Research Article Open Access
Apoptosis and Local Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Human Cleft Lip and Palate Disordered Tissue
Benita Krivicka* and Mara PilmaneDepartment of Morphology, Institute of Anatomy and Anthropology, Riga Stradins University, Riga, Latvia
- *Corresponding Author:
- Benita Krivicka
Institute of Anatomy and
Anthropology of Riga Stradins University
Dzirciema street 16, Riga, LV-1007, Latvia
Tel: 37 67320862
E-mail: b.krivicka@inbox.lv
Received Date: November 27, 2014; Accepted Date: January 26, 2015; Published Date: February 2, 2015
Citation: Krivicka B, Pilmane M (2015) Apoptosis and Local Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Human Cleft Lip and Palate Disordered Tissue. J Oral Hyg Health 3:1000170. doi:10.4172/2332-0702.1000170
Copyright: © 2015 Benita Krivicka, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Development of secondary palate involves epithelial- mesenchymal interactions and extensive remodeling of the extracellular matrix of the palatal shelves. Investigations demonstrate that key regulators in mentioned processes are matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). However, the data on distribution and localization of various MMPs and TIMPs, as well as apoptosis in human cleft-affected tissues are still not clear. Therefore in this study we focuse on the relative abundance and localization of MMPs and TIMPs in cleft lip and palate - affected tissue
Materials and methods: The research involved 17 patients with cleft lip and palate at the age from seven years to 49 years. Tissue samples were collected during the surgical nasal correction procedure from the cleft-affected nasal region. Material was proceed for detection of MMP-2, MMP-8, MMP-9, TIMP-2 and TIMP-4 with biotinstreptavidin immunohistochemistry. TUNEL method was applied for detection of apoptosis. Distribution of immunoreactive structures was detected semi quantitatively.
Results: Our results demonstrated that increased expression of MMP-2, variable expression of MMP-9 and slight expression of MMP-8 is characteristic for cleft lip and palate affected cartilage, while clefts disordered bone in general shows slight expression of MMPs and more pronounced apoptosis. Expression of TIMPs, especially - TIMP-4, was more characteristic for cleft lip and palate affected nasal cartilage.
Conclusions: Considered as a whole, our results suggested that the cleft lip and palate affected cartilage seems is more elastic in tissue remodelling, what can probably result in qualitative postoperative healing.
Keywords
Cleft lip and palate; MMP; TIMP; Apoptosis
Introduction
Cleft lip and palate is one of the most common congenital craniofacial defect and occurs in approximately 1 per 700 – 800 newborn infants in Latvia [1,2].
Morphogenesis of the secondary palate is a complex process involving several consecutive events, which occur from embryonic day E13.5 to E15.5 in the mouse and between the 6th and 10th week of gestation in humans [3]. The main part of secondary palate is formed by two shelf-like outgrowths from the maxillary prominences. Mentioned palatal primordia are covered by medial edge epithelium (MEE) [4]. Palatal shelves grow downward vertically along the side of the tongue. Subsequently, the palatal shelves are raised above the tongue, which moves downward as the mandible elongates, and then they grow towards each other until make contact and adhere at the midline along the MEE. Finally, the epithelium disappears, thus allowing the complete palatal fusion [5]. All mentioned processes basicly are regulated by genes and growth factors. Thus, fibroblast growth factor 10 (FGF10) is a crucial mesenchymal signal that is required for palatal outgrowth, while expression of sonic hedgehog (Shh) is detected in epithelium and depends on FGF10 [6]. Despite a lot of studies, the exact fate of the epithelia in the midline epithelial seam MES is still controversial, and three major pathways have been proposed for their disappearance: apoptosis, migration to the oral or nasal side of the palate, and epithelial-mesenchymal transformation (EMT) [7,8]. Interestingly, the EMT plays a central role in generating the cranial neural crest cells, and recent investigations demonstrate the expression of neural crest stem cell markers in human palatal cells [9,10]. Moreover, compared to palatal shelf fusion, little is known yet about the molecular mechanism of palatal shelf elevation [3].
Fundamentally development of secondary palate involves epithelial-mesenchymal interactions and extensive remodeling of the extracellular matrix of the palatal shelves. Investigations demonstrate that one of the key regulators in mentioned processes are matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [3].
The Human Genome Project has identified more than 550 genes encoding proteases, of which over 185 use a zinc driven hydrolytic mechanism, the zincins. The metzincin super family of zinc endopeptidases includes several families of enzymes, among them - MMPs that are involved in the regulation of the extracellular environment, governing cell - extracellular matrix (ECM) and cell–cell interactions in fundamental ways [11].
The human MMPs are a family of 23 enzymes that facilitate turnover and breakdown of the ECM in both physiology and pathology. MMPs are divided into several groups: collagenases, gelatinases, stromelysins, membrane-type MMPs, and glycosylphosphatidylinositol-anchored enzymes [12]. Collagenases (MMP-, MMP-8, MMP-13 and MMP-18) cleave interstitial collagens I, II and III into characteristic 3/4 and 1/4 fragments but they can digest other ECM molecules and soluble proteins. Gelatinases (MMP-2 and MMP-9) readily digest gelatin and a number of ECM molecules including type IV, V and XI collagens, laminin, aggrecan core protein, decorin and fibronectin [13,14]. In turn, fibronectin fragments influence cytokine and nitric oxide production by chondrocytes as well as the levels of MMPs produced [11]. MMPs can also release growth factors from the ECM or the cell surface. They can modify both cell–cell and cell–ECM interactions by the proteolysis of cell surface growth factor and adhesion receptors [1,12].
TIMPs, consisting of 184-194 amino acids, are inhibitors of MMPs [14]. The first TIMP was described in 1975 as a protein, which was able to inhibit collagenase activity. Since then, 3 new TIMPs have been discovered and have been designated TIMP-2, TIMP-3 and TIMP-4. Besides, TIMPs seem to play a role in the regulation of apoptosis [15].
Studies show that several MMPs – MMP-2, MMP-9, MMP-13 and TIMP-2 are expressed in developing palate tissue [16]. Moreover, palatal shelves from E14.0 wild type mouse embryos failed to fuse when cultured in close proximity in the presence of synthetic MMP inhibitor or excess TIMP-2 [3].
However, irrespective of many researches, the data on distribution and localization of various MMPs and TIMPs, as well as apoptosis in human cleft-affected tissues are still not clear. Therefore in this study we focuse on the relative abundance and localization of MMPs and TIMPs in cleft lip and palate-affected tissue.
Material and Methods
The research is based on the material of cleft lip and palate patients, which was gathered within a period of time from 2003 to 2009 at the Cleft Lip and Palate Centre of the Institute of Stomatology of the Riga Stradins University. Permission of the Ethics Committee: decision of the RSU Ethics Committee dated 22nd May 2003.
The research involved 17 patients with cleft lip and palate at the age from seven years to 49 years. 15 patients had unilateral cleft lip and palate, one – bilateral cleft lip and palate, one – bilateral cleft lip and left cleft palate. Tissue samples were collected during the surgical nasal correction procedure from the cleft-affected nasal region.
Tissue were fixed for a day in mixture of 2% formaldehide and 0,2% picric acid in 0,1 M phosfate buffer (ph 7,2). After samples were rinsed in thyroide buffer containing 10% sacharose for 12 hours, then embedded into paraffin and cut in 6-7 µm thin sections. Sections were proceeded for detection of matrix metalloproteinase 2 (MMP-2, sc-53630, work dilution: 1:100, Santa Cruz Biotechnology, Inc., USA), matrix metalloproteinase 8 (MMP-8, sc-80206, work dilution: 1:50, Santa Cruz Biotechnology, Inc., USA), matrix metalloproteinase 9 (MMP-9, sc-10737, work dilution: 1:250, Santa Cruz Biotechnology, Inc., USA), tissue inhibitor of matrix metalloproteinases 2 (TIMP-2, sc-21735, work dilution 1:50, Santa Cruz Biotechnology, Inc., USA), tissue inhibitor of matrix metalloproteinases 4 (TIMP-4, orb106543, work dilution: 1:200, Biorbyt Limited, UK) by use of Hsu et al. (1981) biotin – streptavidin immunohistochemical method [17]. For TUNEL method we used In situ Cell Death Detection Kit (Pod cat No 11684817910, Roche Diagnostics, Germany) in accordance to Negoescu et al. [18]. Routine histological staining with haemotoxylin and eosin was developed for each case to get review picture of the slide. Distributions of immunoreactive structures were detected semiquantitatively [19,20]. The quantity of structures was analyzed in five visual fields of one section. Scale was as following: "0" – no positive structures found in visual field, "0/+" – occasional positive structures seen in visual field, "+" – few immunoreactive structures seen in visual field, "++" – moderate number of immunoreactive structures seen in visual field, "+++" – numerous immunoreactive structures seen in visual field, and "++++" – abundance of immunoreactive structures seen in visual field.
Results were illustrated using Leica DC 300F camera and the image processing and analysis software Image-Pro plus Version 6.0.
Results
Expression of MMP-2 was observed in the hard tissue of all patients and it was more prominent exactly in the cartilage (Table 1).
| N° | MMP-2 | MMP-8 | MMP-9 | TIMP-2 | TIMP-4 | TUNEL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cartilage | bone | cartilage | bone | cartilage | bone | cartilage | bone | cartilage | bone | cartilage | bone | |
| 1 | ++/+++ | ND | +/++ | ND | ++/+++ | ND | 0/+ | ND | +++ | 0/+ | + | ++ |
| 2 | ++/+++ | 0/+ | - | - | ++ | - | 0/+ | - | +++ | - | - | ++ |
| 3 | +/++ | 0/+ | - | 0/+ | +/++ | - | - | + | 0/+ | ND | + | ++ |
| 4 | +++ | 0/+ | - | - | +++ | 0/+ | +++ | 0/+ | ++ | - | +++ | +++ |
| 5 | ND | + | ND | 0/+ | ND | + | ND | 0/+ | ND | - | ND | ++ |
| 6 | +++ | - | 00/+ | - | ++ | 0/+ | 0/+ | - | +++ | - | - | ++ |
| 7 | +++ | 0/+ | 0/+ | - | + | - | - | - | 0/+ | - | - | ++ |
| 8 | ++/+++ | ND | + | ND | ++/+++ | ND | + | ND | ++ | ND | + | ND |
| 9 | ND | 0/+ | ND | - | ND | - | ND | - | ND | - | ND | ++ |
| 10 | ND | ++ | ND | 0/+ | ND | - | ND | 0/+ | ND | - | ND | ++ |
| 11 | +++ | + | 0/+ | 00/+ | ++ | 0/+ | - | - | + | - | - | +++ |
| 12 | ++ | 0/+ | 0/+ | - | +++ | - | - | - | ++ | - | + | +/++ |
| 13 | +++ | - | 0/+ | - | +/++ | - | 0/+ | - | + | - | + | ++ |
| 14 | ++/+++ | 0/+ | + | 0/+ | + | - | - | - | +/++ | - | + | ++ |
| 15 | ++/+++ | - | 0/+ | - | ++ | - | 0/+ | - | ++ | - | ++ | ++ |
| 16 | ++/+++ | + | + | 0/+ | + | 0/+ | - | - | ++ | - | + | ++ |
| 17 | ++/+++ | - | 0/+ | 0/+ | +++ | - | - | - | ++ | - | ++ | ++ |
| Common | ++/+++ | 0/+ | 0/+ | 0 - 0/+ | ++ | 0 | 0 - 0/+ | 0 | ++ | 0 | + | ++ |
Table 1: Semiquantitative distribution of immunoreactive structures in the material of patients with cleft lip and palate.
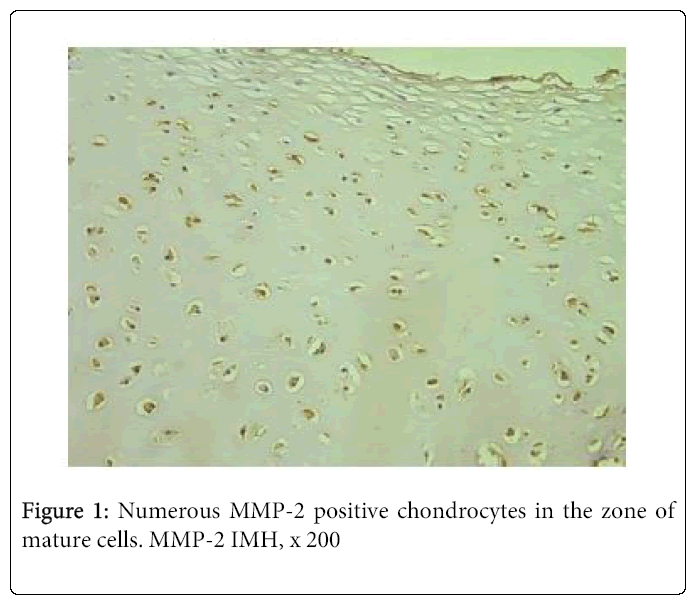
Although the relative number of positive cartilage cells varied from few to moderate (+/++) to numerous, mainly we observed moderate to numerous (++/+++) and numerous positive structures (Figure 1).
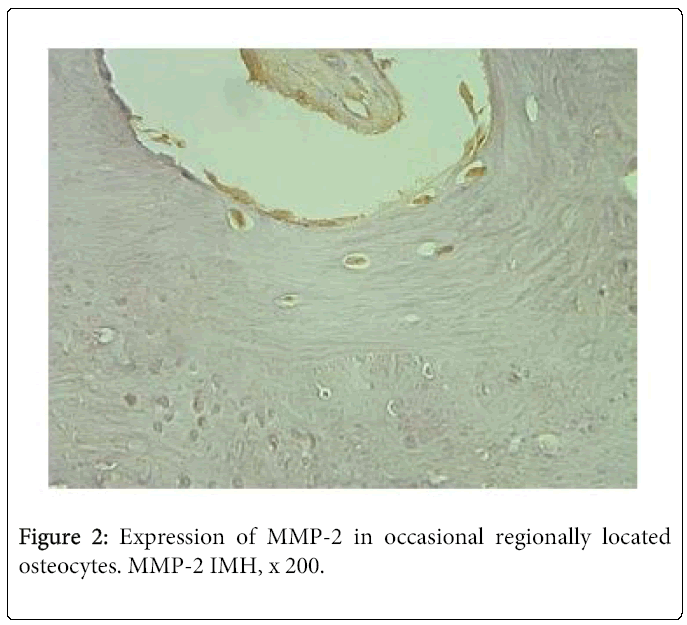
Frequently immunoreactivity was detected not only in the zone of mature chondrocytes, but also in the zone of proliferation. By contrast, MMP-2 immunostaining was much reduced in the bone. Thus, we saw moderate number of immunoreactive osteocytes in one case and few – in three cases. Seven tissue samples demonstrated occasional positive bone cells (Figure 2). We didn’t find any positive structure in six cases.
MMP-8 positive cells we found in the cleft lip and palate affected cartilage of 11 patients and in the cleft lip and palate affected bone of seven patients. Tissue samples mainly demonstrated occasional immunoreactive chondrocytes and osteocytes.
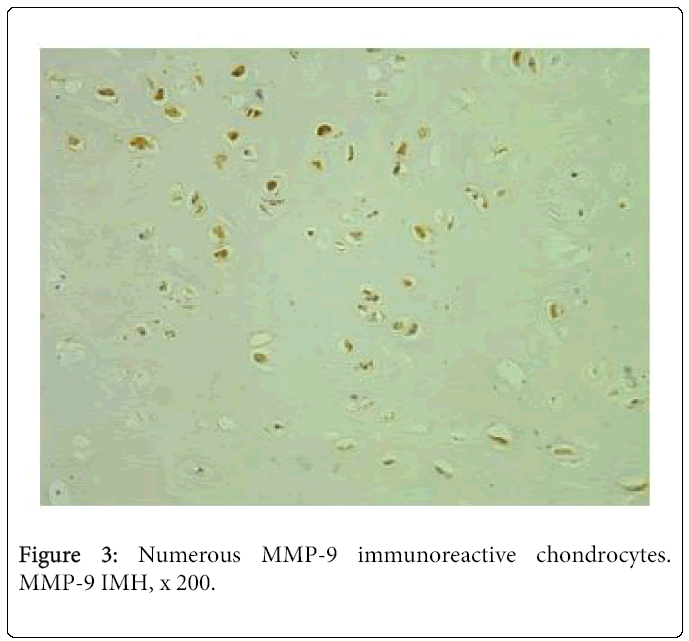
Expression of MMP-9 in the cleft-affected nasal cartilage was very variable. We saw numerous positive structures in three cases (Figure 3), moderate to numerous – in two cases, moderate – in four cases and few to moderate positive structures – in two cases. Three samples demonstrated occasional immunoreactive cells of cartilage. MMP-9 positive osteocytes were seen in the tissue of five patients. There were occasional positive structures in four cases and few – in one case.
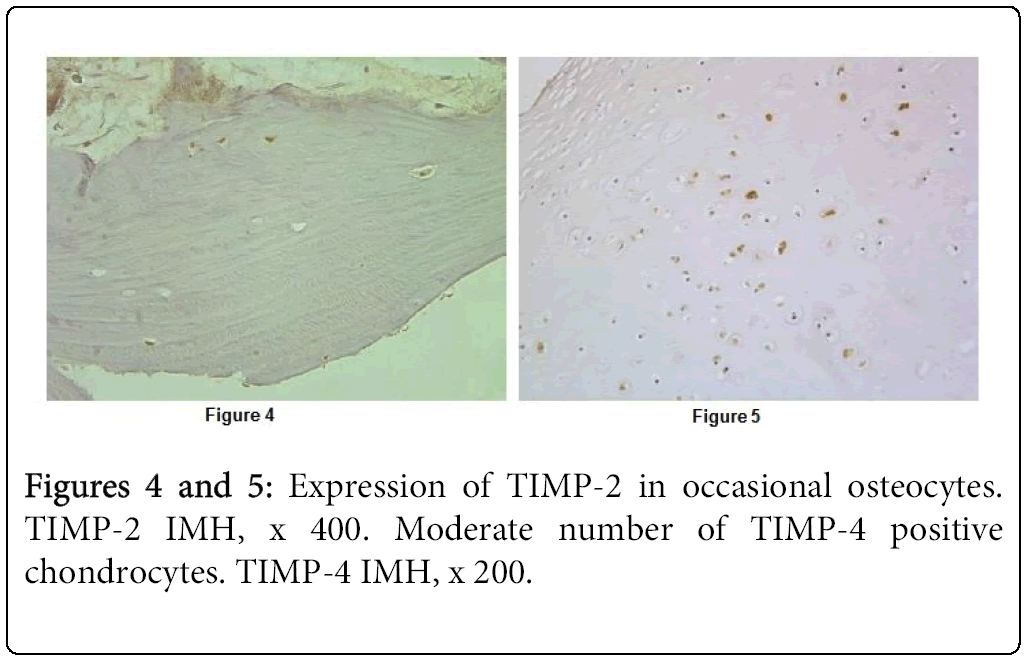
TIMP-2 immunoreactivity was detected in the cartilage of seven patients and in the bone of four patients. Number of TIMP-2 containing cartilage and bone cells mainly varied from occasional too few (Figure 4). Just only one tissue section showed numerous positive chondrocytes as well as chondroblasts. Expression of TIMP-4 we found in all tissue samples with cleft lip and palate affected cartilage and it was very variable. We saw numerous positive cells in three cases and moderate number of positive cells in six cases (Figure 5). In several cases expression was regional. In opposite, immunoreactivity of TIMP-4 in the bone was seen in occasional osteocytes of the one child.
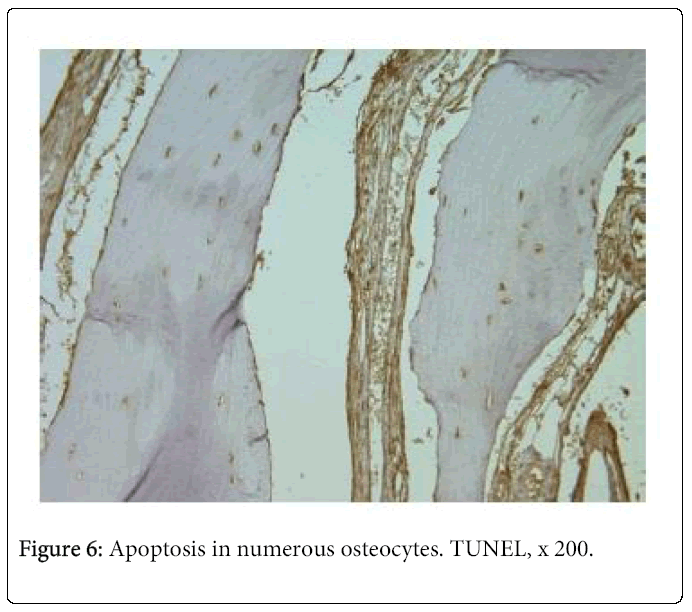
TUNEL demonstrated apoptosis in the hard tissue of all patients. Two tissue samples showed numerous positive osteocytes (Figure 6) and 13 – moderate number of positive osteocytes. Apoptosis in the cleft lip and palate affected nasal cartilage was much less pronounced. We found numerous positive structures in the tissue of one patient, moderate number – in the tissue of two patients, and few – in the tissue of six patients. Tissue samples of four patients gave negative results.
Discussion
Morphogenesis of the secondary palate in mammals involves a sequence of essential steps, among them the elevation of the two palatal shelves from a horizontal to a vertical position above the tongue, followed by their fusion at the midline [3]. The processes of the morphological changes are directed by epithelial-mesenchymal interactions. They result partly from remodeling of the extracellular matrix of the palatal shelves – synthesis and degradation of components of ECM [5]. Studies show that strict regulators of mentioned processes are MMPs and TIMPs [5,16,21]. Moreover, cleavage of ECM proteins by MMPs can also release ECM-bound growth factors, but TIMPs have not only MMPs-inhibiting, but also growth-promoting and antiapoptotic activities [5,22].
Due to the broad spectrum of their substrate specificity, MMPs contribute to the homeostasis of many tissues and participate in several physiological processes, such as bone remodelling, angiogenesis, immunity and wound healing. MMP activity is tightly controlled at the level of transcription, pro-peptide activation and inhibition by tissue inhibitors of MMPs. Dysregulated MMP activity leads to pathological conditions such as arthritis, inflammation and cancer, thus highlighting MMPs as promising therapeutic targets [23].
A healthy cartilage ECM is in a state of dynamic equilibrium, with a balance between synthesis and degradation [12]. It is known, that degradation of the ECM of cartilage is orchestrated by MMPs, which degrade two major structural components of cartilage extracellular matrix, the proteoglycan aggrecan and type II collagen. This makes these enzymes key in the process of cartilage collagen turnover [12,14]. The collagenolytic MMPs, among them MMP-2 and MMP-8, are all produced by chondrocytes [11].
Using immunohistochemistry and semiquantitative grading method, Tetlow with colleges investigated expression of MMPs in normal and osteoarthritic human cartilage [24]. They didn’t see any MMP-2 and MMP-9 positive structures in healthy cartilage, but MMP-8 immunoreactivity was slight and rare. In opposite, osteoarthritic cartilage demonstrated prominent expression of MMP-8 in superficial and deep zones, while immunoreactivity of MMP-2 was moderate and MMP-9 stained variable number of cells in rare cases.
Our study showed pronounced expression of MMP-2 in cleft affected nasal cartilage, while immunoreactivity of MMP-8 in mentioned tissue was slight. Thus, exactly MMP-2, but not MMP-8, is responsible for degradation of collagen in our congenital anomaly affected tissue material. Moreover, low immunoreactivity of MMP-8 possible is perspective signal in cartilage remodeling, because increased expression of MMP-8 is associated by increased production of proinflammatory cytokines by chondrocytes [24]. Very variable number of MMP-9 immunoreactive cartilage cells probable is due the individual features of the tissue.
MMPs are associated with bone matrix turnover, involving virtually all the cell types present [11]. MMPs regulate bone morphogenesis and coordinate not only matrix degradation, but also the recruitment and differentiation of endothelial cells, osteoclasts and osteoprogenitors. During endochondral ossification MMP-9 null mouse shows decrease trabecular bone formation, chondrocyte apoptosis and vascular invasion [25]. Investigations also demonstrate that increased expression of MMP-2 and MMP-9 promotes bone formation and remodeling with very good healing of the cranial defects [26]. As these MMPs are most prominent also in our patients' cartilage, we suggest about active remodeling of nasal hard tissue region of them.
Our findings demonstrated that expression of MMPs in cleft affected bone was light and rare. Despite the fact, that MMP-8 is particularly active on type I collagen [27], we didn’t see MMP-8 positive bone cells in eight cases and MMP-9 positive bone cells in ten cases. Immunoreactivity of MMP-2 was more frequent. It therefore seems likely that following remodeling abilities of the cleft affected bone are weak, what finally may result in poor tissue quality.
MMP activity is regulated by a group of endogenous proteins, called, tissue inhibitor of metalloproteinases (TIMPs) that bind to active and alternative sites of the activated MMP. The TIMPs are secreted proteins, but are localised at the cell surface in association with membrane bound proteins, including metalloproteinases [11]. Thus, the balance between MMPs and TIMPs are critical for the eventual ECM remodelling in the tissue.
Our results showed very variable expression of TIMP-4 and mainly slight and rare expression of TIMP-2 in cleft affected nasal cartilage, while bone demonstrated mainly occasional TIMP-2 positive cells in rare case. TIMP-4 immunoreactivity was detected in the occasional osteocytes of the one cleft palate patient. In accordance to literature, TIMP-1 and TIMP-2 inhibit the activity of a broad range of MMPs. TIMP-4 has a more restricted inhibitory activity that primary blocks MMP-2 to a lesser extent MMP-9 [28]. TIMP-4 inhibits major MMPs implicated in arthritic tissue damage. There is also data, that increases TIMP-4 expression in cartilage of osteoarthritic patients is a possible remodeling response [29]. Thus we can suppose that exactly cleft lip and palate affected cartilage demonstrates readiness for restructuring and reshaping of ECM, where most important is TIMP-4, and then following succesfull tissue healing after the plastic surgery.
TUNEL demonstrated variable number of apoptotic cells in the cleft lip and palate affected cartilage of nine patients. Apoptosis of the osteocytes was observed in the tissue of all patients and it was more prominent. Apoptosis or the programmed cell death plays a key role in tissue homeostasis, embryogenesis, immunologic competence, and normal skeletal development at the growth plate. Cell death in the form of apoptosis appears to be linked to matrix degradation in cartilage [30]. Investigations on patients with traumatic nasal septum also show increased apoptosis. Thus our findings confirm above mentioned presumption that cleft lip and palate affected cartilage is more capable for qualitative remodeling, because apoptosis might be the factor leading to cartilage resorption and weakness.
Osteocytes play a critical role in the regulation of bone remodelling by translating strain due to mechanical loading into biochemical signals transmitted through the interconnecting lacuno-canalicular network to bone lining cells on the bone surface. Osteocyte apoptosis directly affects mentioned process and remarkably reduces it. Such a significant reduction in the signal at the bone lining cells may explain a possible mechanism that leads to the increased remodelling [31]. However in our case weak expression of MMPs is negative indicator for following bone remodelling.
Thus we conclude:
â?Ź Increased expression of MMP-2, variable expression of MMP-9 and slight expression of MMP-8 is characteristic for cleft lip and palate affected cartilage, while clefts disordered bone in general shows slight expression of MMPs and more pronounced apoptosis,
â?Ź Expression of TIMPs, especially - TIMP-4, is more characteristic for cleft lip and palate affected nasal cartilage,
â?Ź Considered as a whole, our results suggest that the cleft lip and palate affected cartilage seems is more elastic in tissue remodelling, what can probably result in qualitative postoperative healing.
Acknowledgement
Research was supported by grant of Riga Stradins University„ The longitudinal research on morphpathogenesis of facial clefts”.
References
- Akota I, Barkane B, Grasmane N (2001) Incidence of congenital malformations in Latvia from 1960 to 1997. ProcLatv Med Acad: 166-170.
- Pilmane M, A Mahers GH (2006) Medical embryology. Riga: Riga StradinsUniversity.
- Gkantidis N, Blumer S, Katsaros C, Graf D, Chiquet M (2012) Site-specific expression of gelatinolytic activity during morphogenesis of the secondary palate in the mouse embryo. PLoS One 7: e47762.
- Dudas M, Li WY, Kim J, Yang A, Kaartinen V (2007) Palatal fusion - where do the midline cells go? A review on cleft palate, a major human birth defect. ActaHistochem 109: 1-14.
- Hirata A, Katayama K, Tsuji T, Natsume N, Sugahara T, et al. (2013) Heparanase localization during palatogenesis in mice. Biomed Res Int 2013: 760236.
- Bush JO, Jiang R (2012) Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development 139: 231-243.
- Iseki S (2011) Disintegration of the medial epithelial seam: is cell death important in palatogenesis? Dev Growth Differ 53: 259-268.
- Okano J, Suzuki S, Shiota K (2006) Regional heterogeneity in the developing palate: morphological and molecular evidence for normal and abnormal palatogenesis. CongenitAnom (Kyoto) 46: 49-54.
- Bueno DF, Sunaga DY, Kobayashi GS, Aguena M, Raposo-Amaral CE, et al. (2011) Human stem cell cultures from cleft lip/palate patients show enrichment of transcripts involved in extracellular matrix modeling by comparison to controls. Stem Cell Rev 7: 446-457.
- Widera D, Zander C, Heidbreder M, Kasperek Y, Noll T, et al. (2009) Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 27: 1899-1910.
- Murphy G, Lee MH (2005) What are the roles of metalloproteinases in cartilage and bone damage? Ann Rheum Dis 64 Suppl 4: iv44-47.
- Milner JM, Rowan AD, Cawston TE, Young DA (2006) Metalloproteinase and inhibitor expression profiling of resorbing cartilage reveals pro-collagenase activation as a critical step for collagenolysis. Arthritis Res Ther 8: R142.
- Kuang B, Dai J, Wang QY, Song R, Jiao K, et al. (2013) Combined degenerative and regenerative remodeling responses of the mandibular condyle to experimentally induced disordered occlusion. Am J OrthodDentofacialOrthop 143: 69-76.
- Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562-573.
- Verstappen J, Von den Hoff JW (2006) Tissue inhibitors of metalloproteinases (TIMPs): their biological functions and involvement in oral disease. J Dent Res 85: 1074-1084.
- Blavier L, Lazaryev A, Groffen J, Heisterkamp N, DeClerck YA, et al. (2001) TGF-beta3-induced palatogenesis requires matrix metalloproteinases. Mol Biol Cell 12: 1457-1466.
- Hsu SM, Raine L, Fanger H (1981) The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J ClinPathol 75: 816-821.
- Negoescu A, Guillermet C, Lorimier P, Brambilla E, Labat-Moleur F (1998) Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed Pharmacother 52: 252-258.
- Pilmane M (1997) Immunohistochemical and radioimmunohistochemical investigations of lung diffuse neuroendocrine system in humans with different respiratory diseases. Riga: Latvian Medicine Academy: 13.
- Tobin G, Luts A, Sundler F, Ekström J (1990) Peptidergic innervation of the major salivary glands of the ferret. Peptides 11: 863-867.
- de Oliveira Demarchi AC, Zambuzzi WF, Paiva KB, da Silva-Valenzuela Md, Nunes FD, et al. (2010) Development of secondary palate requires strict regulation of ECM remodeling: sequential distribution of RECK, MMP-2, MMP-3, and MMP-9. Cell Tissue Res 340: 61-69.
- Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8: 221-233.
- Löffek S, Schilling O, Franzke CW (2011) Series "matrix metalloproteinases in lung health and disease": Biological role of matrix metalloproteinases: a critical balance. EurRespir J 38: 191-208.
- Tetlow LC, Adlam DJ, Woolley DE (2001) Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 44: 585-594.
- Ortega N, Behonick D, Stickens D, Werb Z (2003) How proteases regulate bone morphogenesis. Ann N Y AcadSci 995: 109-116.
- Rocha CA, Cestari TM, Vidotti HA, de Assis GF, Garlet GP, et al. (2014) Sintered anorganic bone graft increases autocrine expression of VEGF, MMP-2 and MMP-9 during repair of critical-size bone defects. J Mol Histol 45: 447-461.
- Umlauf D, Frank S, Pap T, Bertrand J (2010) Cartilage biology, pathology, and repair. Cell Mol Life Sci 67: 4197-4211.
- Stamenkovic I (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200: 448-464.
- Huang W, Mabrouk ME, Sylvester J, Dehnade F, Zafarullah M (2011) Enhanced expression of tissue inhibitor of metalloproteinases-4 gene in human osteoarthritic synovial membranes and its differential regulation by cytokines in chondrocytes. Open Rheumatol J 5: 81-87.
- Garar K, Polat G, Ozcan C, Arslan E, Vayisolu Y, et al. (2007) The role of apoptosis in traumatic versus nontraumatic nasal septal cartilage. PlastReconstrSurg 119: 1773-1776.
- Jahani M, Genever PG, Patton RJ, Ahwal F, Fagan MJ (2012) The effect of osteocyte apoptosis on signalling in the osteocyte and bone lining cell network: a computer simulation. J Biomech 45: 2876-2883.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 15060
- [From(publication date):
March-2015 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 10390
- PDF downloads : 4670