Aortopulmonary Window in an Infant with Type I Osteogenesis Imperfecta: Case Report
Received: 04-Oct-2017 / Accepted Date: 03-Nov-2017 / Published Date: 14-Nov-2017 DOI: 10.4172/2572-4983.1000138
Abstract
Some studies have shown an association between osteogenesis imperfecta and congenital heart diseases, but only those involving changes in the connective tissue of heart structures, such as heart valves, chordae tendineae, fibrous rings, ventricular septum and aortic root (dilatation). The concomitant presence of osteogenesis imperfecta and aortopulmonary window has not been reported in the specialized literature, rendering the present case report uncommon. We report the case of a male infant aged 2 months and 15 days, diagnosed with type I osteogenesis imperfecta and type I aortopulmonary window, submitted to surgery to completely repair his heart disease. In addition, we provide a literature review and discuss the clinical and surgical approaches to this infant, emphasizing that previous multidisciplinary planning is essential for a successful outcome.
Keywords: Congenital cardiopathy; Osteogenesis imperfecta
Abbreviations
OI: Osteogenesis Imperfecta; ICU: Neonatal Intensive Care Unit
Introduction
Osteogenesis imperfecta (OI) is a genetically heritable disease characterized by imperfect bone formation, which leads to bone fragility and bone mass loss [1]. This occurs because both the structure and function of type 1 collagen are affected by gene mutations [2]. OI has an incidence of approximately 1 per 15,000-20,000 births, and a heterogeneous clinical presentation, ranging from asymptomatic to lethal [3].
Initially, OI was classified by Sillence, et al. [4,5] into 4 types based on its clinical characteristics and severity. Recently, types V, VI, VII and VIII [6,7] have been added, and, despite having no defect in the collagen gene, they are characterized by bone frailty (Table 1).
| Type | Clinical expression | Typical clinical aspects |
|---|---|---|
| I | Mild | Normal or slightly shorter-than-average stature, bluish sclera, no dental changes. |
| II | Lethal | Multiple and severe fractures in the ribs and long bones at birth, severe deformities. Flat and hypodense bones, dark blue sclera. |
| III | Severe | Marked short stature, triangular facial shape, severe scoliosis, grayish sclera, dentinogenesis imperfecta. |
| IV | Moderate | Moderate short stature, mild-to-moderate scoliosis, white or grayish sclera, dentinogenesis imperfecta. |
| V | Moderate | Mild-to-moderate short stature, normal sclera, no dentinogenesis imperfecta, dislocation of the radial head, calcification of the interosseus membrane, hypertrophic calluses. |
| VI | Moderate to severe | Moderate short stature, scoliosis, normal sclera, no dentinogenesis imperfecta, excessive osteoid and bone lamellae as fish scale. |
| VII | Moderate | Mild short stature, short humerus and femurs, coxa vara, normal sclera and teeth. |
| VIII | Severe/Lethal | Severe short stature, extreme bone fragility, very similar to types II and III, but with a different genetic cause. |
Source: Clinical protocol and therapeutic guidelines. Ministry of Health. Ministerial order SAS/MS no 1306, November 22, 2013, Brazil.
Table 1: Classification of Osteogenesis imperfecta.
The association of OI with congenital heart disease leads to changes in the connective tissue [4,8]. Such changes have a structural impact on the cardiovascular system, affecting heart valves, chordae tendineae, fibrous rings, interventricular septum, arteries and the aorta [9,10]. Publications on severe cardiac structural changes in patients with OI have been infrequent, most studies reporting on aortic root dilatation and valvular dysfunction in those patients [11]. The major medical journals lack reports on the association of OI with congenital aortopulmonary window.
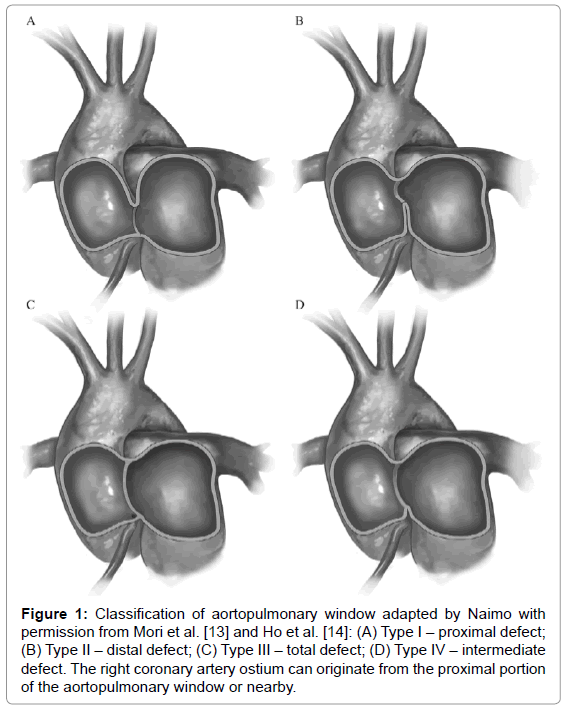
The aortopulmonary window is rare, accounting for approximately 0.15% of all congenital heart diseases. It results from the abnormal septation of the aortopulmonary trunk during embryogenesis, leading to a communication defect between the ascending aorta and the pulmonary trunk or artery [12]. According to Mori’s classification [13], adapted by Ho [14], the types of aortopulmonary window are as follows: type I (proximal – 70%); type II (distal – 20%); type III, (5%), a combination of types I and II, simulating a truncus arteriosus [15]; and type IV, the intermediate defect (Figure 1). In approximately 80% of the cases, identification of the aortopulmonary window can be hindered by its association with another cardiac anomaly [16].
Figure 1: Classification of aortopulmonary window adapted by Naimo with permission from Mori et al. [13] and Ho et al. [14]: (A) Type I – proximal defect; (B) Type II–distal defect; (C) Type III–total defect; (D) Type IV–intermediate defect. The right coronary artery ostium can originate from the proximal portion of the aortopulmonary window or nearby.
We report the case of an infant with OI and congenital heart disease, submitted to surgical repair, its relevance lying in the presence of OI as an underlying bone disorder. In addition, the clinical, anesthetic and surgical approaches are discussed.
Case Report
The patient is a male twin infant, born by Cesarean delivery at term and birth weight of 2460 g. His mother has type I OI. He was admitted to the neonatal intensive care unit (ICU) because of respiratory distress in the first hours following birth.
In the neonatal ICU, he required ventilation with positive pressure and nasal prong. As the dyspnea persisted, he was submitted to several inconclusive echocardiographies and to ineffective treatments. The infant was, then, referred for new imaging tests at a specialized hospital, where an echocardiogram raised the suspicion of heart disease. A tomographic angiography confirmed that by evidencing proximal aortopulmonary window and increased pulmonary flow. Diuretic was then initiated, resulting in improvement of the respiratory findings, and progressive ventilator weaning to room air. The infant was then referred to a specialized hospital that provided heart surgery.
His preoperative echocardiogram revealed patent foramen ovale, mild tricuspid regurgitation, significant overload of the left cavities, dilated pulmonary artery and veins, wide 9.0 mm aortopulmonary window, and preserved myocardial function. His preoperative laboratory tests were normal.
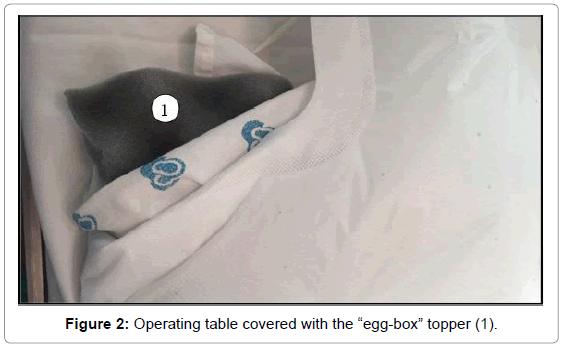
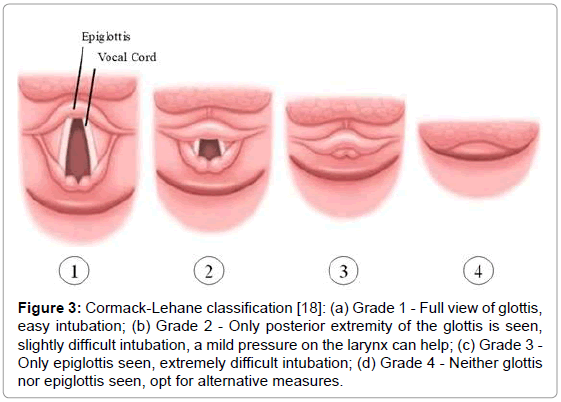
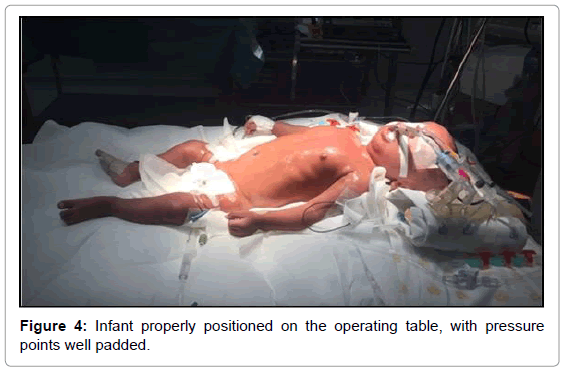
On the third day of admission, the infant was sent to the operating room for total repair of his heart disease. He was carefully placed on the operating table, which was topped with an “egg-box” mattress (Figure 2). The infant was properly positioned for surgery, with pads placed under the pressure points. Orotracheal intubation was performed with a flexible tube with neither difficulty (Cormack 3–Figure 3) [17]. The left femoral vein was punctured, and invasive arterial pressure measurement was installed in the right femoral artery (Figure 4).
Figure 3: Cormack-Lehane classification [18]: (a) Grade 1 - Full view of glottis, easy intubation; (b) Grade 2 - Only posterior extremity of the glottis is seen, slightly difficult intubation, a mild pressure on the larynx can help; (c) Grade 3 - Only epiglottis seen, extremely difficult intubation; (d) Grade 4-Neither glottis nor epiglottis seen, opt for alternative measures.
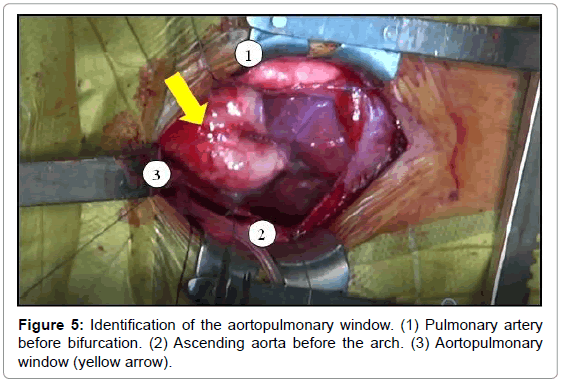
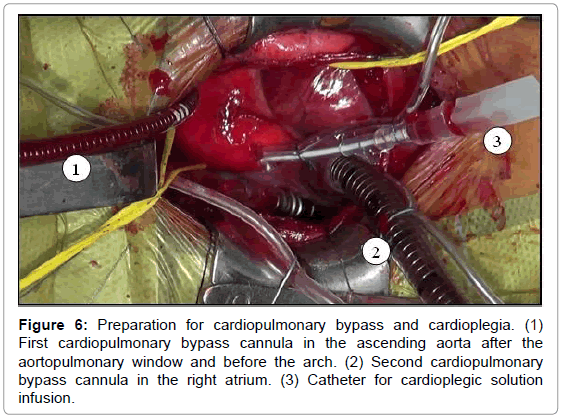
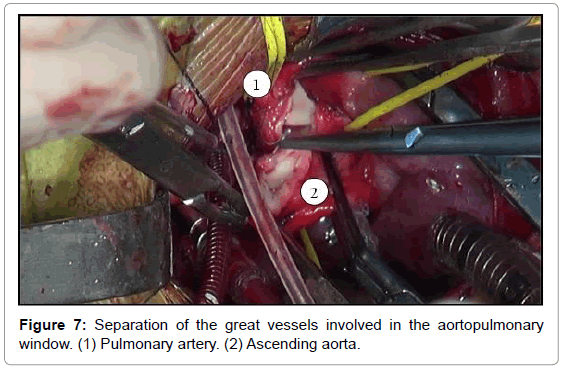
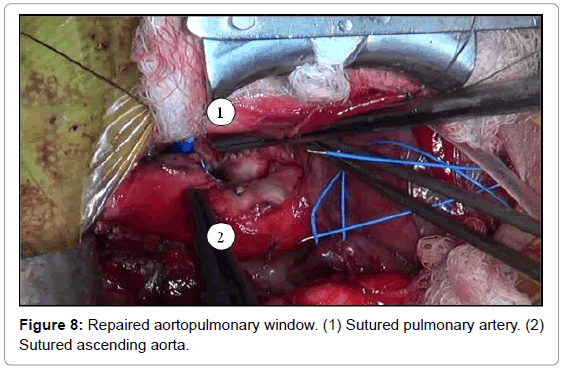
The aortopulmonary window was corrected with aortoplasty and enlargement of the pulmonary artery at the site of connection to the aorta, by using primary suture (Figures 5-8). The infant received intraoperative red blood cell concentrate, platelets, albumin, and recombinant fibrinogen. The surgery was uneventful.
In the immediate postoperative period, massive bleeding trough the mediastinal tube was identified. Hemostasis review in the operating room revealed surgical site bleeding. The addition of an absorbable hemostatic agent stopped the bleeding, and the infant left the operating room after 2 h.
The control echocardiogram evidenced closed aortopulmonary window, with no residual shunt, minimum aortic regurgitation, and mild overload of the left cavities with mild myocardial dysfunction. By the tenth postoperative day, the infant was on a full diet and breathing room air, being transferred then to his hospital of origin.
There the infant was discharged at the age of 4 months and 8 days, breathing room air, and suckling breast milk properly.
Discussion
Aortopulmonary window is a non-restrictive communication between two great arteries, with increased pulmonary flow, rapidly leading to heart failure and pulmonary hypertension. It was first described in 1830 by Eliotson and its first successful surgical repair was reported by Gross at Boston Children’s Hospital, in 1952 [18].
The prenatal diagnosis of aortopulmonary window is difficult [19]. After birth, the diagnosis requires echocardiography, which can raise the suspicion of an aortopulmonary window or even evidence it. Tomographic angiography can aid in establishing the diagnosis and providing information about the precise location of the defect. Furthermore, it can evidence the absence or presence of an anomalous origin of the right coronary artery from the aortopulmonary window [20]. Association with the clinical signs supports the imaging exams.
Patients older than 6 months are usually submitted to cardiac catheterization before surgery to estimate the extent of pulmonary hypertension and its reversibility [21].
The treatment of aortopulmonary window is surgical. To avoid irreversible pulmonary hypertension, surgery should occur as early as possible, preferably before the age of 6 months. The repair can range from a simple suture in small communications to the need for a vascular prosthesis. When the defect is early diagnosed and in the absence of other malformations, intraoperative mortality is low, the long-term prognosis is satisfactory, and the reoperation rate is low [22]. If the diagnosis is delayed, pulmonary resistance should be assessed, because progression to an irreversible pulmonary vascular disease contraindicates surgery [23]. The major postoperative complication is stenosis of the pulmonary branches [24]. The prognosis of nonsurgically treated patients is poor, with 40% mortality in the first year of life, the other 60% dying during childhood because of congestive heart failure [25].
It is worth noting the need for multidisciplinary strategic planning prior to surgery for such infants, emphasizing the clinical, anesthetic and surgical approaches, to avoid fractures and complications inherent in OI. Find below the precautions the medical team should take to prevent pitfalls.
Bone fractures
Bone fragility and bone mass loss predispose patients with OI to fractures. In addition to careful moving, it is extremely important to properly position those patients on the operating table [26] (Figure 4), which should be covered with an “egg-box” topper, and to apply pads to all pressure points (Figure 2).
Catheter placement for invasive blood pressure measurement should be considered. For venous puncture and venous catheter placement, mild pressure should be applied, and the use of tourniquets should be avoided.
Bleedings
Patients with OI have increased capillary fragility, decreased factor VIII level, and defective platelet aggregation induced by endothelial collagen [27,28]. Bleeding can occur despite the normal results of coagulation exams, hindering the prediction of intra- and postoperative bleeding [29]. Thromboelastogram might not be useful. Thus, the infusion of platelets and hemostatic components is always recommended to those patients during surgery [30].
Malignant hyperthermia
Malignant hyperthermia is a pharmacogenetic disorder, whose crisis is triggered during general anesthesia by halogenated compounds, such as halothane, isoflurane, sevoflurane and desflurane, as well as by the muscle relaxant, succinylcholine. Anticholinergic drugs have been reported as possible inducers [31].
Patients are usually asymptomatic, and the crisis begins suddenly. The clinical signs and symptoms of malignant hyperthermia are progressive body temperature increase reaching up to 44°C; cardiovascular changes; excessive sweating; muscle stiffness; dark urine due to myoglobinuria; and general bleeding [32]. Some case reports of hyperthermia in patients with OI are not of the malignant form, but of a hypermetabolic state of unknown origin [33]. The supposed explanation for that is abnormal central regulation of body temperature or abnormal cell metabolism [34]. Patients with OI and hyperthermia can be controlled with body cooling, increased oxygen offer, and intravenous administration of sodium bicarbonate, vasoactive amines, and dantrolene sodium. If not properly and early treated, malignant hyperthermia can have a 70% mortality rate, which can be reduced to 9% with the use of dantrolene sodium [26].
Difficult orotracheal intubation and ventilation
Intubation: Cervical hyperextension should be avoided, because dislocation of the atlanto-axial joint or fracture of its bones can occur, in addition to basilar invagination [35]. The best and safest method is fiberoptic intubation [36], in addition to applying shoulder pads to prevent any cervical dislocation. Another alternative is the use of a laryngeal mask, and, in extreme cases, urgent tracheostomy. Intubation should be rapid, avoiding mucosal injury, and jaw and dental fractures [37].
Ventilatory support: Patients with OI often have kyphoscoliosis and thoracic cage deformities. Scoliosis occurs in only 26% of the individuals with OI under the age of 5 years, increasing to 82% of older children [38]. Those changes lead to limitations of the respiratory function [39], with a reduction in vital capacity and lung compliance, and hypoxemia due to inefficient ventilation/perfusion ratio [34]. This might require, during mechanical ventilation, higher oxygen concentration and greater positive end-expiratory pressure. Other ventilatory parameters recommended for those infants are: tidal volume of 4-8 ml/kg, and inspiratory pressure lower than 30 cm H2O [40].
Conclusion
Because OI can lead to intra- and post-operative complications, previous multidisciplinary planning is essential for the surgical success of patients with that disease. Prevention of pitfalls with measures already established increases the safety and success of the procedure.
Contributors’ Statement
Dr. Arnet and Dr. Pereira conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. Magalhães, Dr. Airoza, Dr Barcelos and Dr. Berg collected data and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- Glorieux FH (2008) Osteogenesis Imperfecta. Best Practice & Research Clin Rheumat 22: 85-100.
- Weinstein SL, Buckwalter AJ (2000) Tecidos músculo-esqueléticos e sistema músculo-esquelético. Sao Paulo: Manole 13-68.
- Van Dijk FS, Sillence DO (2014) Osteogenesis Imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet 164: 1470-1481.
- Sillence DO, Senn A, Danks DM (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16: 101-116.
- Sillence DO (1981) Osteogenesis Imperfect: an expanding panorama of variants. Clin Ortop 159: 11-25.
- Rauch F, Glorieux FH (2004) Osteogenesis Imperfecta. Lancet 363: 1377-1385.
- Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, et al. (2007) Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet 39: 359-365.
- Shapiro JR, Rowe DW (1982) Clinical research in Osteogenesis Imperfecta. AAOS symposium on heritable disorders of connective tissue. St Louis, CV Mosby Co.
- Millington-Sanders C, Meir A, Lawrence L, Stolinski C (1998) Structure of chordae tendineae in the left ventricle of the human heart. J Anat 192: 573-581.
- Vouyouka AG, Pfeiffer BJ, Liem TK, Taylor TA, Mudaliar J, et al. (2001) The role of type I collagen in aortic wall strength with a homotrimeric. J Vasc Surg 33: 1263-1270.
- Stein D, Kloster FE (1997) Valvular heart disease in osteogenesis imperfecta. Am Heart J 94: 637.
- Rowe RD (1978) Aortopulmonary septal defect. In: Keith JD, Rowe RD, Vlad P (eds.) Heart Disease in infancy and childhood, McMillan, New York, USA.
- Mori K, Ando M, Takao A, Ishikawa S, Imai Y (1978) Distal type of aortopulmonary window. Br Heart J 40: 681-689.
- Ho SY, Gerlis LM, Anderson C (1994) The morphology of aortopulmonary windows with regard to their classification and morphogenesis. Cardiol Young 4: 146-155.
- Tiraboshi R, Salomone G, Crupi G (1998) Aortopulmonary window in the first year of life: Report on 11 surgical cases. Ann Thorac Surg 46: 438-441.
- Faulkner LS, Oldham RR, Atwood GF, Graham TP (1974) Aortopulmonary window, ventricular septal defect, and membranous pulmonary atresia with a diagnosis of truncus arteriosus. Chest 65: 351-353.
- Cormack R, Lehane J (1984) Difficult tracheal intubation in obstetrics. Anaesthesia 39: 1105 -1111.
- Gross RE (1952) Surgical closure of an aortic septal defect. Circulation 5: 858-863.
- Barnes ME, Mitchell ME, Tweddell JS (2011) Aortopulmonary window. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 14: 67-74.
- Costa TJ, Damry N, Jacquemart C, Christophe C (2014) Aortopulmonary Window: a rare congenital heart disease. JBR-BTR 97: 356-357.
- Melby SJ, Gandhi SK (2009) Current treatment of aortopulmonary window. Curr Treat Options Cardiovasc Med 11: 392-395.
- Naimo PS, Young MS, d’Udekem Y (2014) Outcomes of aortopulmonary window repair in children: 33 years of experience. Ann Thorac Surg 98: 1674-1679.
- Schena F, Capelleri A, Picciolli I, Mayer A, Francescato G, et al. (2015) A case report of aortopulmonary window in an infant born to a diabetic mother. J Card Cases 12: 173-175.
- McElhinney DB, Reddy VM, Tworetzky W, Silverman NH, Hanley FL (1998) Early and late results after repair of aortopulmonary septal defect and associated anomalies in infants <6 months of age. Amer J Cardiol 81: 195-201.
- Tkebuchava T, von Segesser LK, Vogt PR, Bauersfeld U, Jenni R, et al. (1997) Congenital aortopulmonary window: diagnosis, surgical technique and long-term results. Europ J Cardio-thoracic Surgery 11: 293-297.
- Libman RH (1981) Anesthetic considerations for the patient with osteogenesis imperfecta. Clin Orthop Relat Res 159: 123-125.
- Evensen SA, Myhre L, Stormorken H (1984) Haemostatic studies in osteogenesis imperfecta. Scand J Haematol 33: 177-179.
- Hathaway WE, Solomons CC, Ott JE (1972) Platelet function and pyrophosphates in osteogenesis imperfecta. Blood 39: 500-509.
- Wong RS, Follis FM, Shively BK, Wernly JÃ (1995) Osteogenesis imperfecta and cardiovascular diseases. Ann Thorac Surg 60: 1439-1443.
- Stehling (1996) Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 84: 732-747.
- Karabiyik L, Parpuco M, Kurtipek O (2002) Total intravenous anaesthesia and the use of an intubating laryngeal mask airway in a patient with osteogenesis imperfecta. Acta Anaesthesiol Scand 46: 618-621.
- Rampton AJ, Kelly DA, Shanahan EC, Ingram GS (1984) Occurrence of malignant hyperpyrexia in a patient with osteogenesis imperfecta. Br J Anaesthesiol 56: 1443-1445.
- Porsborg P, Astrup G, Bendixen D, Lund AM, Ording H (1996) Osteogenesis imperfecta and malignant hyperthermia: is there a relationship? Anaesthesia 51: 863-865.
- Cho E, Dayan SS, Marx GF (1992) Anaesthesia in a parturient with osteogenesis imperfecta. Br J Anaesth 68: 422-423.
- Maya D, Nayyar BM, Patra P (1981) Anaesthetic management of a case of Osteogenesis imperfecta. Clin Orthop Relat Res 159: 123-125.
- Rodrigo C (1995) Anesthesia for maxillary and mandibular osteotomies in osteogenesis imperfecta. Anesth Prog 42: 17-20.
- Ingrid O, Lauren PR (2010) Anesthetic implications for the patient with Osteogenesis imperfecta. AANA J 78: 47-53.
- Papneja C, Tandon MS, Singh D, Ganjoo P (2010) Anaesthetic management in a patient of osteogenesis imperfecta with basilar invagination. Internet J Anesthesiol 78: 47-53.
- Widmann RF, Bitan FD, Laplaza FJ, Burke SW, DiMaio MF, et al. (1999) Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine 24: 1673-1678.
- Mercer M (2000) Anaesthesia for the patient with respiratory disease. Update Anaesth 12: 1-3.
Citation: Arnet V, Pereira S, Magalhães J, Airoza C, Berg T, et al. (2017) Aortopulmonary Window in an Infant with Type I Osteogenesis Imperfecta: Case Report. Neonat Pediatr Med 3: 138. DOI: 10.4172/2572-4983.1000138
Copyright: ©2017 Arnet V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5476
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 4645
- PDF downloads: 831