Application of Acellular Amniotic Scaffold Following Total Ankle Replacement: A Retrospective Comparison
Received: 23-Aug-2018 / Accepted Date: 28-Sep-2018 / Published Date: 05-Oct-2018 DOI: 10.4172/2329-910X.1000275
Keywords: Acellular; Amniotic membrane; Connective tissue matrix; Dehydrated; Placental tissue; Regenerative healing; Total ankle replacement
Introduction
Breakdown of an operative incision resulting in a wound is one of the most devastating complications following total ankle replacement (TAR). The ankle joint is particularly prone to incisional complications following TAR, given the avascular nature of the anterior envelop and motion restrictions after surgery. Wynn et al. reported wound dehiscence in 39% of patients in the early postoperative period [1]. Following TAR, the surgeon must carefully weigh the risk of soft tissue disruption with the risk of soft tissue contracture and loss of joint motion. It is important to initiate return to activity early in the postoperative period to prevent soft tissue contracture and loss of range of motion. In deciding when to initiate ankle joint motion, foot and ankle surgeons must consider each patient individually. The ankle incision must be epithelialized to safely begin weight bearing and initiate range of motion exercise to reduce the risk of dehiscence. Consequently, interventions capable of establishing a favorable healing environment and preventing wound dehiscence following TAR are of increasing interest.
The use of human amniotic membranes are becoming more popular due to the increasing evidence, demonstrating their ability to promote epithelialization, decrease postoperative inflammation and scarring, and improve pain; while potentially preventing infection and aiding in the revascularization of tissues [2-15]. There are no studies to date that have applied amniotic membrane following total ankle replacement (TAR) as a proactive means of reducing surgical incisional healing complications. Given the paucity of clinical evidence, the purpose of this study is to compare the incidence of surgical wound dehiscence in patients who underwent TAR with placement of an acellular dehydrated human amniotic membrane with patients who did not receive an acellular dehydrated human amniotic membrane (Biovance®, Celularity Inc.).
The amniotic membrane is the inner layer of the placenta, which comes into direct contact with the fetus. It serves as a barrier, forming the amniotic cavity and supporting the growing fetus. The amnion is the innermost layer, measuring approximately 0.02-0.05 mm in thickness and is composed of 5 layers [6]. The first layer is the epithelium, a single row of columnar cells which produce vasoactive peptides, growth factors, cytokines and proteins which are stored in the mesenchyme. This layer is often removed during processing of the amniotic membrane in order to enhance contact with the wound base. A thick basement membrane is just deep to this layer, followed by the mesenchymal tissue, which is composed of the compact layer, fibroblast layer, and finally the spongy layer. The spongy layer separates the amnion from the chorion and facilitates easy blunt dissection between the two components. Although amniotic membrane does not contain any lymphatics, blood vessels or nerves, it is highly metabolically active [6].
The use of human amniotic membranes has been documented for wound care in the early 1900’s, and its popularity has increased in recent years due to successful outcomes and an increased understanding of mechanisms through which it influences the body’s ability to heal surgical and non-surgical wounds [2-4,6-10,13-15]. The use of amniotic membranes is well documented in the treatment of full- and partialthickness burns, acute and chronic wounds, vascular and diabetic ulcers, and prevention of inflammation and adhesions following surgical insult [2-9]. A detailed explanation of the exact molecular interactions is beyond the scope of this article, but a brief overview of many of the ways in which amnion supports skin epithelialization and wound healing are present.
Human amniotic membranes promote epithelialization by enhancing epithelial cell proliferation, migration, and differentiation. Bench top data on acellular dehydrated amnion scaffolding demonstrates a surge of several growth factors at the surgical site, including epidermal growth factor (EGF), keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and hepatocyte growth factor receptor (HGF-R), which all have been shown to aid in epithelialization [6,10]. These characteristic have the potential to positively impact the healing environment following TAR, and prevent one of the most devastating complications, incision dehiscence. Enhancing the rate of epithelialization allows for a more predictable healing environment following initiation of return to activity.
One of the most studied effects of amniotic membrane is its ability to inhibit inflammation [8]. Its lack of human leukocyte antigens (HLA)-A, B, C or DR antigens on the surface of the cells allows it to be used without concern of the host mounting an immune response [6]. Within the mesenchyme, the amniotic membrane contains interleukins (IL) 4, 6, 8 and 10, which decrease inflammation by inhibiting markers of inflammation, including interferon gamma (IFN-gamma), IL-2, IL- 3, TNF-alpha, and granulocyte macrophage colony stimulating factor (GM-CSF), as well as other enzymes involved in the inflammatory cascade. They also contain tissue inhibitors of metalloproteinase (TIMP)-1, 2 and 4, which have the effect of down-regulating matrix metalloproteinase 2 and 9, and IL-1 receptor antagonist [6,8].
The healing of skin, the ability to minimize scar, the ability to decrease inflammation are just some of the ways the human amniotic membranes can positively affect the body in the post-fetal development situation.
Methodology
A retrospective chart review was performed to identify consecutive patients that underwent TAR with (treatment group) and without (control group) application of a decellularized, dehydrated, human amniotic membrane. Contributory patient demographics and co morbidity data were recorded. These consisted of patient age (years), body mass index (BMI), gender (male/female), and operative limb (left/ right).
For inclusion, patients were at least 18 years of age at the time of surgery, had exhausted all forms of conservative treatment, and elected to undergo TAR with or without the application of a decellularized, dehydrated human amniotic membrane. All procedures were performed by one surgeon (SAB). Exclusion criteria included postoperative acute traumatic injury that could potentially interfere with wound healing as well as patients undergoing TAR revision. The protocol was approved by our Institutional Review Board and the informed consent was waived. Data was recorded into a password protected secure database. The confidentiality and privacy of individuals was ensured and maintained.
Electronic medical records were reviewed to identify patient who had undergone a TAR. To evaluate our primary endpoint, patients who experienced postoperative wound dehiscence were identified and recorded. Incisional dehiscence was categorized as superficial not penetrating beyond the subcutaneous fat) or deep (penetrating deep to the subcutaneous tissue). Infected wounds were also identified and recorded. Wounds were classified as infected if the wound margins were erythematous and had one or both of the following: active purulence or necrosis of underlying soft tissues. As secondary study endpoints, the time to wound healing, the time to physical therapy initiation, and the duration of physical therapy were compared between the two groups. A wound was considered healed when the incision was completely epithelialized with no surrounding erythema.
Statistical analyses were performed to compare patient demographics, the incidence of incisional dehiscence, the incidence of infection, healing time, the time to physical therapy initiation, and the duration of physical therapy between the two groups. Nominal variables were compared using Pearson’s Chi-Square test. The proportions of the two groups were compared to evaluate whether the control group experienced more cases of dehiscence or infection. Time to heal, time to physical therapy initiation, and the duration of physical therapy sessions was compared between the two groups using an independent samples t-test. Pearson product-moment correlations were conducted to measure the relationship between two variables. Statistical significance was set at the 5% level (p ≤ 0.05). Data presented as mean ± standard deviation and count (%).
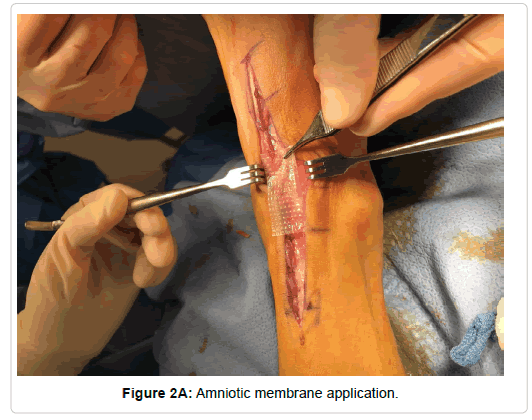
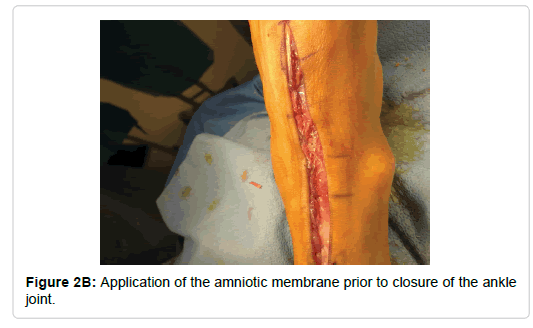
With reference to surgical technique, a traditional anterior approach was utilized for all total ankle replacement except for the two Zimmer total ankles. An anterior approach just lateral to the midline was made approximately 10 cm proximal to the ankle joint extending distally to the level of the talonavicular joint. The extensor retinaculum was then sharply incised with care being taken to identify sheath of the tibialis anterior and leave it completely intact. With the tibialis anterior being retracted medially and the remaining contents laterally, the periosteal capsular structures were then sharply reflected medially and laterally thus exposing ankle joint. Following the implantation of the prosthetic, the periosteal and capsular structures were re-approximated with 2-0 monocryl suture. A 4 cm by 4 cm Biovance amniotic scaffold (Figure 1) was placed at the flexion point of the ankle joint and the extensor retinaculum was closed with 2-0 monocryl (Figure 2). The skin was re-approximated with 3-0 nylon in a horizontal mattress suturing technique. For the two Zimmer total ankles, a lateral approach was used and the amniotic scaffolding was placed subcuticular prior to skin closure.
Results
Ninety-four patients (60.9 ± 10.9 years, 55 men) met the inclusion criteria. Forty-seven (50.0%) patients were in the treatment group, and forty-seven (50.0%) patients were included in the control group. Eight different TAR implants were used (Table 1). The most common TAR implants included the CadenceTM Total Ankle System (Integra Life Sciences, Plainsboro, NJ) (22 [23.4%]) and STARTM Ankle (Stryker, Mahwah, NJ) (43 [45.7%]). In comparing patient demographics (Table 2), the treatment group was found to be significantly older (63.5 ± 10.1 years) than the control group (58.3 ± 11.1 years) at the time of surgery (P=0.020). Otherwise, the two groups were similar in terms of patient demographics, including BMI (P=0.821), gender (P=0.143), operative limb (P=0.836), smoking status (P=0.307), Diabetes Mellitus (P=0.740), and Neuropathy (P=1.00) as shown in Table 1.
| Implant | Manufacturer | All Patients | Treatment | Control |
|---|---|---|---|---|
| CadenceTM Total Ankle System | Integra LifeSciences, Plainsboro, NJ | 22 (23.4) | 21 (44.7) | 1 (2.1) |
| INBONETM Total Ankle System | Wright Medical, Memphis, TN | 3 (3.2) | - | 3 (6.4) |
| INFINITYTM Total Ankle System | Wright Medical, Memphis, TN | 6 (6.4) | - | 6 (12.8) |
| INFINITYTM Tibia & INBONETM Talus | Wright Medical, Memphis, TN | 10 (10.6) | - | 10 (21.3) |
| Integra® XT | Integra LifeSciences, Plainsboro, NJ | 1 (1.1) | - | 1 (2.1) |
| Salto Talaris® Ankle | Integra LifeSciences, Plainsboro, NJ | 7 (7.4) | 3 (6.4) | 4 (8.5) |
| STARTM Ankle | Stryker, Mahwah, NJ | 43 (45.7) | 23 (48.9) | 20 (42.6) |
| Trabecular MetalTM Total Ankle | Zimmer Biomet, Warsaw, IN | 2 (2.1) | 2 (4.3) | |
| Total | 94 (100.0) | 47 (100.0) | 47 (100.0) |
Table 1: Total ankle replacement implant. Data presented as count (%).
| Patient Demographics | All Patients | Treatment | Control | P-Value |
|---|---|---|---|---|
| Patients | 94 (100.0) | 47 (50.0) | 47 (50.0) | |
| Age (years) | 60.9 ± 10.9 | 63.5 ± 10.1 | 58.3 ± 11.1 | 0.020 |
| BMI (kg/m2) | 31.8 ± 5.6 | 31.6 ± 4.9 | 31.9 ± 6.2 | 0.821 |
| Gender | 0.200 | |||
| Men | 55 (58.5) | 31 (66.0) | 24 (51.1) | |
| Women | 39 (41.5) | 16 (34.0) | 23 (48.9) | |
| Operative Side | 0.832 | |||
| Left | 43 (45.7) | 22 (46.8) | 21 (44.7) | |
| Right | 51 (54.3) | 25 (53.2) | 26 (55.3) | |
| Smoking Status* | 0.307 | |||
| Former Smoker | 13 (14.0) | 9 (19.6) | 4 (8.5) | |
| Non-Smoker | 65 (69.9) | 30 (65.2) | 35 (74.5) | |
| Smoker | 15 (16.1) | 7 (15.2) | 8 (17.0) | |
| Co-morbidities | ||||
| Diabetes Mellitus | 10 (10.6) | 6 (12.8) | 4 (8.5) | 0.740 |
| Neuropathy | 1 (1.1) | 1 (2.1) | 1.00 | |
| *Smoking status was not available for one patient in the treatment group. | ||||
Table 2: Patient demographics. Data presented as mean ± SD and count (%).
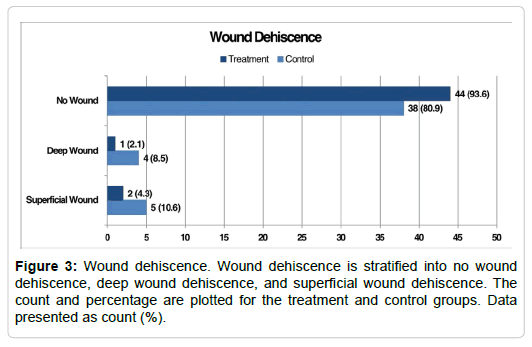
In total, there were 12 (12.8%) cases of incisional dehiscence with 3 (6.4%) in the treatment group and 9 (19.1%) in the control group (Figure 3, Table 3). Of the 3 cases of dehiscence in the treatment group, 2 (4.3%) were considered superficial wounds and 1 (2.1%) deep. Of the nine cases of dehiscence in the control group, 5 (10.6%) were considered superficial and 4 (8.5%) deep. In comparing population proportions, incisional dehiscence was experienced significantly more by the control group (9 [19.1%]) than the treatment group (3 [6.4%], P=0.032). However, when dehiscence was further stratified into superficial or deep wounds, there was no significant difference in the proportion of deep wounds (P=0.804) or superficial wounds (P= 0.119) between the two groups. There was not a significant correlation between group and wound dehiscence (r=0.19, P=0.065). However, there was a significant correlation between TAR implant type and wound dehiscence (r=0.25, P=0.015, Table 4). Wound dehiscence progressed to infection in 8 (8.5%) patients. In comparing population proportions, there was no significant difference in incidence of infection between the treatment group (2 [4.3%]) and control group (6 [12.8%], P=0.069). There was not a significant correlation between group and infection (r=0.15, P=0.142). All cases of infection were treated and healed with the exception of one patient who is continuing local wound care. Three (37.5%) patients received oral antibiotics and Epsom salts soaks without the need for surgical intervention for superficial infections, 3 (37.5%) underwent surgical debridement with application of biovance and 1 (12.5%) underwent surgical debridement without the application of a decellularized, dehydrated human amniotic membrane for deep infection, and 1 (12.5%) received intravenous antibiotics for six weeks, surgical debridement, and a polyethylene exchange for a more extensive deep infection (Table 5). The patient that required IV antibiotics with a polyethylene exchange was unique in that they had a relatively uncomplicated postoperative course until postoperative week four. At which time, he developed a delayed wound dehiscence. He reported that a physical therapist who was treating him at the time removed a small stable scab over his anterior ankle incision. This caused a new open wound, which worsened over several days, and subsequently required the surgical intervention described. At the time this article is being written, he is three months postoperative and continues to have a small, stable superficial wound.
| Outcomes | All Patients | Treatment | Control |
|---|---|---|---|
| Wound Dehiscence | 12 (12.8) | 3 (6.4) | 9 (19.1) |
| Superficial Wound | 7 (7.4) | 2 (4.3) | 5 (10.6) |
| Deep Wound | 5 (5.3) | 1 (2.1) | 4 (8.5) |
| No Wound Dehiscence | 82 (87.2) | 44 (96.3) | 38 (80.9) |
| Total | 94 (100.0) | 47 (100.0) | 47 (100.0) |
Table 3: Wound dehiscence. Data presented count (%).
| Implant | Manufacturer | All Patients | Treatment | Control |
|---|---|---|---|---|
| CadenceTM Total Ankle System | Integra LifeSciences, Plainsboro, NJ | 1 (8.3) | 1 (33.3) | 0 (0.0) |
| INBONETM Total Ankle System | Wright Medical, Memphis, TN | 1 (8.3) | 0 (0.0) | 1 (11.1) |
| INFINITYTM Total Ankle System | Wright Medical, Memphis, TN | 1 (8.3) | 0 (0.0) | 1 (11.1) |
| INFINITYTM Tibia & INBONETM Talus | Wright Medical, Memphis, TN | 3 (25.0) | 0 (0.0) | 3 (33.3) |
| Integra® XT | Integra LifeSciences, Plainsboro, NJ | 1 (8.3) | 0 (0.0) | 1 (11.1) |
| Salto Talaris® Ankle | Integra LifeSciences, Plainsboro, NJ | 2 (16.7) | 0 (0.0) | 2 (22.2) |
| STARTM Ankle | Stryker, Mahwah, NJ | 3 (25.0) | 2 (66.7) | 1 (11.1) |
| Trabecular MetalTM Total Ankle | Zimmer Biomet, Warsaw, IN | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 12 (100.0) | 3 (100.0) | 9 (100.0) |
Table 4: Total ankle replacement implant and wound dehiscence. Data presented as count (%).
| Treatment | All patients |
|---|---|
| Antibiotics and epsom salt soaks | 3 (37.5) |
| Surgical debridement with application of Biovance | 3 (37.5) |
| Surgical debridement | 1 (12.5) |
| IV antibiotics, surgical debridement, and poly exchange | 1 (12.5) |
| Total | 8 (100.0) |
Table 5: Infection treatment summary. Data presented count (%).
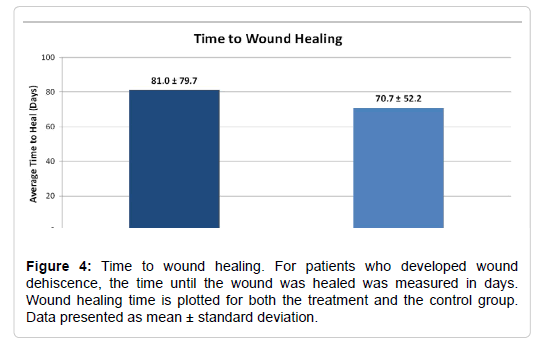
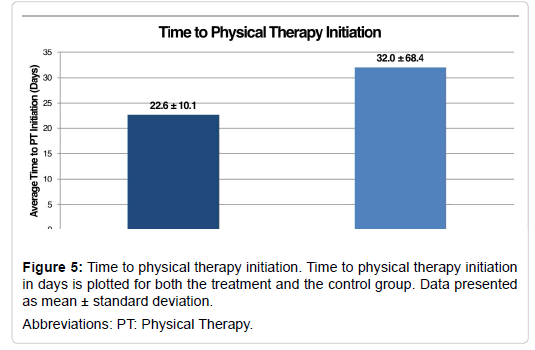
Our secondary outcomes measures included the time to wound healing, the time to physical therapy initiation, and the duration of physical therapy (Table 6). The time to wound healing was evaluated for patients who experienced surgical incision dehiscence. The time to wound healing was similar for the treatment group and the control group (81.0 ± 79.7 days vs. 70.7 ± 52.2 days, respectively; P=0.85, Figure 4). This data is somewhat misleading as there was one patient in the treatment group who had 173 days to wound closure. There was not a significant correlation between group and the time to wound healing (r=0.13, P=0.196).While there was no statistically significant difference in the time to physical therapy initiation (P = 0.36), the treatment group began physical therapy approximately 10 days earlier than the control group (treatment: 22.6 ± 10.1 days vs. control: 32.0 ± 68.4 days, Figure 5). There was not a significant correlation between group and the time to physical therapy initiation (r=0.10, P=0.354). Initiation of physical therapy data was unavailable for one patient in the treatment group. The duration of physical therapy was similar for the treatment group (16.6 ± 9.1 visits) and the control group (16.5 ± 9.4 visits, P=0.94). There was not a significant correlation between group and the duration of physical therapy initiation (r=0.01, P=0.947). The duration of physical therapy data was unavailable for one patient in the treatment group and five patients in the control group.
| Secondary Outcome Measures | Treatment | Control | P-Value |
|---|---|---|---|
| Time to Wound Healing (days) | 81.0 ± 79.7 | 70.7 ± 52.2 | P = 0.85 |
| Time to Physical Therapy Initiation (days) | 22.6 ± 10.1 | 32.0 ± 68.4 | P = 0.36 |
| Duration of Physical Therapy (visits) | 16.6 ± 9.1 | 16.5 ± 9.4 | P = 0.95 |
Table 6: Secondary outcome measures. Data presented as mean ± standard deviation.
Discussion
While there is inherently some incidence of dehiscence related to surgical technique, we feel this is minimized within our study due to surgeon’s experience and the understanding of the importance of preservation of soft tissue envelope. Previous studies reported by the author demonstrated a wound complication rate of 14% with focus on preservation of tissue alone [16]. This complication rate is much improved from earlier published studies by Myerson which reported upwards of 32% wound complication rate before techniques improved [17]. Our data demonstrate a dehiscence rate decrease from 19.1% to 6.4% with the use of an amniotic scaffold. It is important to note that our data included all patients classified as having a delay in wound closure, and we are aggressive in our treatment. A few of the superficial dehiscence patients could have been classified as delayed skin healing as we did not distinguish these patients separately.
As expected, there were no cases of dehiscence noted with the lateral approach system from Zimmer. There was, however, a significantly higher dehiscence rate of 31% with the Infinity and INBONE systems. We attribute this somewhat to the technique required to retract all the soft tissue with pins in order to properly fit the jig, which is not an issue with the other systems. The Integra Cadence and STAR total ankles had the lowest rates of dehiscence of 4.5% and 7% respectively. These two were the most commonly implanted systems with all the Integra Cadence patients being in the treatment group except for one. This further validates our data due to this system having the lowest rate of dehiscence upon all systems with an anterior approach.
Human amniotic membranes have many desirable properties that have the potential to positively impact the healing environment following TAR and prevent incision dehiscence. This retrospective comparative study found a statistically significant decrease in the cases of incisional dehiscence with the use of a dehydrated, acellular human amniotic membrane. These findings adhered to expectation and suggest that the dehydrated, acellular human amniotic membrane may help prevent cases of surgical wound dehiscence following total ankle replacement. While the underlying mechanisms were not investigated in this study, the results are in line with those previously reported. While not statistically significant, this study also showed that patients treated with a dehydrated, acellular human amniotic membrane began physical therapy approximately 10 days earlier than their control counterparts. This is significant due to the importance of early range of motion with physical therapy for a successful outcome and reduction of stiffness following TAR. Early mobilization is further supported throughout literature with a notably faster attainment of short term mobility milestones, increase in functional outcomes, and decrease in costs with total joint replacements [11].
Despite promising preliminary findings, there were inherent weaknesses, which could threaten the validity of our conclusions. The retrospective nature of the study increases the potential for bias and also limits the data to that which is contained within the patients’ medical records. To more fully evaluate the effect of a dehydrated, acellular human amniotic membrane on incisional healing and the incidence of incisional dehiscence, a study should be prospectively designed that also evaluates patient functionality. However, this topic is especially relevant given the potential for amniotic membranes to reduce wound healing complications following TAR.
Conflicts of Interest and Source of Funding
No funding was received. Dr. Brigido SA serves as a consultant for Celularity Inc and Stryker Orthopaedics. None of the aforementioned companies had any knowledge or influence in study design, protocol, or data collection. For the remaining authors, none were declared.
Acknowledgements
None.
References
- Wynn AH, Wilde AH (1992) Long-term follow-up of the Conaxial (Beck-Steffee) total ankle arthroplasty. Foot Ankle 13: 303-306.
- Zelen CM, Serena TE, Denoziere G, Fetterolf DE (2013) A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 10: 502-507.
- Zelen CM, Serena TE, Snyder RJ (2014) A prospective, randomised, comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J 11: 122-128.
- Sheikh ES, Sheikh ES, Fetterolf DE (2013) Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J 11: 711-717.
- Niknejad H, Paeini Vayghan G, Tehrani FA, Khayat-Khoei M, Peirovi H (2013) Side dependent effects of the human amnion on angiogenesis. Placenta 34: 340-345.
- Fairbairn NG, Randolph MA, Redmond RW (2014) The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg 67: 662-675.
- Riordan NH, George BA, Chandler TB, McKenna RW (2015) Case report of non-healing surgical wound treated with dehydrated human amniotic membrane. J Transl Med 13: 242.
- Zelen CM, Snyder RJ, Serena TE, Li WW (2015) The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: A review. Clin Podiatr Med Surg 32: 135-146.
- Loeffelbein DJ, Rohleder NH, Eddicks M, Baumann CM, Stoeckelhuber M, et.al (2014) Evaluation of human amniotic membrane as a wound dressing for split-thickness skin graft donor sites. Biomed Res Int 2014: 572183.
- Riboh JC, Saltzman BM, Yanke AB, Cole BJ (2016) Human amniotic membrane-derived products in sports medicine: basic science, early results, and potential clinical applications. Am J Sports Med 44: 2425-2434.
- Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE (1998) Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA 279: 847–852.
- Manuelpillai U, Tchongue J, Lourensz D, Vaghjiani V, Samuel CS, et.al. (2010) Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4- treated mice. Cell Transplant 19: 1157-1168.
- Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ et al. (2013) Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 10: 493-500.
- ElHeneidy H, Omran E, Halwagy A, Al-Inany H, Al-Ansary M, et al. (2016). Amniotic membrane can be a valid source for wound healing. Int J Womens Health 8:225-231.
- Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, et al. (2014) Angiogenic properties of dehydrated human amnion/chroion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell 6: 1-10.
- Bleazey ST, Brigido SA, Protzman NM (2013) Perioperative complications of a modular stem fixed-bearing total ankle replacement with intramedullary guidance. J Foot Ankle Surg 52: 36–41.
- Myerson MS, Mroczek K (2003) Perioperative complications of total ankle arthroplasty. Foot Ankle Int 24: 17–21.
Citation: Brigido SA, Riniker ML, Protzman NM, Constant DD (2018) Application of Acellular Amniotic Scaffold Following Total Ankle Replacement: A Retrospective Comparison. Clin Res Foot Ankle 6: 275. DOI: 10.4172/2329-910X.1000275
Copyright: © 2018 Brigido SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3978
- [From(publication date): 0-2018 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 3108
- PDF downloads: 870