RJ(2001) Foundations of Colloid Science. Oxford University Press,New York, USA
- Crittenden JCT, RR,Hand DW, Tchobanoglous G (2005) Water Treatment: Principals and Design.(3rdedition) Hoboken, New Jersey, USA.
- Quagraine EK, Peterson HG, Headley JV(2005) In Situ Bioremediation of Naphthenic Acids Contaminated Tailing Pond Waters in the Athabasca Oil Sands Region-Demonstrated Field Studies and Plausible Options. J ENVIRON SCI HEAL 40:685-722.
- Siddique,Fedorak PM, MacKinnon MD, Foght JM (2007) Metabolism of BTEX and naphtha compounds to methane in oil sands tailings.Environ SciTechnol 41: 2350-2356.
- Fedorak PM, Coy DL, Dudas MJ, Simpson MJ, Renneberg AJ, et.al.( 2003)Microbially-mediated fugitive gas production from oil sands tailings and increased tailings densification rates. J ENVIRON SCI HEAL 2(3):199-211.
- Voordouw (2013) Interaction of oil sands tailings particles with polymers and microbial cells: First steps toward reclamation to soil.Biopolymers 99: 257-262.
- Guo H (2003) The lack of a specific association between arsenic in drinking water and hepatocellular carcinoma.J Hepatol 39: 383-388.
- Li X, Yang Q, Huang K, Zeng GM, Liao DX, et al. (2007) Screening and characterization of a bioflocculant produced by Aeromonas sp.Biomed Environ Sci 20: 274-278.
- Lünsdorf,Erb RW, Abraham WR, Timmis KN (2000) 'Clay hutches': a novel interaction between bacteria and clay minerals.Environ Microbiol 2: 161-168.
- SalloumM,Dudas MJ, Fedorak PM (2002) Microbial reduction of amended sulfate in anaerobic mature fine tailings from oil sand.Waste Manag Res 20: 162-171.
- Holowenko F, MacKinnon MD, Fedorak PM (2000) Methanogens and sulfate-reducing bacteria in oil sands fine tailings waste.Can J Microbiol 46: 927-937.
- Golby S1, Ceri H, Gieg LM, Chatterjee I, Marques LL, et al. (2012) Evaluation of microbial biofilm communities from an Alberta oil sands tailings pond.FEMS MicrobiolEcol 79: 240-250.
- Brown D, Ramos-Padrón E, Gieg L, Voordouw G (2013) Effect of calcium ions and anaerobic microbial activity on sedimentation of oil sands tailings. International Biodeterioration& Biodegradation 81:9-16.
- Ramos-Padrón E1, Bordenave S, Lin S, Bhaskar IM, Dong X, et al. (2011) Carbon and sulfur cycling by microbial communities in a gypsum-treated oil sands tailings pond.Environ SciTechnol 45: 439-446.
- Chen M, Walshe G, Chi FruE, Ciborowski JJH, Weisener CG (2013) Microcosm assessment of the biogeochemical development of sulfur and oxygen in oil sands fluid fine tailings. Applied Geochemistry 37:1-11.
- Nasim T, Bandyopadhyay A (2012) Introducing different poly (vinyl alcohol)s as new flocculant for kaolinated waste water. Separation and Purification Technology 88:87-94.
- UcbeyiaySahinkaya H, Ozkan A (2011) Investigation of shear flocculation behaviors of colemanite with some anionic surfactants and inorganic salts. Separation and Purification Technology 80:131-139.
- Besra L, Sengupta DK, Roy SK, Ay P (2003) Influence of surfactants on flocculation and dewatering of kaolin suspensions by cationic polyacrylamide (PAM-C) flocculant. Separation and Purification Technology 30:251-264.
- Singh BP, Besra L, Reddy PSR, Sengupta DK(1998) Use of surfactants to aid the dewatering of fine clean coal. Fuel 77:1349-1356.
- Chhatre S, Purohit H, Shanker R, Khanna P (1996) Bacterial consortia for crude oil spill remediation. Water Science and Technology 34:187-193.
- Mulligan CN1 (2005) Environmental applications for biosurfactants.Environ Pollut 133: 183-198.
- Banat IM1, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants.ApplMicrobiolBiotechnol 53: 495-508.
- Rahman KSM, Rahman TJ, McClean S, Marchant R, Banat IM(2002)RhamnolipidBiosurfactant Production by Strains of Pseudomonas aeruginosa Using Low-Cost Raw Materials. Biotechnology Progress 18:1277-1281.
- Xu Q1, Nakajima M, Liu Z, Shiina T (2011) Biosurfactants for microbubble preparation and application.Int J MolSci 12: 462-475.
- Urum K1, Pekdemir T (2004) Evaluation of biosurfactants for crude oil contaminated soil washing.Chemosphere 57: 1139-1150.
- Rodrigues L1, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine.J AntimicrobChemother 57: 609-618.
- Mulligan CN (2014) Enhancement of Remediation Technologies with Biosurfactants.Biosurfactants: Research Trends and Applications, CRC Press, Taylor & Francis Group USA: 239-254.
- Foght J, Dunfield P (2013) The Impact of Genomics Sciences on the Oil and Gas Industry- Part 1: The Good, the Bad and the Hungry: Micro-organisms and Their Effects on Oil Sands Tailings Ponds. In:Petroleum Technology alliance of Canada (PTAC) Calgary, Alberta,Canada.
- Wang S, Mulligan CN (2009)Rhamnolipidbiosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochemistry44:296-301.
- Clifford JS1, Ioannidis MA, Legge RL (2007) Enhanced aqueous solubilization of tetrachloroethylene by a rhamnolipidbiosurfactant.J Colloid Interface Sci 305: 361-365.
- Abbasi-Garravand E (2012) Removal of Cr (VI) and Cr (III) From Water by Reduction and Micellar Enhanced Ultrafiltration Techniques MSc Thesis. Concordia University, Montreal, Quebec, Canada.
- Saborimanesh N, Mulligan CN (2015) Effect of sophorolipidbiosurfactnat on oil biodegradation by the natural oil degrading bacteria on the weathered biodiesel, diesel and light crude oil. Bioremediation and Biodegradation, 6:314.
- Cookson J(1995) Design and Applications. Bioremediation Engineering,McGraw-Hill Professional,New York,USA.
- Youssef N (2006) Purification, Structure, Activity Relationship and In-situ Production in Oil Reservoirs. Bacillus LipopeptideBiosurfactants, PhD Thesis, UNIVERSITY OF OKLAHOMA, Norman, Oklahoma, USA.
- Eckert WF, Masliyah JH, Gray MR, Fedorak PM (1996) Prediction of sedimentation and consolidation of fine tails. AIChE Journal42:960-972.
- Jalali F (2007) Enhancement Bioremediation of a Soil Contaminated With Both Petroleum Hydrocarbons and Heavy Metals With In-Soil Biosurfactant Production. MSc Thesis, Concordia University, Montreal, Quebec.
- Walworth JL(1995), Reynolds CM: Bioremediation of a petroleum-contaminated cryic soil: Effects of phosphorus, nitrogen, and temperature. Journal of Soil Contamination 4:299-310.
- http://www.horiba.com: Access date: 16-08-2014.
- http://www.somatco.com/ZM3-U-G_D390-2.pdf: Access date: 16-08-2014.
- (Author name not mentioned) (2006) Annual Book of ASTM Standards. ASTM International, West Conshohocken, USA.
- McInerney MJ1, Javaheri M, Nagle DP Jr (1990) Properties of the biosurfactant produced by Bacillus licheniformis strain JF-2.J IndMicrobiol 5: 95-101.
- Abbasi-Garravand E, Mulligan CN (2014) Using micellar enhanced ultrafiltration and reduction techniques for removal of Cr(VI) and Cr(III) from water. Separation and Purification Technology 132:505-512.
- Siddique T1, Kuznetsov P1, Kuznetsova A1, Arkell N1, Young R2, et al. (2014) Microbially-accelerated consolidation of oil sands tailings. Pathway I: changes in porewater chemistry.Front Microbiol 5: 106.
- Bouchez-Naïtali M1, Rakatozafy H, Marchal R, Leveau JY, Vandecasteele JP (1999) Diversity of bacterial strains degrading hexadecane in relation to the mode of substrate uptake.J ApplMicrobiol 86: 421-428.
- Kaczorek E1, Chrzanowski L, Pijanowska A, Olszanowski A (2008) Yeast and bacteria cell hydrophobicity and hydrocarbon biodegradation in the presence of natural surfactants: rhamnolipides and saponins.BioresourTechnol 99: 4285-4291.
- Prabhu Y1, Phale PS (2003) Biodegradation of phenanthrene by Pseudomonas sp. strain PP2: novel metabolic pathway, role of biosurfactant and cell surface hydrophobicity in hydrocarbon assimilation.ApplMicrobiolBiotechnol 61: 342-351.
- Krasowska A1, Sigler K2 (2014) How microorganisms use hydrophobicity and what does this mean for human needs?Front Cell Infect Microbiol 4: 112.
- Torres S1, Pandey A, Castro GR (2011) Organic solvent adaptation of Gram positive bacteria: applications and biotechnological potentials.BiotechnolAdv 29: 442-452.
- Baumgarten T1, Sperling S, Seifert J, von Bergen M, Steiniger F, et al. (2012) Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation.Appl Environ Microbiol 78: 6217-6224.
- Al-Tahhan RA1, Sandrin TR, Bodour AA, Maier RM (2000) Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates.Appl Environ Microbiol 66: 3262-3268.
- Rehm B (2009) Applications and Perspectives. Microbial Production of Biopolymers and Polymer Precursors,Caister Academic.
- Huysman F, Verstraete W (1993) Effect of cell surface characteristics on the adhesion of bacteria to soil particles. Biology and Fertility of Soils 16:21-26
- Warren LJ (1992)Colloid Chemistry in Mineral Processing. (1stedn) Elsevier, New York, USA, 309-329.
- Song S1, Lopez-Valdivieso A, Reyes-Bahena JL, Bermejo-Perez HI, Trass O (2000) Hydrophobic Flocculation of Galena Fines in Aqueous Suspensions.J Colloid Interface Sci 227: 272-281.
- Song S, Lopez-Valdivieso A, Reyes-Bahena JL, Lara-Valenzuela C (2001)Floc Flotation of Galena and Sphalerite Fines. Minerals Engineering, 14: 87-98.
- Bressler D, Cardenas M, Fedorak P. M, Guigard S, Gupta R, et.al (2010) Microorganisms in oil sand tailings ponds influence the properties and behaviour of mature fine tailings. In: 2nd International Oil Sands Tailings Conference, Edmonton, Alberta, Canada.
- Tenney MW, Stumm W (1965) Chemical flocculation of microorganisms in biological waste treatment.J Water Pollut Control Fed 37: 1370-1388.
- Chen F (2007) Bacterial Auto-Aggregation and C-Aggragation in activated sludge. PhD Thesis, Clemson University, South Carolina
- Yu GH1, He PJ, Shao LM (2009) Characteristics of extracellular polymeric substances (EPS) fractions from excess sludges and their effects on bioflocculability.BioresourTechnol 100: 3193-3198.
- Morse JW1, Arvidson RS, Lüttge A (2007) Calcium carbonate formation and dissolution.Chem Rev 107: 342-381.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13171
- [From(publication date):
July-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12088
- PDF downloads : 1083