Application of a Protease Inhibitor for the Treatment of Viral Respiratory Infections: Acceptable Concentrations of the Protease Inhibitor Nafamostat and Ammonium Chloride for Direct Administration to the Respiratory Epithelium of Mice
Received: 10-Jan-2022 / Manuscript No. JIDT-22-51397 / Editor assigned: 12-Jan-2022 / PreQC No. JIDT-22-51397(PQ) / Reviewed: 24-Jan-2022 / QC No. JIDT-22-51397 / Revised: 24-Jan-2022 / Manuscript No. JIDT-22-51397 (R) / Accepted Date: 26-Jan-2022 / Published Date: 01-Feb-2022 DOI: 10.4172/2332-0877.1000487
Abstract
Back ground: Enveloped viruses invade cells by fusing the viral envelope with the cell membrane. Most viral fusion proteins require specific host protease(s) to activate their fusion activity. Many influenza viruses and severe acute respiratory syndrome associated coronaviruses use transmembrane serine protease TMPRSS2 for activation. Protease inhibitor nafamostat suppresses TMPRSS2, thereby interfering with the viral infection in-vitro. However, no successful application of nafamostat for the treatment of respiratory viral infection has been reported. This is because no method has been established to deliver nafamostat to the respiratory epithelium. Additionally, many coronaviruses have another infectious pathway, in which the virus is engulfed in endosome and activated by endosomal protease(s) and acidification. Ammonium chloride is known to block this pathway in-vitro , by interfering with the endosomal acidification. The present study has done to explore the method to safely deliver these reagents by assessing whether adverse effects occur when the reagents are administered to the respiratory epithelium in mice.
Methods: To ass ess adverse effects, inbred mice were intranasally administered the reagents 2~20 μL/day for a week under anesthesia. Mice were daily observed and change in the body weight was used as a health status barometer. At the end of experiment, the serum biochemical examination was done.
Results: The solution of 200 μM nafamostat and 74 mM ammonium chloride could be intranasally administered 20 μL/day for 1 week to adult C57BL/6 mice without any visible adverse effects. Biochemical data on these mice were within the normal range.
Conclusion: Since 1 μM nafamostat and 50 mM ammonium chloride are known to efficiently suppress the viral invasion to cell in-vitro, nafamostat is highly expected to show inhibitory effect in the virus-infected mice, and ammonium chloride may be also available to treat the virus-infected mice. The present study encourages future researches in infected mice and to apply these reagents for the clinical treatment.
Keywords: Nafamostat; Protease inhibitor; Respiratory viral infection; SARS-CoV-2
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronavirus Disease 2019; TP: Total Protein (g/dL); ALB: Albumin (g/dL); BUN: Blood Urea Nitrogen (mg/dL); Cre: Creatinine (mg/dL); Na: Natrium (mEq/L); K: Kalium (mEq/L); Cl: Chlorine (mEq/L); Ca: Calcium (mg/dL); IP: Inorganic phosphate (mg/dL); Mg: Magnesium (mg/dL); AST: Angiotensin Sensitivity Test (IU/L); ALT: Alanine Aminotransferase (IU/L); ALP: Alkaline Phosphatase (IU/L); LDH: Lactate Dehydrogenase (IU/L); TBil: Total Bilirubin (mg/dL)
Introduction
Enveloped respiratory viruses invade cells by fusing the viral envelope with the cell membrane through the action of viral fusiogenic proteins. Most fusiogenic proteins are activated by proteases of host organisms, through a process called “cleavage activation of viral fusion activity” [1-8]. Thus, most respiratory viruses require a specific host protease to infect the host animal cells. Severe acute respiratory syndrome coronavirus (SARS-CoV) and the SARS-CoV-2 (so-called COVID-19 virus) are no exception [9-11]. The spike protein S of SARS-CoV and SARS-CoV-2 first bind to Angiotensin-Converting Enzyme2 (ACE2) on the cell membrane. Thereafter, Transmembrane Serine Protease2 (TMPRSS2) on the cell surface cleaves the S protein, unleashing the fusion capability of S, and leading to cell invasion [9-11]. TMPRSS2 and related proteases are also essential for many other human respiratory viruses, including influenza [6-8] and are known to be inhibited by protease inhibitors camostat mesylate and nafamostat mesylate [12-14].
Recently, an in vitro study revealed that nafamostat mesylate (nafamostat) suppressed cell invasion by SARS-CoV-2 even at an extremely low concentration of 1 μM [15]. Nafamostat is widely used as a protease inhibitor in clinical settings, and the safety of long-term use of nafamostat has been established; moreover, TMPRSS2 a target of nafamostat, is not an enzyme essential for biogenesis and homeostasis [16]. These findings indicate that nafamostat is a potential drug candidate for the treatment of respiratory viral infection. However, SARS-CoV and SARS-CoV-2 can infect cells even in the absence of TMPRSS2 through an alternative pathway, although the infection efficiency is approximately 1/100 compared to that in the presence of TMPRSS2 [17]. In the alternative pathway, the viruses are engulfed in the endosome and cause the cleavage activation of S by endosomal proteases and acidification, thereby entering the cytoplasm via the endosomal membrane [18,19]. In principle, this pathway could be blocked with inhibitors against endosomal protease cathepsin L and related proteases, such as E-64d [11,18,19], but this avenue is not available in vivo because endosomal proteases are essential for homeostasis in organisms. Instead of the protease inhibitor, NH4Cl may be available to block this pathway by temporarily interfering with the acidification of endosomes. Indeed, 50 mM NH4Cl has been shown to efficiently inhibit the invasion of SARS-CoV-2 via endosomes in vitro [11]. Although NH4Cl has long been clinically used for controlling the pH of intravenous solutions, no case has been reported in which NH4Cl is used to suppress viral infections in vivo. In this study, we assessed whether adverse effects occurred when nafamostat and NH4Cl were administered directly to the respiratory epithelium and defined their acceptable concentrations.
Materials and Methods
Mice and reagents
Weanling C57BL/6JJmsSlc female mice (3-week-old) were purchased from Charles River Laboratories Japan, Inc. Five to six mice were raised in each cage. Nafamostat mesylate for injection (Nichi-Iko Pharmaceutical Co. Ltd.) was dissolved in 5% glucose solution (as a working solution, 200 μM) and used for the experiments after appropriate dilution with saline. Ammonium chloride (guaranteed reagent) was purchased from FUJIFILM Wako Pure Chemical Corporation. Isoflurane inhalation solution (Pfizer Japan Inc.) was used for anesthesia.
Intranasal administration with nafamostat and NH4 Cl solutions
Under isoflurane anesthesia, weanling (3-week-old) and adult (5- week-old) mice were intranasally administered 2 μL and 20 μL of the solution, respectively, once daily for 1 week. Administration into the nasal cavity is a method generally applied in experiments on influenza infection, and it is known that administration of 2 μL of solution leads to the solution remaining in the upper respiratory tract, and that administration of 20 μL of solution leads to the solution reaching the lower respiratory tract [20] .
Observational details
The mice were observed for 12-14 days after administration into the nasal cavity. Each mouse was identified by a marker, and changes in body weight, fur, and behavior (such as crouching) were observed daily. Changes in the body weight of inbred mice have been used extensively as a health status barometer and as an excellent index for both sensitivity and reproducibility. If the body weight of the mouse decreased at a rate exceeding 20% at the start of the experiment and was unlikely to recover, the mouse was euthanized.
After the completion of the experiment, the mice were euthanized under heavy anesthesia with isoflurane inhalation. A cardiac puncture was conducted with a group of mice, and Oriental Yeast Co. Ltd. was requested to perform biochemical tests on the mice from which a necessary amount of serum was obtained.
Statistical analysis
The Statcel4 program was used for the statistical analysis. All variables were tested for normality of distribution and analyzed using a parametric test (i.e., repeated measure two-factor ANOVA). Both tests were upper side, and the significance level was set at P<0.01.
Results
Experiment 1 Effect of administration of nafamostat into the nasal cavity
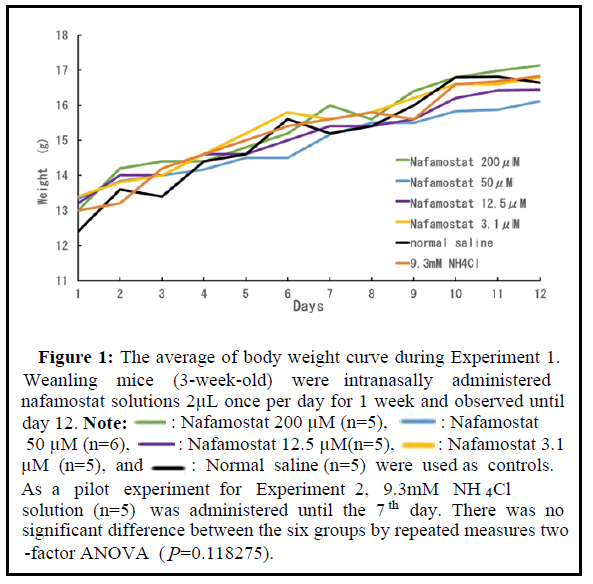
The nafamostat solution (200 μM) was sequentially diluted with saline to prepare solutions of 50, 12.5, and 3.1 μM for intranasal inoculation. Each weanling mouse was administered 2 μL once per day for 1 week. One group (five mice in a cage) was administered saline as a control, and the other was administered 9.3 mM NH4Cl solution, which was a pilot experiment for administering NH 4 Cl in the following experiment. The change in the mean body weight of each group is shown in Figure 1. All the mice used in this experiment exhibited steady growth, and the final mean body weight on day 14 was approximately 17 g in all groups. The mice showed favorable fur and active movement. The mice administered NH4Cl showed frequent rubbing of their nose immediately after administration. No other pain symptoms owing to administration were observed under the conditions of Experiment 1. This result suggests that the administration of 200 μM nafamostat into the nasal cavity is acceptable in weanling mice.
Figure 1: The average of body weight curve during Experiment 1. Weanling mice (3-week-old) were intranasally administered nafamostat solutions 2μL once per day for 1 week and observed until day 12. Note:
 : Nafamostat 200 μM (n=5),
: Nafamostat 200 μM (n=5), : Nafamostat
50 μM (n=6),
: Nafamostat
50 μM (n=6),  : Nafamostat 12.5 μM(n=5),
: Nafamostat 12.5 μM(n=5),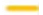 : Nafamostat 3.1 μM (n=5), and
: Nafamostat 3.1 μM (n=5), and 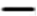 : Normal saline (n=5) were used as controls. As a pilot experiment for Experiment 2, 9.3mM NH4Cl solution (n=5) was administered until the 7th day. There was no significant difference between the six groups by repeated measures two -factor ANOVA (P=0.118275).
: Normal saline (n=5) were used as controls. As a pilot experiment for Experiment 2, 9.3mM NH4Cl solution (n=5) was administered until the 7th day. There was no significant difference between the six groups by repeated measures two -factor ANOVA (P=0.118275).
Experiment 2 Effect of the administration of nafamostat 200 μM plus NH4Cl into the respiratory tract through the nasal cavity
Based on the results of Experiment 1, in Experiment 2, 5-week-old adult mice were administered a mixture of 200 μM nafamostat and various concentrations of NH4 Cl (20 μL per day for 1 week). The concentrations of NH 4 Cl in the mixtures were 74, 37, 18.5, and 9.3 mM. As a control group, mice were administered normal saline.
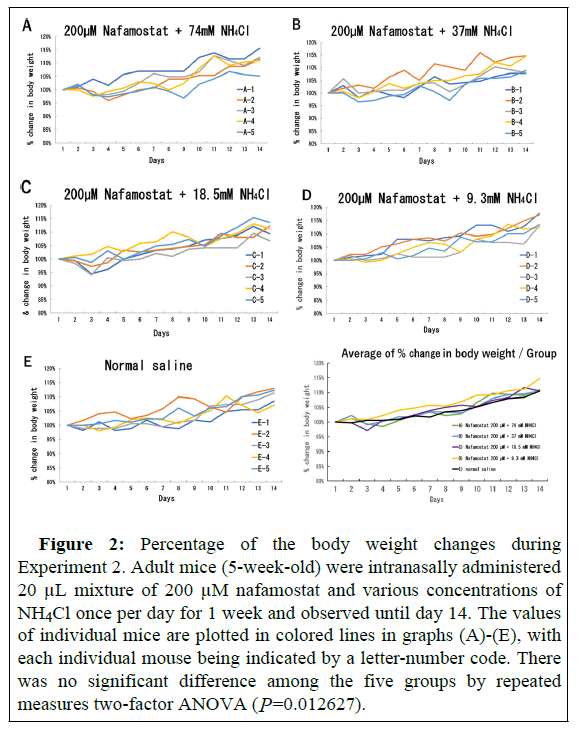
Body weight change in individual mice was expressed as a percentage of the body weight at the beginning of Experiment 2 and is shown in Figure 2. Some mice administered 200 μM nafamostat plus 74, 37, and 18.5 mM NH4Cl (Figure 2A, 2B, and 2C, respectively) showed transiently decreased weight (by 1 g or less) by the 3rd day of administration. However, the mice exhibited a steady weight gain on the 4th day of administration and showed the same weight gain as the control mice at the end of the observation period. The mice administered 200 μM nafamostat plus 9.3 mM NH4Cl and the control mice did not exhibit any decrease in weight and gradually increased in weight (Figures 2D and 2E).
Figure 2: Percentage of the body weight changes during Experiment 2. Adult mice (5-week-old) were intranasally administered 20 μL mixture of 200 μM nafamostat and various concentrations of NH4Cl once per day for 1 week and observed until day 14. The values of individual mice are plotted in colored lines in graphs (A)-(E), with each individual mouse being indicated by a letter-number code. There was no significant difference among the five groups by repeated measures two-factor ANOVA (P=0.012627).
In addition to the above groups, one group (three mice) was administered 2 × 20 μL of 200 μM nafamostat plus 74 mM NH4Cl without anesthesia per day, and another group administered (three mice) was 37 μL of 200 μM nafamostat alone under anesthesia without NH4Cl per day. In the former group, the mice resisted intranasal administration and spat some of the inhaled doses. In the latter group, the solution was observed to run out of the nasal cavity several times, probably because the inoculum volume was too high. All mice showed favorable fur and active movements and exhibited a steady weight gain identical to that of the control mice (data not shown).
Although all mice showed favorable fur and active movements, the mice administered 200 μM nafamostat plus 18.5 mM NH4Cl (Group C) seemed to be docile with low physical activity. To examine their health status more precisely, blood was drawn by cardiac puncture after the observation period and sera were separated for biochemical tests that were performed by a testing company. The amount of serum required for testing was obtained from four out of five mice in Groups A, C, and E (detailed in Table 1).
| Experiment 1 (3-week-old mice) | Experiment 2 (5-week-old mice) | ||||||
|---|---|---|---|---|---|---|---|
| Group (mice) | Inoculum | Concentration | Volume | Nafamostat Concentration | NH4Cl Concentration | Inoculum Volume | |
| A (n=5) | Nafamostat | 3.1 µM | 2 µL | 200 µM | 74 mM | 20 µL | |
| B (n=5) | Nafamostat | 12.5 µM | 2 µL | 200 µM | 37 mM | 20 µL | |
| C (n=5) | Nafamostat | 200 µM | 2 µL | 200 µM | 18.5 mM | 20 µL | |
| D (n=5) | NH4Cl | 9.3 mM | 2 µL | 200 µM | 9.3 mM | 20 µL | |
| E (n=5) | Saline | 0 | 2 µL | 0 | 0 | 20 µL | |
| F (n=6) | Nafamostat | 50 µM | 2 µL | F1 (n=3) | 200 µM | 0 | 37 µL |
| F2 (n= 3)* | 200 µM | 74 mM | 2x20 µL | ||||
Note: *: Without Anesthesia
Table 1: Mice groups in Experiment 1 and 2.
As shown in the Table 2, two out of four mice administered 200 μM nafamostat plus 18.5 mM NH4Cl (Group C) showed higher liver function markers than the normal ranges. The mice in Group C were also administered 200 μM nafamostat at 2 μL for 1 week at the weanling age (see Table 1) in Experiment 1. The high-dose dose administration at that time and the double administration of a high dose of nafamostat throughout Experiments 1 and 2 were considered to have stressed the liver of the mice. The laboratory data on the mice of Group A were all within the normal ranges as observed in the control mice of Group C.
| Group | A | C | E | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | 1 | 2 | 3 | 4 | 1 | 2 | 4 | 5 | 1 | 3 | 4 | 5 |
| TP | 4.4 | 4.4 | 4.6 | 4.7 | 4.6 | 4.6 | 4.6 | 4.8 | 4.4 | 4.4 | 4.6 | 4.5 |
| ALB | 3.1 | 3.2 | 3.4 | 3.4 | 3.2 | 3.4 | 3.4 | 3.6 | 3.6≥ | 3.4 | 3.6 | 3.3 |
| BUN | 20.6 | 19 | 23.8 | 23.7 | 21.6 | 20.4 | 28.2 | 35.4 | 20 | 18.4 | 24.2 | 17.1 |
| Cre | 0.09 | 0.1 | 0.14 | 0.1 | 0.14 | 0.12 | 0.14 | 0.21 | 0.2> | 0.12 | 0.1 | 0.15> |
| Na | 149 | 150 | 148 | 146 | 150 | 148 | 144 | 150 | 152 | 148 | 148 | 150 |
| K | 3.5 | 3.4 | 4.2 | 4.1 | 4.8 | 4 | 4.4 | 4.2 | 3.6≥ | 4 | 3 | 4.5 |
| Cl | 111 | 104 | 104 | 111 | 106 | 100 | 100 | 102 | 100 | 104 | 102 | 102 |
| Ca | 10 | 9.2 | 9.4 | 10 | 9.6 | 9.4 | 9.6 | 10.5 | 9.6 | 9.2 | 9.8 | 9.3 |
| IP | 9 | 7.2 | 7.8 | 9.3 | 10.4 | 6.4 | 6.8 | 9.9 | 8 | 7.6 | 7 | 9 |
| Mg | 2.8 | 2.8 | 3.4 | 2.9 | 3.4 | 2.6 | 3 | 3.3 | 2.4 | 2.4 | 2.6 | 2.7 |
| AST | 89 | 98 | 48 | 45 | 722 | 168 | 88 | 99 | 56 | 58 | 74 | 96 |
| ALT | 22 | 20 | 20 | 23 | 276 | 54 | 24 | 30 | 20 | 20 | 24 | 24 |
| ALP | 701 | 586 | 622 | 675 | 594 | 682 | 590 | 594 | 612 | 622 | 686 | 708 |
| LDH | 283 | 368 | 378 | 282 | 1374 | 540 | 606 | 408 | 204 | 254 | 240 | 483 |
| T- BIL | 0.15 | 0.22 | 0.06 | 0.06 | 0.12 | 0.04 | 0.1 | 0.03 | 0.12 | 0.12 | 0.08 | 0.15 |
Table 2: Biochemical data on the sera obtained from mice in Groups A, C, and E.
Table 2 explains Biochemical data on the sera obtained from mice in Groups A, C, and E.
Group and mouse numbers are the same as those in Figure 2. Group A) 200 μM nafamostat+74 mM NH4Cl, Group C) 200 μM nafamostat+18.5 mM NH4Cl, and Group E) Control (saline).
The underlined numerals indicate liver function markers higher than the normal ranges. These results reveal that the protease inhibitor nafamostat 200 μM at 20 μL/day for 1 week could be administered directly to the respiratory epithelium of adult mice without any adverse effects, and that 74 mM NH4Cl could also be administered in the same manner.
Discussion
The above acceptable concentration of nafamostat (200 μM), is 200-fold higher than the effective in vitro concentration of 1 μM, which inhibits the viral fusion of SARS-CoV-2 efficiently [15] and the acceptable concentration of NH4Cl (74 mM) adequately covers the effective in vitro concentration of 50 mM [11]. Nafamostat is widely used as a protease inhibitor in clinical settings, and ammonium is a metabolite that is ubiquitous in organisms and is known to be rapidly detoxified in the liver. Nafamostat and NH4Cl administrations may transiently modify some of the functions of organisms, but they are not antivirals that directly act on the viruses. Therefore, logically, the virus resistant to nafamostat or NH4Cl may hardly emerge. From this perspective, both reagents are good candidates for therapeutic drugs for the respiratory viral infections including COVID-19. The next step should be to conduct an experiment of administering the drugs to influenza virus-infected mice to confirm whether the drugs exert therapeutic effects within the acceptable concentration ranges. C57BL mice are sensitive to human influenza virus 20). If therapeutic effects are confirmed in C57BL mice infected with influenza virus, this strategy is highly likely to be developed to combat many respiratory infections with the viruses that utilize TMPRSS2 for their cleavage activation. C57BL/6 mice are sensitive to mouse-adapted SARS-CoV, and Yoshikawa, et al. reported that the knockout of TMPRSS2 gene in C57BL/6 mice reduced the severity of lung pathology after infection with SARS-CoV [21], suggesting TMPRSS2 involves in the severity of SARS.
In the animal models for experimental infection with SARS-CoV-2, many researchers utilize hamsters instead of mice [22], because SARS-CoV-2 isolates replicate efficiently in the lungs of hamsters, causing severe lung injury that share characteristics with human COVID-19 cases [23-25]. Therefore, when the optimal condition for therapeutic effect of the intranasal administration will have been revealed through the experiment of influenza virus-infected mice, the therapeutic effect against SARS-CoV-2 infection could be examined in the infection model using hamsters.
Unfortunately, Shinshu University, to which the author belongs, has no Biosafety Level (BSL) 2 facilities enabling animal experiments using influenza viruses. Therefore, we hope that further experiments will be conducted to confirm the effect of nafamostat plus NH4Cl against influenza virus infection at institutions with a BSL2 animal experimental setup, using the data of the present study. Further, it is desirable to directly confirm the therapeutic effect of nafamostat plus NH4Cl against COVID-19 in institutions where SARS-CoV-2 can be handled.
Recently, a strategy similar to that proposed in the present study was attempted by Li, et al. using hACE2-transduced and transgenic mice [26]. They reported that intranasal nafamostat treatment (0.3-3 mg/kg) before or shortly after SARS-CoV-2-infection significantly reduced virus titer in the lung, whereas intraperitoneal treatment (20 mg/kg) showed no significant effect. They also found that prophylactic intranasal treatment (3 mg/kg) reduced mortality in hACE2-trangenic mice [26]. The amount of intranasal nafamostat administered in the present study was 200 μM and 20 μl/mouse (17 g), corresponding to 0.127 mg/kg. In the experiment by Li, et al. nafamostat treatment was performed once (two hours before infection) for prophylactic purposes [26]; however, in the present study, 1 week of treatment after infection was assumed to have a therapeutic effect. Regarding the application of nafamostat and NH4Cl in humans, administration as a nasal spray into the nasal cavity and via gargling and mouth-rinsing in the oral cavity can be considered in the early phase of infection, and as a nebulizer into the respiratory tract in the middle phases of infection. The method of administration is simple, and nafamostat and NH4Cl are much cheaper to manufacture than vaccines and antivirals. Confirmation of the therapeutic effect of nafamostat plus NH4Cl would give great hope to developing countries with inadequate medical facilities.
Conclusion
The results of this study showed that a protease inhibitor, nafamostat (200 μM) at 20 μL/day for 1 week could be administered directly to the respiratory epithelium of adult mice without any adverse effects and that NH4Cl (74 mM) could be administered in the same manner. If it is confirmed that influenza virus-infected mice can be treated with these drugs, it is highly likely that these drugs can be applied for the treatment of SARS-CoV-2 infection, which invades cells with the aid of the same protease via the same route.
Acknowledgements
The author gratefully acknowledges Dr. M. Ohuchi (Professor Emeritus of Kawasaki Medical School, Japan) for his advice on the planning of this study, the staff of the Institute of Experimental Animals, Shinshu University, for their technical support, and Dr. Y. Kawakami (Professor Emeritus of Shinshu University, Japan) for his advice on the preparation of this manuscript. I would like to acknowledge the English language translation and editing support provided by CRL Inc. and Simon Elderton.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors Contributions
S. N. contributed to the conception and design of this study, collected data, performed the statistical analysis, and drafted the manuscript.
Ethics Approval and Consent to Participate
This study was conducted in an experimental setting at the Institute of Experimental Animals, Shinshu University, on obtaining experimental design approval (approval number 020040), according to the regulations at Shinshu University for animal experiments and the guidelines for raising and storing experimental animals and relieving distress.
Consent for Publication
Not applicable.
Availability of Data and Materials
Not applicable
Competing Interests
The author has declared that no competing interest exists.
References
- Ohuchi M, Homma M (1976) Trypsin action on the growth of Sendai virus in tissue culture cells. IV. Evidence for activation of Sendai virus by cleavage of a glycoprotein. J Virol 18:1147-1150.
- Nagai Y, Klenk HD, Rott R (1976) Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology 72:494-508.
[Crossref] [Google scholar] [PubMed]
- Garten W, Bosch FX, Linder D, Rott R, Klenk HD (1981) Proteolytic activation of the influenza virus hemagglutinin: The structure of the cleavage site and the enzymes involved in cleavage. Virology 115:361-374.
[Crossref] [Google scholar] [PubMed]
- Kawaoka Y, Webster RG (1988) Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci USA 85:324-328.
[Crossref] [Google scholar] [PubMed]
- Klenk HD, Rott R (1988) The molecular biology of influenza virus pathogenicity. Adv Virus Res 34: 247-281.
[Crossref] [Google scholar] [PubMed]
- Bottcher E, Matrosovich T, Beyerle M, Klenk HD, Garten W, et al. (2006) Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol 80:9896-9898.
[Crossref] [Google scholar] [PubMed]
- Shirogane Y, Takeda M, Iwasaki M, Ishihuro N, Takeuchi H, et al. (2008) Efficient multiplication of human metapneumovirus in Vero cells expressing the transmembrane serine protease TMPRSS2. J Virol 82:8942-8946.
- Abe M, Tahara M, Sakai K, Yamaguchi H, Kanou K, et al. (2013) TMPRSS2 is an activating protease for respiratory parainfluenza viruses. J Virol 87:11930-11935.
[Crossref] [Google scholar] [PubMed]
- Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, et al. (2010) Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol 84:12658-12665.
[Crossref] [Google scholar] [PubMed]
- Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, et al. (2011) Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol 85:4122-4134.
[Crossref] [Google scholar] [PubMed]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280.
[Crossref] [Google scholar] [PubMed]
- Lee MG, Kim KH, Park KY, Kim JS (1996) Evaluation of anti-influenza effects of camostat in mice infected with non-adapted human influenza viruses. Arch Virol 141:1979-1989.
[Crossref] [Google scholar] [PubMed]
- Yamaya M, Shimotai Y, Hatachi Y, Kalonji NL, Tando Y, et al. (2015) The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells. Pulm Pharmacol Therapeut 33:66-74.
[Crossref] [Google scholar] [PubMed]
- Yamaya M, Shimotai Y, Ohkawara A, Bazarragchaa E, Okamatsu M, et al. (2021) The clinically used serine protease inhibitor nafamostat reduced influenza virus replication and cytokine production in human airway epithelial cells and viral replication in mice. J Med Virol 93:3484-3495.
[Crossref] [Google scholar] [PubMed]
- Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, et al. (2020) Nafamostat mesylate blocks activation of SARS-Co-2: New treatment option for COVID-19. Antimicrob Agents Chemother 64:e00754-e00820.
[Crossref] [Google scholar] [PubMed]
- Kim TS, Heinlein C, Hackman RC, Nelson PS. (2006) Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol Cell Biol 26:965-975.
[Crossref] [Google scholar] [PubMed]
- Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, et al. (2020) Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci USA 117:7001-7003.
[Crossref] [Google scholar] [PubMed]
- Simmons G, Gosalia DN, Rennekamp A, Reeves JD, Diamond SL, et al. (2005) Inhibitors of cathepsin L prevent severe acute respiratory coronavirus entry. Proc Natl Acad Sci USA 102:11876-11881.
[Crossref] [Google scholar] [PubMed]
- Bosch JB, Bartelink W, Rottier PJM (2008) Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol 82:8887-8890.
[Crossref] [Google scholar] [PubMed]
- Tokunaga H, Ushirogawa H, Ohuchi M (2011) The pandemic (H1N1) 2009 influenza virus is resistant to mannose-binding lectin . Virol J 8:50.
[Crossref] [Google scholar] [PubMed]
- Iwata-Yoshikawa N, Okumura T, Shimizu Y, Hasegawa H, Takeda M, et al. (2019) TMPRSS2 contributes to virus spread and immunopathology in the airway of murine models after coronavirus infection. J Virol 93:e01815-e01818.
[Crossref] [Google scholar] [PubMed]
- Schwedhelm P, Kusnick J, Heinl C, Schönfelder G, Bert B (2021) How many animals are used for SARS-CoV-2 research? An overview on animal experimentation in pre-clinical and basic research. EMBO Rep 22:e53751.
[Crossref] [Google scholar] [PubMed]
- Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, et al. (2020) Syrian hamsters as a small animal model for SARS-CoC-2 infection and countermeasure development. Proc Natl Acad Sci USA 117:16587-16595.
[Crossref] [Google scholar] [PubMed]
- Sia SF, Yan LM, Chin AWH, Fung K, Choy, KT, et al. (2020) Pathogenesis and transmission of SARS-C0V-2 in golden hamsters. Nature 583:834-838.
[Crossref] [Google scholar] [PubMed]
- Trimpert J, Vladimirova D, Dietert K, Abdelgawad A, Kunec D, et al. (2020) The Roborovski Dwarf hamster is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 infection. Cell Rep 33:108488.
[Crossref] [Google scholar] [PubMed]
- Li K, Meyerholz DK, Bartlett JA, McCray Jr PB. (2021) The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2 Pulmonary infection in mouse models of COVID-19. mBio 12:e00970-e01021.
[Crossref] [Google scholar] [PubMed]
Citation: Nakagomi S (2022) Application of a Protease Inhibitor for the Treatment of Viral Respiratory Infections: Acceptable Concentrations of the Protease Inhibitor Nafamostat and Ammonium Chloride for Direct Administration to the Respiratory Epithelium of Mice. J Infect Dis Ther 10:487. DOI: 10.4172/2332-0877.1000487
Copyright: © 2022 Nakagomi S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3399
- [From(publication date): 0-2022 - Oct 20, 2025]
- Breakdown by view type
- HTML page views: 2752
- PDF downloads: 647